Abstract
Blood vessels and neurons use similar guidance cues to control their behaviour during embryogenesis. The semaphorin SEMA3A was originally identified as a repulsive cue for developing axons that acts by signalling through receptor complexes containing NRP1 and A-type plexins. SEMA3A also competes with the VEGF164 isoform of vascular endothelial growth factor for binding to NRP1 to modulate the migration of endothelial cells in vitro. Surprisingly, we have found that SEMA3A and semaphorin-signalling through NRP1 were not required for blood vessel development in the mouse. Moreover, we found that there was no genetic interaction between SEMA3A and VEGF164 during vasculogenesis or angiogenesis. Our observations suggest that in vivo vascular NRP1 preferentially confers VEGF164 signals, whilst axonal NRP1 preferentially transmits SEMA3A signals.
Keywords: semaphorin, VEGF, VEGF164, neuropilin, angiogenesis, vasculogenesis, blood vessel, nerve, axon
Introduction
Developing blood vessels and axons branch throughout the vertebrate body and employ similar cell biological mechanisms to invade and navigate within tissues. For example, endothelial tip cells send out filopodia to explore their territory for guidance cues, just like axon growth cones (Gerhardt et al. 2003). Several recent studies have also explored the idea that axon guidance cues control blood vessel branching. For example, ephrin/EPH ligand/receptor pairs, netrins with their UNC and DCC receptors and neuropilin 1 (NRP1) with its ligands have all been implicated in neuronal and vascular patterning (Eichmann et al. 2005). In this chapter, we will discuss recent published data as well as our own unpublished observations to determine the relative contribution of two different NRP1 ligands to neuronal and vascular development, the class 3 semaphorin SEMA3A and an isoform of vascular endothelial growth factor termed VEGF164 (Raper 2000; Ruhrberg 2003).
Two working models have previously been put forward that implicate SEMA3A in vascular patterning (Fig. 1). According to one model (Fig. 1A), SEMA3A binds to an endothelial receptor composed of NRP1 and a plexin (Serini and Bussolino 2004). Originally, it was thought that the class 3 semaphorin receptor contained an A-type plexin, but recent in vitro work has shown that plexin D1 can also cooperate with NRP1 to form a SEMA3A receptor (Gitler et al. 2004). Moreover, in zebrafish plexin D1 controls blood vessel branching, with SEMA3A2, the zebrafish homolog of murine SEMA3A, being a possible ligand for plexin D1 (Torres-Vazquez et al. 2004). Interestingly, at least in the mouse, NRP1 is not required for semaphorin-signalling through plexin D1 (Gu et al. 2005). According to an alternative model (Fig. 1B), SEMA3A controls vascular patterning by competing with VEGF164 for NRP1 binding (Miao et al. 1999). In this model, VEGF164 acts through a receptor composed of NRP1 and VEGFR2 (alternative names FLK1 and KDR) to stimulate endothelial cell migration and proliferation, and SEMA3A inhibits this pathway. To evaluate the suitability of either model to explain the molecular control of vascular patterning, we examined the genetic requirement for SEMA3A during vascular morphogenesis and blood vessel branching in the mouse, and compared the phenotypes of SEMA3A null mutants to the known defects of mutants lacking VEGF164 alone or both VEGF164 and SEMA3A. We find that neither model is suitable to describe a mechanism operating in vascular development in the mouse, as SEMA3A and semaphorin-signalling through neuropilins are not essential for blood vessel formation or angiogenic vessel branching.
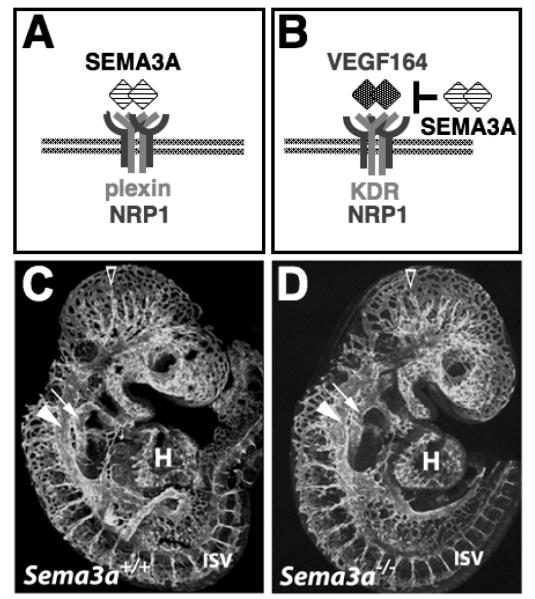
Fig. 1. Working models that have been put forward to explain how SEMA3A affects blood vessel growth.
According to model (A), SEMA3A binds to an endothelial receptor composed of NRP1 and a plexin. According to model (B), SEMA3A competes with VEGF164 binds to an endothelial receptor composed of NRP1 and KDR (also known as VEGFR2 or FLK1). (C,D) Visualisation of the endomucin-positive cardiovasculature in stage-matched littermate embryos expressing (C) or lacking (D) SEMA3A in a CD1 background at 9.5 dpc, when vessel networks have extended throughout the embryo and have begun to remodel into the large head vessels (open arrowhead); the anterior cardinal vein (arrowhead) and dorsal aorta (arrow) are clearly visible.
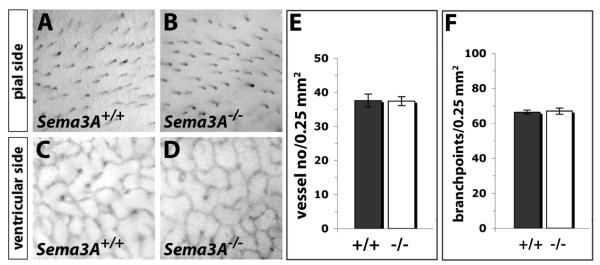
Firstly, we found that the vasculature of SEMA3A null mutants at E9.5 and E10.5 was indistinguishable from that of stage-matched wild type littermates in both genetic backgrounds examined, C57Bl/6 and CD1 mice (Fig. 1C,D and data not shown; unpublished observations). Specifically, we observed normal vascular remodelling in the head and identified a paired dorsal aorta and paired anterior cardinal veins in all mutants. At both developmental stages, heart development and formation of the pharyngeal arch arteries appeared normal. These observations suggest that SEMA3A is not required for the formation of blood vessel networks. Secondly, we determined if vessel branching in the hindbrain was reduced in the absence of SEMA3A, because (a) brain vessels are known to be affected severely by loss of NRP1, (b) vessel branching is readily quantifiable in this model system, and (c) SEMA3A is expressed in the hindbrain (Kawasaki et al. 1999; Chilton and Guthrie 2003; Gerhardt et al. 2003; Schwarz et al. 2004). We found that hindbrain vessels formed normal networks in the absence of SEMA3A in either the C57Bl/6 or CD1 background: At E12.5, a similar number of vessels had branched from the perineural vascular plexus into the hindbrain on its pial side, and a similar number of branch points was present in the vessel plexus that formed in the subventricular zone (C57Bl/6 data shown in Fig. 2; CD1 data not shown; unpublished observations). Our findings contrast those of another group that recently described vessel defects in SEMA3A null mutants in the CD1 background (Serini et al. 2003), even though Serini and co-workers reported to have analysed mice carrying the same gene-targeting event we have examined (Taniguchi et al. 1997). We therefore confirmed that our SEMA3A null mutants contained the correct gene targeting event by examining their nerve patterning. In agreement with previously published observations, we found that loss of SEMA3A caused extensive axon defasciculation of the cranial and limb nerves in both CD1 and C57Bl/6 backgrounds.
Fig. 2. Loss of Sema3A does not impair brain vascularisation.
Visualisation (A-F) and quantification (G,E) of vessel branching on the pial side (A-C) and in the subventricular zone (D-F) of PECAM-stained 12.5 dpc littermate hindbrains expressing and lacking SEMA3A in a C57/Bl6 background.
The alternative model of SEMA3A function in the vasculature suggests that SEMA3A influences blood vessel endothelium by competing with VEGF164 for NRP1 binding (Miao et al. 1999). To test this model, we have compared vascular pattering in single mutants lacking either VEG164 or SEMA3A to compound mutants lacking both VEGF164 and SEMA3A, and mutants with an imbalanced expression of these two NRP1 ligands. The VEGF mutants we analysed carry a deletion of exons 6 and 7 of the Vegfa gene; this mutation prevents expression of the VEGF164, but not the VEGF120 isoform and therefore circumvents the embryonic lethality observed in mice lacking all VEGF isoforms (Carmeliet et al. 1999). Hindbrains lacking VEGF164 expression contain larger vessels with fewer branch points compared to wild type littermates. This phenotype is caused by an altered distribution of VEGF in the extracellular matrix in the absence of heparin-binding isoforms (Ruhrberg et al. 2002), but it is also similar to the phenotype of mutants lacking NRP1 specifically in the vasculature (Gu et al. 2003) and our unpublished observations).
Importantly, we found that the loss of SEMA3A neither increased nor ameliorated the severity of vascular defects caused by loss of VEGF164 in whole embryos at E9.5 or in the hindbrain model at E12.5 (unpublished observations). Specifically, the vessel branching defect of hindbrains lacking VEGF164 was not affected by abolishing SEMA3A expression, and all compound mutants contained a paired dorsal aorta and paired cardinal vein. These observations suggested that the presence of VEGF120 is sufficient to drive angioblast migration and the assembly of the major vessels in the mouse. Moreover, they demonstrated that SEMA3A does not cooperate with VEGF164 to direct either vasculogenesis or angiogenesis in the mouse, even though SEMA3A1 guides angioblasts to the sites where the paired dorsal aorta will form in zebrafish (Shoji et al. 2003).
To extend our observations on the role of NRP1 ligands in neuronal and vascular patterning to a region of the body where nerves and blood vessels are normally co-patterned, we have examined developing mouse limbs at E12.5. In this organ system, a vascular network develops throughout the limbs prior to axon invasion, but a second vascular network extends alongside growing axons into the interdigital regions. We have found that (a) NRP1 mutants display defects in both nerve and vascular patterning in the limb, with extensive axon defasciculation and reduced vessel branching; (b) SEMA3A mutants phenocopy the axonal defect of NRP1 mutants, but show normal vessel patterning; (c) axonal growth appears at least grossly normal in limbs lacking VEGF164, whilst vessel patterning is disturbed like in NRP1 mutants. Only the simultaneous deletion of both NRP1 ligands impairs both axon and vessel growth and recreates a full NRP1 defect. Our findings suggest that VEGF164 and SEMA3A control distinct patterning events. In agreement with this idea, we have previously shown that SEMA3A and VEGF164 pattern distinct compartments of the facial nerve in the mouse, with SEMA3A, but not VEGF164 controlling the behaviour of facial nerve axons in the branchial arches, and VEGF164, but not SEMA3A guiding the migration of the facial branchiomotor neuron somata inside the hindbrain (Schwarz et al. 2004).
Lastly, we would like to draw attention to our observation that, at the time points we have observed, excess axonal branching in SEMA3A mutants does not result in the induction of ectopic vessels, and that loss of vessel branches in VEGF120 mutants does not impair axon branching. It therefore appears that both types of networks initially grow independently of each other, and that the co-patterning observed in the adult, exemplified for example by the development of a vasa nervorum, must be set up at later developmental stages.
Acknowledgements
We thank Drs Masahiko Taniguchi, Hajime Fujisawa, David D. Ginty, Alex L. Kolodkin and David T. Shima for providing mouse strains. C. R. is funded by the UK Medical Research Council (MRC) and J. M. V holds a PhD studentship from the Fundação para a Ciência e Tecnologia (SFRH/BD/17812/2004).
References
- Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D’Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5(5):495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev Dyn. 2003;228(4):726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19(9):1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7(1):107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5(1):45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307(5707):265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126(21):4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146(1):233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10(1):88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C. Growing and shaping the vascular tree: multiple roles for VEGF. Bioessays. 2003;25(11):1052–1060. doi: 10.1002/bies.10351. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, Taniguchi M, Kolodkin AL, Ginty DD, Shima DT, Ruhrberg C. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18(22):2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Bussolino F. Common cues in vascular and axon guidance. Physiology (Bethesda) 2004;19:348–354. doi: 10.1152/physiol.00021.2004. [DOI] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424(6947):391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130(14):3227–3236. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19(3):519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7(1):117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]