Abstract
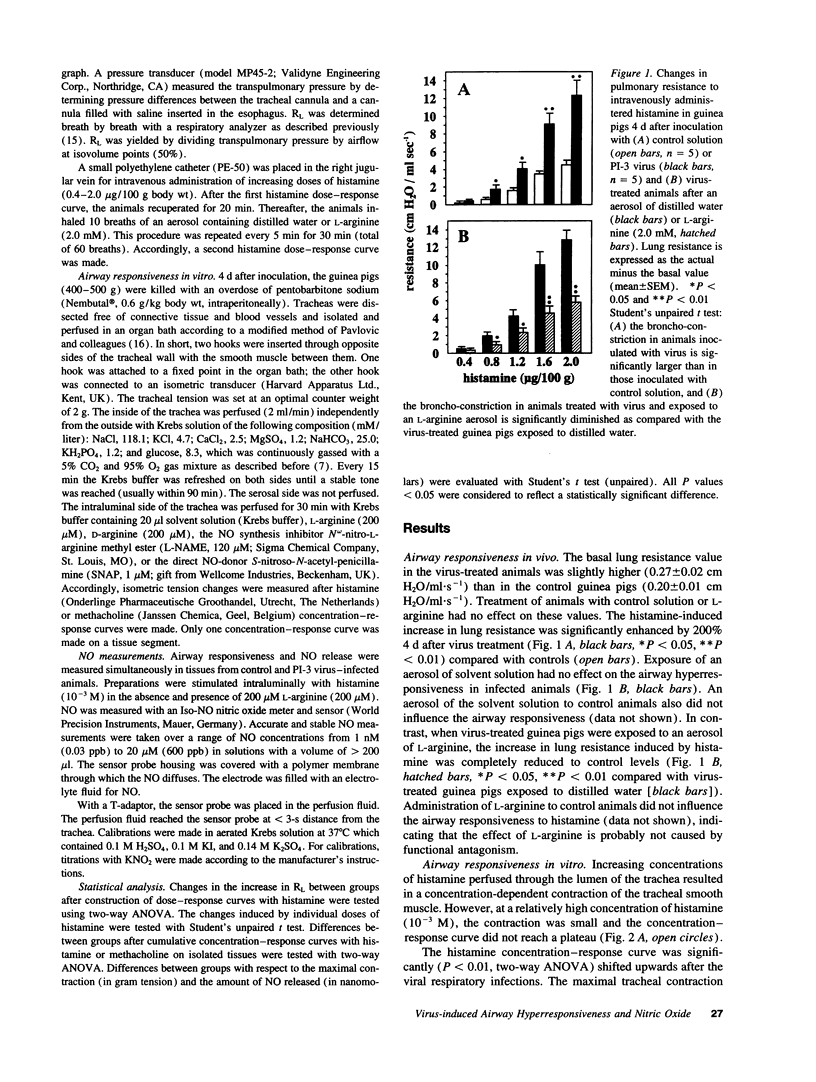
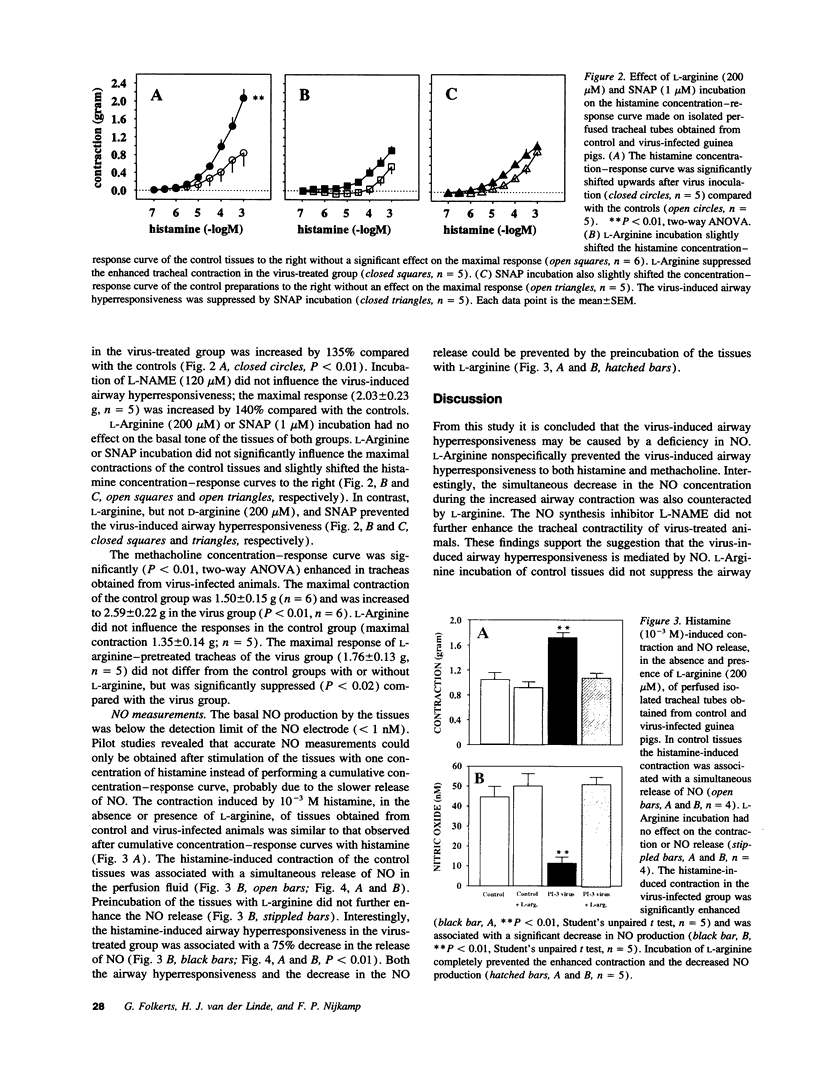
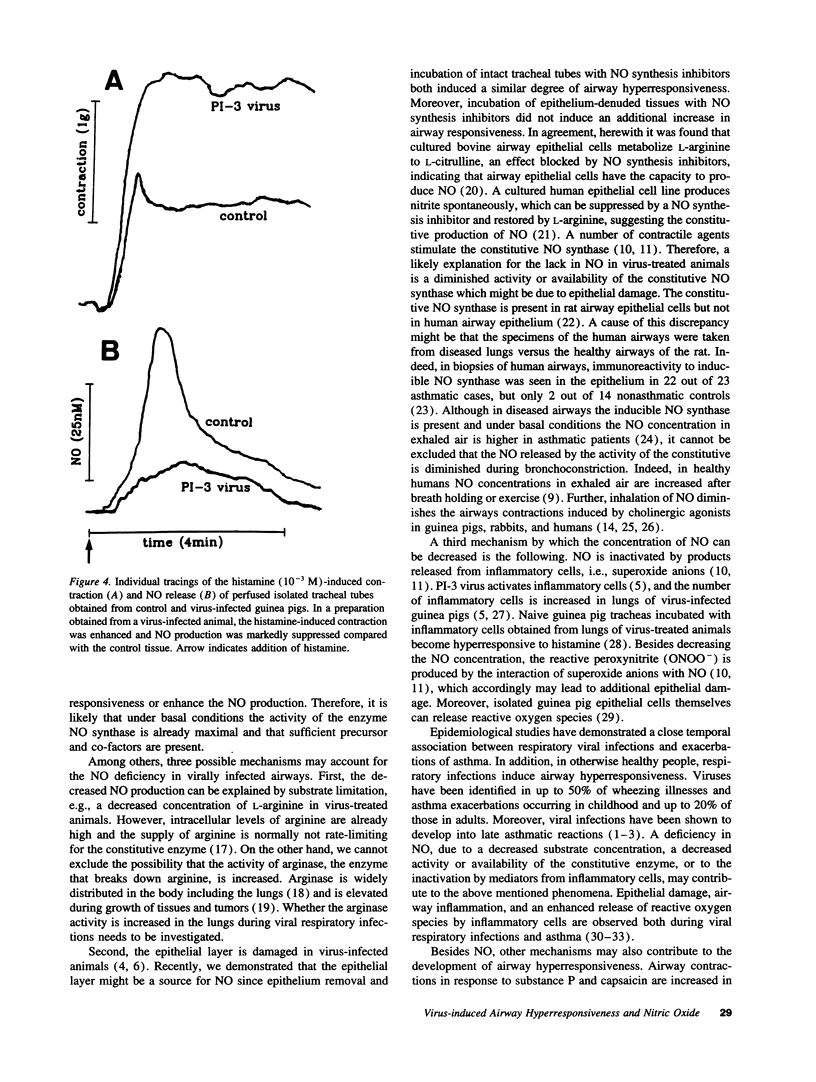
Intratracheal inoculation of parainfluenza type 3 virus to guinea pigs induces a marked increase in airway responsiveness in vivo and in vitro. In spontaneously breathing anesthetized guinea pigs inhalation of an aerosol containing the nitric oxide (NO) precursor L-arginine (2.0 mM) completely prevented the virus-induced airway hyperresponsiveness to histamine. In addition, perfusion of L-arginine (200 microM) or the direct NO-donor S-nitroso-N-acetyl-penicillamine (SNAP, 1 microM) through the lumen of tracheal tubes from infected animals prevented the increase in airway responsiveness to histamine or the cholinoceptor agonist methacholine. The NO synthase inhibitor N omega-nitro-L-arginine methyl ester (L-NAME, 120 microM) did not further increase the virus-induced airway hyperresponsiveness. In additional experiments, NO was measured with an Iso-NO nitric oxide meter and sensor. Stimulation of control tissues in vitro with histamine (10(-3) M) resulted in a contraction with a simultaneous release of NO (44.5 +/- 5.4 nM). The release of NO was markedly reduced by 75% (P < 0.01, 11.4 +/- 3.1 nM) in tracheas from virus-infected animals that demonstrated enhanced contractile responses. Preincubation of tissues from virus-treated guinea pigs with L-arginine (200 microM) completely prevented the enhanced contraction and simultaneously returned the NO production to control values (51.2 +/- 3.4 nM). An NO deficiency might be causally related to the development of airway hyperresponsiveness after a viral respiratory infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminlari M., Vaseghi T. Arginase distribution in tissues of domestic animals. Comp Biochem Physiol B. 1992 Oct;103(2):385–389. doi: 10.1016/0305-0491(92)90309-f. [DOI] [PubMed] [Google Scholar]
- Bardin P. G., Johnston S. L., Pattemore P. K. Viruses as precipitants of asthma symptoms. II. Physiology and mechanisms. Clin Exp Allergy. 1992 Sep;22(9):809–822. doi: 10.1111/j.1365-2222.1992.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J., Belvisi M. G. Nitric oxide and lung disease. Thorax. 1993 Oct;48(10):1034–1043. doi: 10.1136/thx.48.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse W. W. Viral infections and allergic disease. Clin Exp Allergy. 1991 Jan;21 (Suppl 1):68–71. doi: 10.1111/j.1365-2222.1991.tb01708.x. [DOI] [PubMed] [Google Scholar]
- Calhoun W. J., Reed H. E., Moest D. R., Stevens C. A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992 Feb;145(2 Pt 1):317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Dupuy P. M., Shore S. A., Drazen J. M., Frostell C., Hill W. A., Zapol W. M. Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest. 1992 Aug;90(2):421–428. doi: 10.1172/JCI115877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerts G., De Clerck F., Reijnart I., Span P., Nijkamp F. P. Virus-induced airway hyperresponsiveness in the guinea-pig: possible involvement of histamine and inflammatory cells. Br J Pharmacol. 1993 Apr;108(4):1083–1093. doi: 10.1111/j.1476-5381.1993.tb13509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerts G., Henricks P. A., Slootweg P. J., Nijkamp F. P. Endotoxin-induced inflammation and injury of the guinea pig respiratory airways cause bronchial hyporeactivity. Am Rev Respir Dis. 1988 Jun;137(6):1441–1448. doi: 10.1164/ajrccm/137.6.1441. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Van Esch B., Janssen M., Nijkamp F. P. Virus-induced airway hyperresponsiveness in guinea pigs in vivo: study of broncho-alveolar cell number and activity. Eur J Pharmacol. 1992 Dec 1;228(4):219–227. doi: 10.1016/0926-6917(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Verheyen A. K., Geuens G. M., Folkerts H. F., Nijkamp F. P. Virus-induced changes in airway responsiveness, morphology, and histamine levels in guinea pigs. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1569–1577. doi: 10.1164/ajrccm/147.6_Pt_1.1569. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Verheyen A., Janssen M., Nijkamp F. P. Virus-induced airway hyperresponsiveness in the guinea pig can be transferred by bronchoalveolar cells. J Allergy Clin Immunol. 1992 Sep;90(3 Pt 1):364–372. doi: 10.1016/s0091-6749(05)80016-8. [DOI] [PubMed] [Google Scholar]
- Folkerts G., Verheyen A., Nijkamp F. P. Viral infection in guinea pigs induces a sustained non-specific airway hyperresponsiveness and morphological changes of the respiratory tract. Eur J Pharmacol. 1992 Sep 1;228(2-3):121–130. doi: 10.1016/0926-6917(92)90021-4. [DOI] [PubMed] [Google Scholar]
- Folkerts G., van der Linde H. J., Omini C., Nijkamp F. P. Virus-induced airway inflammation and hyperresponsiveness in the guinea-pig is inhibited by levodropropizine. Naunyn Schmiedebergs Arch Pharmacol. 1993 Aug;348(2):213–219. doi: 10.1007/BF00164801. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. E., Leone A. M., Persson M. G., Wiklund N. P., Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991 Dec 16;181(2):852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- Högman M., Frostell C. G., Hedenström H., Hedenstierna G. Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis. 1993 Dec;148(6 Pt 1):1474–1478. doi: 10.1164/ajrccm/148.6_Pt_1.1474. [DOI] [PubMed] [Google Scholar]
- Högman M., Frostell C., Arnberg H., Hedenstierna G. Inhalation of nitric oxide modulates methacholine-induced bronchoconstriction in the rabbit. Eur Respir J. 1993 Feb;6(2):177–180. [PubMed] [Google Scholar]
- Jacoby D. B., Tamaoki J., Borson D. B., Nadel J. A. Influenza infection causes airway hyperresponsiveness by decreasing enkephalinase. J Appl Physiol (1985) 1988 Jun;64(6):2653–2658. doi: 10.1152/jappl.1988.64.6.2653. [DOI] [PubMed] [Google Scholar]
- Jansen A., Drazen J., Osborne J. A., Brown R., Loscalzo J., Stamler J. S. The relaxant properties in guinea pig airways of S-nitrosothiols. J Pharmacol Exp Ther. 1992 Apr;261(1):154–160. [PubMed] [Google Scholar]
- Kharitonov S. A., Yates D., Robbins R. A., Logan-Sinclair R., Shinebourne E. A., Barnes P. J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994 Jan 15;343(8890):133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- Kinnula V. L., Adler K. B., Ackley N. J., Crapo J. D. Release of reactive oxygen species by guinea pig tracheal epithelial cells in vitro. Am J Physiol. 1992 Jun;262(6 Pt 1):L708–L712. doi: 10.1152/ajplung.1992.262.6.L708. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993 Oct;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- McCall T., Vallance P. Nitric oxide takes centre-stage with newly defined roles. Trends Pharmacol Sci. 1992 Jan;13(1):1–6. doi: 10.1016/0165-6147(92)90002-n. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., Folkerts G. Nitric oxide and bronchial reactivity. Clin Exp Allergy. 1994 Oct;24(10):905–914. doi: 10.1111/j.1365-2222.1994.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., van der Linde H. J., Folkerts G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro. Role of the epithelium. Am Rev Respir Dis. 1993 Sep;148(3):727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- Pattemore P. K., Johnston S. L., Bardin P. G. Viruses as precipitants of asthma symptoms. I. Epidemiology. Clin Exp Allergy. 1992 Mar;22(3):325–336. doi: 10.1111/j.1365-2222.1992.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic D., Fournier M., Aubier M., Pariente R. Epithelial vs. serosal stimulation of tracheal muscle: role of epithelium. J Appl Physiol (1985) 1989 Dec;67(6):2522–2526. doi: 10.1152/jappl.1989.67.6.2522. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Wiklund N. P., Gustafsson L. E. Endogenous nitric oxide in single exhalations and the change during exercise. Am Rev Respir Dis. 1993 Nov;148(5):1210–1214. doi: 10.1164/ajrccm/148.5.1210. [DOI] [PubMed] [Google Scholar]
- Robbins R. A., Hamel F. G., Floreani A. A., Gossman G. L., Nelson K. J., Belenky S., Rubinstein I. Bovine bronchial epithelial cells metabolize L-arginine to L-citrulline: possible role of nitric oxide synthase. Life Sci. 1993;52(8):709–716. doi: 10.1016/0024-3205(93)90232-r. [DOI] [PubMed] [Google Scholar]
- Saban R., Dick E. C., Fishleder R. I., Buckner C. K. Enhancement by parainfluenza 3 infection of contractile responses to substance P and capsaicin in airway smooth muscle from the guinea pig. Am Rev Respir Dis. 1987 Sep;136(3):586–591. doi: 10.1164/ajrccm/136.3.586. [DOI] [PubMed] [Google Scholar]
- Taylor A. A., Stewart G. R. Tissue and subcellular localization of enzymes of arginine metabolism in Pisum sativum. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1281–1289. doi: 10.1016/0006-291x(81)91586-2. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Wong D. T., Sun M., Middleton E., Jr, Vaughan R. S., Ogra P. L. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981 Oct 8;305(15):841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]




