Abstract
Disruption of the mdr2 gene in mice leads to a complete absence of phospholipid from bile (Smit, J. J. M., et al. 1993. Cell. 75:451-462). We have investigated the control of both mdr2 P-glycoprotein (Pgp) expression and bile salt secretion on biliary lipid secretion in the mouse. Lipid secretion was monitored at various bile salt output rates in wild-type mice (+/+), heterozygotes (+/-), and homozygotes (-/-) for mdr2 gene disruption. In (-/-) mice, phospholipid secretion was negligible at all bile salt output rates. In (+/-) mice, a curvilinear relation between bile salt and phospholipid secretion was observed similar to that in (+/+) mice; however, at all bile salt secretion rates phospholipid secretion was reduced compared to (+/+) mice, indicating that mdr2 Pgp exerts a strong control over secretion. Infusion of increasing amounts of taurocholate up to maximal secretory rate led to a decline in the phospholipid and cholesterol secretion in both (+/+) and (+/-) mice in accordance to what has been observed in other species. In contrast, in (-/-) mice cholesterol secretion increased under these conditions while phospholipid output remained extremely low. The increased cholesterol secretion may represent extraction of cholesterol from the canalicular plasma membrane by taurocholate micelles as opposed to the concomitant secretion of both phospholipid and cholesterol in the presence of a functional mdr2 Pgp. Increased bile flow in (-/-) mice could be attributed completely to an increase in the bile salt-independent fraction and may therefore be caused by the bile duct proliferation in these mice.
Full text
PDF
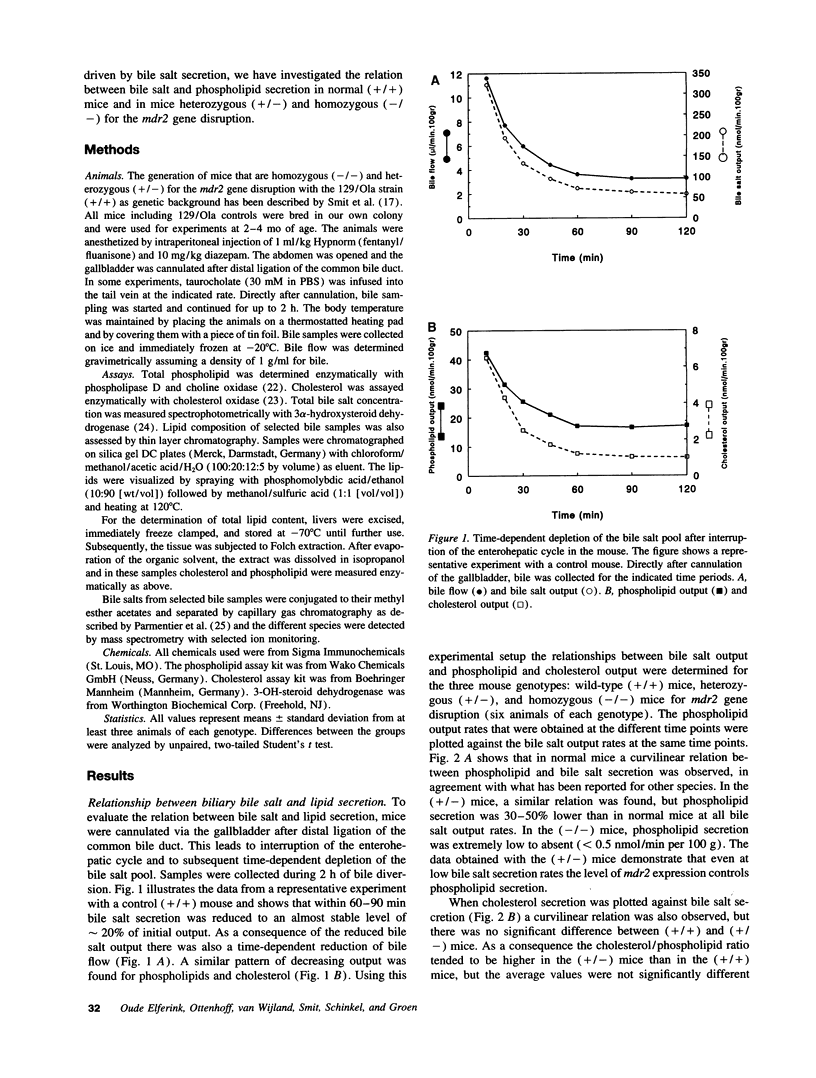
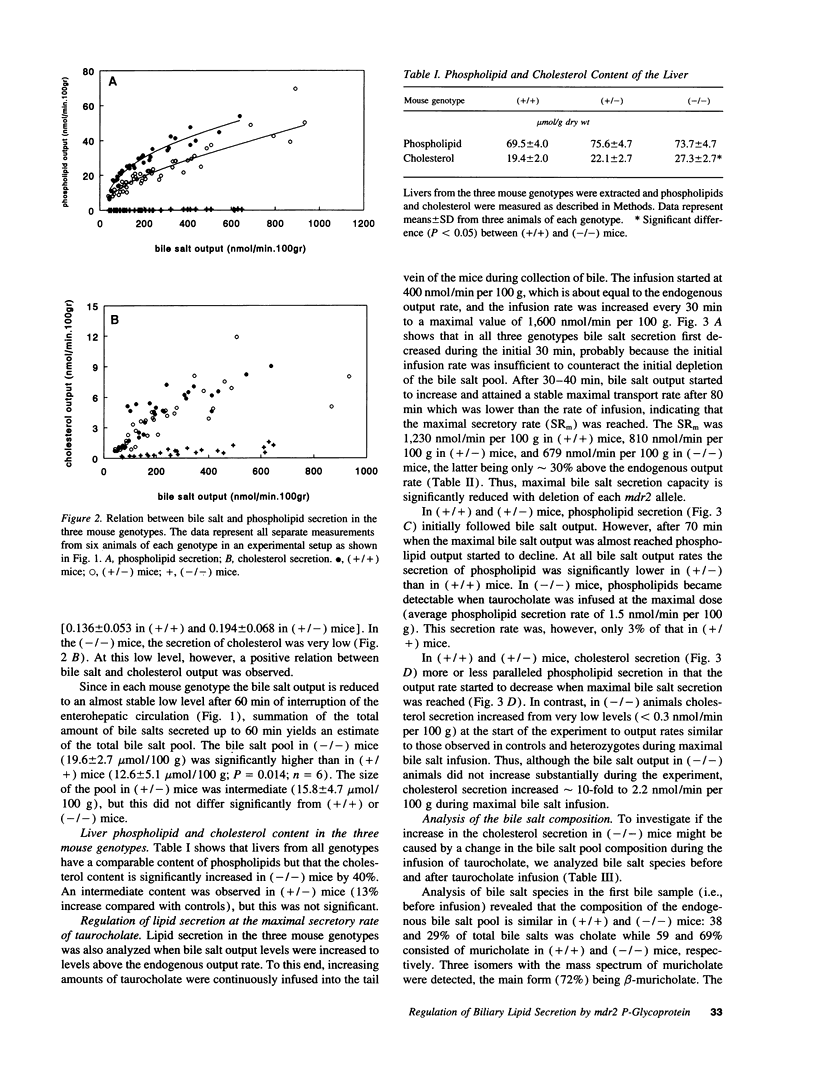
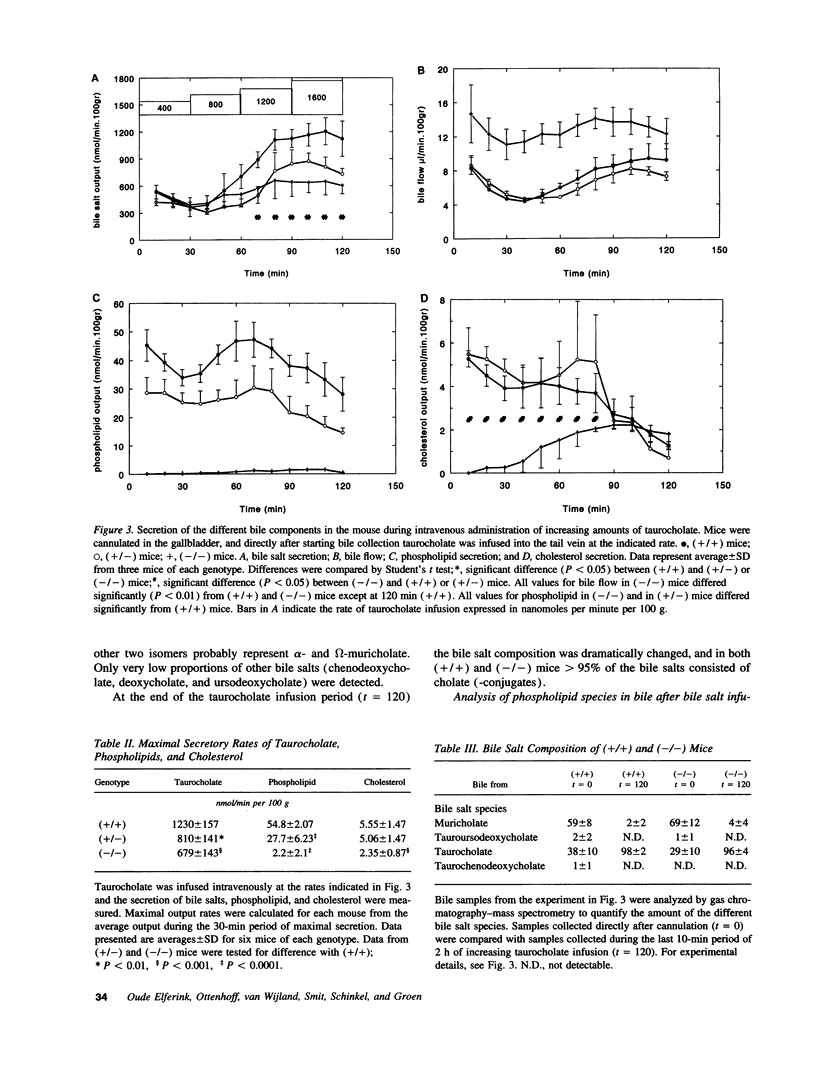



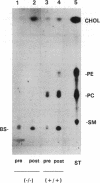
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988 Feb;81(2):569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint J. A., Beeler D. A., Kyriakides E. C., Treble D. H. The effect of bile salts upon lecithin synthesis. J Lab Clin Med. 1971 Jan;77(1):122–133. [PubMed] [Google Scholar]
- Ballatori N., Truong A. T. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992 Nov;263(5 Pt 1):G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Truong A. T. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am J Physiol. 1989 Jan;256(1 Pt 1):G22–G30. doi: 10.1152/ajpgi.1989.256.1.G22. [DOI] [PubMed] [Google Scholar]
- Barnwell S. G., Lowe P. J., Coleman R. The effects of colchicine on secretion into bile of bile salts, phospholipids, cholesterol and plasma membrane enzymes: bile salts are secreted unaccompanied by phospholipids and cholesterol. Biochem J. 1984 Jun 15;220(3):723–731. doi: 10.1042/bj2200723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell S. G., Tuchweber B., Yousef I. M. Biliary lipid secretion in the rat during infusion of increasing doses of unconjugated bile acids. Biochim Biophys Acta. 1987 Nov 21;922(2):221–233. doi: 10.1016/0005-2760(87)90158-5. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter. Cell. 1985 Aug;42(1):51–60. doi: 10.1016/s0092-8674(85)80100-8. [DOI] [PubMed] [Google Scholar]
- Bode C., Zelder O., Goebell H., Neuberger H. O. Choleresis induced by secretin: distinctly increased response in cirrhotics. Scand J Gastroenterol. 1972;7(8):697–699. doi: 10.3109/00365527209180980. [DOI] [PubMed] [Google Scholar]
- Buschman E., Arceci R. J., Croop J. M., Che M., Arias I. M., Housman D. E., Gros P. mdr2 encodes P-glycoprotein expressed in the bile canalicular membrane as determined by isoform-specific antibodies. J Biol Chem. 1992 Sep 5;267(25):18093–18099. [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978 Apr;61(4):998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. Biochemistry of bile secretion. Biochem J. 1987 Jun 1;244(2):249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Rahman K., Kan K. S., Parslow R. A. Retrograde intrabiliary injection of amphipathic materials causes phospholipid secretion into bile. Taurocholate causes phosphatidylcholine secretion, 3-[(3-cholamidopropyl)dimethylammonio]-propane-1-sulphonate (CHAPS) causes mixed phospholipid secretion. Biochem J. 1989 Feb 15;258(1):17–22. doi: 10.1042/bj2580017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Rahman K. Lipid flow in bile formation. Biochim Biophys Acta. 1992 Apr 23;1125(2):113–133. doi: 10.1016/0005-2760(92)90036-u. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Berken C. A., Gollan J. L. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J Lipid Res. 1988 Feb;29(2):144–156. [PubMed] [Google Scholar]
- Crawford J. M., Gollan J. L. Transcellular transport of organic anions in hepatocytes: still a long way to go. Hepatology. 1991 Jul;14(1):192–197. doi: 10.1002/hep.1840140131. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gregory D. H., Vlahcevic Z. R., Prugh M. F., Swell L. Mechanism of secretion of biliary lipids: role of a microtubular system in hepatocellular transport of biliary lipids in the rat. Gastroenterology. 1978 Jan;74(1):93–100. [PubMed] [Google Scholar]
- Gurantz D., Laker M. F., Hofmann A. F. Enzymatic measurement of choline-containing phospholipids in bile. J Lipid Res. 1981 Feb;22(2):373–376. [PubMed] [Google Scholar]
- Hardison W. G., Hatoff D. E., Miyai K., Weiner R. G. Nature of bile acid maximum secretory rate in the rat. Am J Physiol. 1981 Oct;241(4):G337–G343. doi: 10.1152/ajpgi.1981.241.4.G337. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Ng O. C., Ma A., Boyer J. L., Cheng O. Taurocholate stimulates transcytotic vesicular pathways labeled by horseradish peroxidase in the isolated perfused rat liver. Gastroenterology. 1990 Jul;99(1):216–228. doi: 10.1016/0016-5085(90)91251-z. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Homolya L., Holló Z., Germann U. A., Pastan I., Gottesman M. M., Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993 Oct 15;268(29):21493–21496. [PubMed] [Google Scholar]
- Lamri Y., Roda A., Dumont M., Feldmann G., Erlinger S. Immunoperoxidase localization of bile salts in rat liver cells. Evidence for a role of the Golgi apparatus in bile salt transport. J Clin Invest. 1988 Oct;82(4):1173–1182. doi: 10.1172/JCI113714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. J., Barnwell S. G., Coleman R. Rapid kinetic analysis of the bile-salt-dependent secretion of phospholipid, cholesterol and a plasma-membrane enzyme into bile. Biochem J. 1984 Sep 15;222(3):631–637. doi: 10.1042/bj2220631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo M. P., Rigotti A., Nervi F. Secretion of biliary lipids from the hepatocyte. Hepatology. 1990 Sep;12(3 Pt 2):134S–142S. [PubMed] [Google Scholar]
- Mazer N. A., Carey M. C. Mathematical model of biliary lipid secretion: a quantitative analysis of physiological and biochemical data from man and other species. J Lipid Res. 1984 Sep;25(9):932–953. [PubMed] [Google Scholar]
- Montet J. C., Parquet M., Sacquet E., Montet A. M., Infante R., Amic J. beta-Muricholic acid; potentiometric and cholesterol-dissolving properties. Biochim Biophys Acta. 1987 Mar 13;918(1):1–10. doi: 10.1016/0005-2760(87)90002-6. [DOI] [PubMed] [Google Scholar]
- Parmentier G. G., Janssen G. A., Eggermont E. A., Eyssen H. J. C27 bile acids in infants with coprostanic acidemia and occurrence of a 3 alpha,7 alpha,12 alpha-tridhydroxy-5 beta-C29 dicarboxylic bile acid as a major component in their serum. Eur J Biochem. 1979 Dec;102(1):173–183. doi: 10.1111/j.1432-1033.1979.tb06278.x. [DOI] [PubMed] [Google Scholar]
- Ruetz S., Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994 Jul 1;77(7):1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Sagawa H., Tazuma S., Kajiyama G. Protection against hydrophobic bile salt-induced cell membrane damage by liposomes and hydrophilic bile salts. Am J Physiol. 1993 May;264(5 Pt 1):G835–G839. doi: 10.1152/ajpgi.1993.264.5.G835. [DOI] [PubMed] [Google Scholar]
- Sakisaka S., Ng O. C., Boyer J. L. Tubulovesicular transcytotic pathway in isolated rat hepatocyte couplets in culture. Effect of colchicine and taurocholate. Gastroenterology. 1988 Sep;95(3):793–804. doi: 10.1016/s0016-5085(88)80030-1. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov 5;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Swell L., Bell C. C., Jr, Entenman C. Bile acids and lipid metabolism. 3. Influence of bile acids on phospholipids in liver and bile of the isolated perfused dog liver. Biochim Biophys Acta. 1968 Oct 22;164(2):278–284. [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Jones E. A., Sherlock S. Biliary secretion in a patient with cystic dilation of the intrahepatic biliary tree. Gastroenterology. 1968 Jun;54(6):1155–1161. [PubMed] [Google Scholar]
- Uchida K., Akiyoshi T., Igimi H., Takase H., Nomura Y., Ishihara S. Differential effects of ursodeoxycholic acid and ursocholic acid on the formation of biliary cholesterol crystals in mice. Lipids. 1991 Jul;26(7):526–530. doi: 10.1007/BF02536598. [DOI] [PubMed] [Google Scholar]
- Velardi A. L., Groen A. K., Elferink R. P., van der Meer R., Palasciano G., Tytgat G. N. Cell type-dependent effect of phospholipid and cholesterol on bile salt cytotoxicity. Gastroenterology. 1991 Aug;101(2):457–464. doi: 10.1016/0016-5085(91)90025-g. [DOI] [PubMed] [Google Scholar]
- Wheeler H. O., King K. K. Biliary excretion of lecithin and cholesterol in the dog. J Clin Invest. 1972 Jun;51(6):1337–1350. doi: 10.1172/JCI106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- Yousef I. M., Barnwell S., Gratton F., Tuchweber B., Weber A., Roy C. C. Liver cell membrane solubilization may control maximum secretory rate of cholic acid in the rat. Am J Physiol. 1987 Jan;252(1 Pt 1):G84–G91. doi: 10.1152/ajpgi.1987.252.1.G84. [DOI] [PubMed] [Google Scholar]
- Yousef I., Mignault D., Tuchweber B. Effect of complete sulfation of bile acids on bile formation: role of conjugation and number of sulfate groups. Hepatology. 1992 Mar;15(3):438–445. doi: 10.1002/hep.1840150314. [DOI] [PubMed] [Google Scholar]