Abstract
In this study, electrospun poly(ε-caprolactone) (PCL) microfiber scaffolds, coated with cartilaginous extracellular matrix (ECM), were fabricated by first culturing chondrocytes under dynamic conditions in a flow perfusion bioreactor and then decellularizing the cellular constructs. The decellularization procedure yielded acellular PCL/ECM composite scaffolds containing glycosaminoglycan and collagen. PCL/ECM composite scaffolds were evaluated for their ability to support the chondrogenic differentiation of mesenchymal stem cells (MSCs) in vitro using serum-free medium with or without the addition of transforming growth factor-β1 (TGF-β1). PCL/ECM composite scaffolds supported chondrogenic differentiation induced by TGF-β1 exposure, as evidenced in the up-regulation of aggrecan (11.6 ± 3.8 fold) and collagen type II (668.4 ± 317.7 fold) gene expression. The presence of cartilaginous matrix alone reduced collagen type I gene expression to levels observed with TGF-β1 treatment. Cartilaginous matrix further enhanced the effects of growth factor treatment on MSC chondrogenesis as evidenced in the higher glycosaminoglycan synthetic activity for cells cultured on PCL/ECM composite scaffolds. Therefore, flow perfusion culture of chondrocytes on electrospun microfiber scaffolds is a promising method to fabricate polymer/extracellular matrix composite scaffolds that incorporate both natural and synthetic components to provide biological signals for cartilage tissue engineering applications.
Introduction
Articular cartilage serves a vital role in normal joint function, but has a limited capacity for regeneration once injured or damaged due to its avascular nature and sparse cell population. Clinical procedures that penetrate the subchondral bone to trigger an intrinsic wound healing response, such as abrasion or microfracture, typically result in fibrous tissue which lacks the structure and function of native cartilage [1]. Other clinical approaches involving the transplantation of osteochondral grafts or autologous chondrocytes require tissue biopsies that damage otherwise healthy cartilage [2, 3]. Therefore, tissue engineering strategies incorporating scaffolds, cells, and bioactive factors to regenerate functional cartilage tissue have emerged as a promising alternative.
Mesenchymal stem cells (MSCs) and chondrocytes are often targeted for cartilage tissue engineering due to their vital role in native cartilage formation and function. Since their cellular processes are influenced by both physical and biological signals, effective biomaterial scaffolds for cartilage repair must not only act as temporary supports for tissue growth, but also as instructive microenvironments to guide cellular function. Thus extracellular matrix components, either as isolated proteins or with complex compositions, have been investigated as scaffolding materials for cartilage tissue engineering in an effort to stimulate chondrogenesis by culturing cells within a biological microenvironment [4].
Recently, sponge-like scaffolds fabricated using collagen and glycosaminoglycan, or purely from native cartilage extracellular matrix components, have been shown to support MSC chondrogenic differentiation [5, 6]. Although MSCs were exposed to chondroinductive growth factors either during expansion or throughout the culture period, these studies demonstrate the application of natural matrices with promising results. Fabricating cartilaginous scaffolds typically entails reconstituting proteins and may even involve crosslinking to strengthen the matrix; processing conditions which can affect matrix biochemistry. Still these scaffolds, even after prolonged culture in vitro, lack sufficient mechanical properties to support joint function during tissue regeneration [6].
Synthetic polymers on the other hand, are more robust scaffolding materials whose physical properties can be easily controlled through tailored processing conditions. Electrospun polymer scaffolds, in particular, are promising candidates for tissue engineering applications due to their nonwoven fiber mesh structure, which imparts a large surface-to-volume ratio for cell attachment and offers a high interconnected porosity for cell and tissue infiltration. Electrospun poly(ε-caprolactone) (PCL) nanofiber scaffolds have been shown to support the attachment and proliferation of chondrocytes and also MSC differentiation along the chondrogenic lineage when cultured with transforming growth factor-β1 (TGF-β1) [7, 8].
Previously, we have demonstrated that culturing MSCs in a flow perfusion bioreactor on fiber mesh scaffolds in the presence of osteogenic cell culture supplements, promotes the deposition of an extracellular matrix (ECM) containing both structural matrix proteins and bioactive growth factors [9]. Upon decellularization, composite scaffolds containing mineralized matrix were capable of inducing MSC osteogenic differentiation even in the absence of dexamethasone, the cell culture supplement often required to stimulate osteogenic differentiation in vitro [10, 11]. Here, we seek to develop composite scaffolds for cartilage repair by incorporating cartilaginous matrix generated under fluid flow perfusion conditions on electrospun PCL microfiber scaffolds. By employing both natural and synthetic components in a tissue engineering scaffold, we aim to provide a more physiological microenvironment containing both structural and biological signals to guide MSC chondrogenic differentiation, while at the same time maintaining physical scaffolding properties in a controllable polymeric system.
In this study, we fabricate PCL/ECM composite scaffolds consisting of electrospun microfibers coated with cartilaginous extracellular matrix, and evaluate their ability to support the chondrogenic differentiation of MSCs in vitro. For the fabrication of PCL/ECM composite scaffolds, we hypothesized that culturing chondrocytes on PCL scaffolds under dynamic conditions in a flow perfusion bioreactor would stimulate the deposition of cartilaginous ECM that remains even after decellularization. In an effort to evaluate the chondrogenic properties of PCL/ECM composite scaffolds, we hypothesized that PCL/ECM scaffolds would support MSC differentiation along the chondrogenic lineage induced by TGF-β1 exposure, and that the presence of cartilaginous matrix would further enhance this differentiation response by providing cells with a more biological microenvironment compared to plain PCL scaffolds. To investigate our hypotheses, bovine chondrocytes were seeded on electrospun PCL microfiber scaffolds and cultured in a flow perfusion bioreactor. The resulting PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds, which were characterized for their cartilaginous matrix morphology and composition in response to the decellularization procedure. PCL/ECM composite scaffolds as well as plain PCL scaffolds were seeded with rabbit MSCs and cultured in serum-free medium either with our without the addition of TGF-β1. Constructs were evaluated for cellularity, glycosaminoglycan content (GAG) and synthetic activity (GAG/DNA), and gene expression through real-time reverse transcription polymerase chain reaction (RT-PCR), to determine how physical matrix interactions and biochemical signaling influence chondrogenic differentiation in vitro.
Materials and Methods
Electrospinning
Nonwoven PCL microfiber mats were fabricated using a horizontal electrospinning setup with a copper ring to stabilize the electric field as previously described [12]. Mats were electrospun to a targeted fiber diameter of 10 μm using a solution of 14 wt % PCL (Sigma-Aldrich, St. Louis, MO) in a 5:1 volume ratio of chloroform to methanol. PCL with Mn = 73,000 ± 9,000 and Mw = 154,000 ± 26,000 was characterized by gel permeation chromatography (Waters, Milford, MA) from three samples relative to polystyrene. The polymer solution was pumped at a flow rate of 18 mL/h while charged with an applied voltage of 25.5 kV to draw microfibers toward the collector plate [11]. The resulting PCL mat was aerated, inspected for consistent microfiber morphology, and stored in a desiccator.
Scaffold preparation
PCL mats were die-punched into scaffolds 6 mm in diameter with thicknesses between 0.95 and 1.05 mm. As previously characterized through scanning electron microscopy and mercury porosimetry, these scaffolds had an average fiber diameter of 9.86 ± 0.56 μm and a porosity of 87% with an average pore size of 45 μm [11, 12]. Prior to use, PCL scaffolds were sterilized with ethylene oxide gas for 14 h and aerated overnight to remove residual fumes. Scaffolds were pre-wetted by centrifuging through a graded series of ethanol from 100% to 70%, followed by three rinses in phosphate buffered saline (PBS), and incubated in cell culture medium overnight. In preparation for cell seeding, scaffolds were press-fitted into cassettes designed to confine the cell suspension and to be used in the flow perfusion bioreactor [13].
PCL/ECM composite scaffold generation
Chondrocytes were harvested and pooled from cartilage collected from the femoral condyle area of four young calves through tissue obtained from Research 87 (Research 87, Boylston, MA) according to previously established methods [14]. Cartilage was collected from the condyles, washed with PBS, and digested in culture medium containing 2 mg/mL collagenase (Worthington, Lakewood, NJ) while incubating at 37 °C overnight. Chondrocytes were frozen in aliquots of medium containing 20% fetal bovine serum (FBS) and 10% dimethyl sulfoxide (DMSO).
PCL/ECM constructs containing cartilaginous extracellular matrix were generated by culturing chondrocytes on electrospun microfiber scaffolds under dynamic conditions in the flow perfusion bioreactor for 9 days, then decellularized through a freeze and thaw procedure to yield PCL/ECM composite scaffolds, as schematically depicted in Fig. 1. Cryopreserved chondrocytes were first thawed at 37 °C and plated in tissue culture flasks with chondrocyte culture medium consisting of DMEM, supplemented with 10% FBS (Gemini Bio-Products, West Sacramento, CA), 1% non-essential amino acids, 0.4 mM proline, 10 mM HEPES buffer, and 50 mg/L ascorbic acid, also with the addition of penicillin, streptomycin, and fungizone (Invitrogen, Carlsbad, CA). Primary chondrocytes were cultured for 7 days in chondrocyte culture medium with medium changes every 3 days. Chondrocytes were lifted with 0.05% trypsin and suspended in culture medium for seeding onto press-fitted scaffolds at a seeding density of 150,000 cells in 200 μL of medium within each cassette. Scaffolds were incubated with the seeding solution for 2 h then medium was added to fill each cassette. After allowing 24 h for cell attachment, constructs in their cassettes were transferred directly into the flow perfusion bioreactor and cultured for 9 days with medium changes every 3 days. Medium was perfused through the press-fitted constructs at a flow rate of 0.3 mL/min to provide cells with some mechanical stimulation and enhance metabolic transport [13].
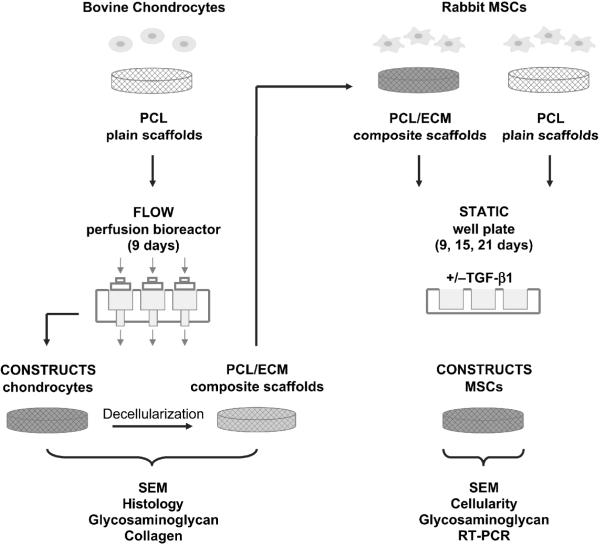
Figure 1.
Schematic representation of the overall experimental design. PCL/ECM constructs were generated through flow perfusion culture of bovine chondrocytes, then decellularized and characterized for matrix morphology and composition in response to the decellularization procedure. PCL/ECM composite scaffolds along with plain PCL polymer scaffolds were seeded with rabbit MSCs and evaluated for their ability to support chondrogenic differentiation in static culture with or without the addition of TGF-β1.
At the end of culture, constructs were rinsed with PBS and stored in 1.5 mL ddH2O at −80 °C. PCL/ECM constructs were decellularized to yield PCL/ECM composite scaffolds via three consecutive cycles of a freeze and thaw procedure, in which constructs were frozen for 10 min in liquid nitrogen then thawed for 10 min in a 37 °C water bath [15]. Even though this decellularization procedure has been shown to yield acellular constructs [16], samples were prepared for scanning electron microscopy, histology, and glycosaminoglycan and collagen assays to assess matrix morphology and composition before and after decellularization. The resulting PCL/ECM composite scaffolds were air-dried overnight, press-fitted into cassettes, sterilized with ethylene oxide gas for 14 h and aerated overnight in preparation for seeding.
Chondrogenic differentiation
MSCs were harvested from bone marrow aspirates taken from the tibiae of six male New Zealand white rabbits (Charles River, Wilmington, MA) weighing 2.8–3.0 kg according to previously established methods [17]. Bone marrow from each leg was aspirated into a 10 mL syringe containing 5,000 U/mL heparin to prevent coagulation. The experimental protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee of Rice University, and all procedures were conducted according to the Principles of Laboratory Animal Care (NIH Publication No. 85-23, Revised 1985). The bone marrow was plated in tissue culture flasks with general expansion medium consisting of DMEM, supplemented with 10% FBS (Gemini Bio-Products, West Sacramento, CA), also with the addition of penicillin, streptomycin, and fungizone (Invitrogen, Carlsbad, CA). Non-adherent cells were washed away after 72 h, and adherent cells were cultured for 14 days in general expansion medium with medium changes every 3 days. After this primary culture period, MSCs were lifted with 0.05% trypsin and pooled from all six rabbits then frozen in aliquots of medium containing 20% FBS and 10% DMSO, in order to reduce variation between individual animals [18].
Cryopreserved MSCs were thawed at 37 °C, plated in tissue culture flasks, and expanded to passage three with general expansion medium for the differentiation study, as schematically illustrated in Fig. 1. MSCs were then trypsinized and seeded onto press-fitted experimental PCL/ECM composite scaffolds and also plain PCL control scaffolds at a density of 150,000 cells in 200 μL of medium within each cassette. Scaffolds were incubated with the seeding solution for 2 h then serum-free chondrogenic medium was added to fill each cassette consisting of DMEM, supplemented with 1% ITS+ Premix (BD Biosciences, San Jose, CA), 10−7 M dexamethasone, 1 mM sodium pyruvate, 50 mg/L ascorbic acid, also with the addition of penicillin, streptomycin, and fungizone (Invitrogen, Carlsbad, CA). After allowing 24 h for cell attachment, constructs were removed from their cassettes and transferred into 24-well plates with 1 mL of medium and cultured under static conditions for 9, 15, and 21 days with serum-free chondrogenic medium either with or without the addition of 10 ng/mL TGF-β1 replenished every 3 days. Eight samples were cultured for each scaffold group (PCL and PCL/ECM) and growth factor treatment (−TGF and +TGF) for each culture time (9, 15, and 21 days), at the end of which samples were rinsed with PBS and stored for later analysis. Three samples were prepared for assessing construct cellularity and glycosaminoglycan content and synthetic activity, one sample was prepared for scanning electron microscopy, and four samples were prepared for assessing gene expression through real-time reverse transcription polymerase chain reaction.
Histology
Samples for histology were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA) then immersed in 70% ethanol prior to embedding in HistoPrep freezing medium (Fisher Scientific, Pittsburgh, PA) and stored at −80 °C. Frozen sections 5 μm thick were cut using a cryostat (Leica Biosystems, Richmond, IL), mounted onto Superfrost Excell glass slides (Fisher Scientific, Pittsburgh, PA), and placed on a 37 °C slide warmer to facilitate adhesion. Sections were stained with hematoxylin to visualize the distribution of cells, and Safranin O to visualize the distribution of cartilaginous extracellular matrix. Images were obtained using a light microscope (Nikon Eclipse E600, Melville, NY) with a video camera attachment (Sony DXC950P, New York, NY).
Biochemical assays
Samples for biochemical assays were digested in 500 μL of a proteinase K solution while incubating in a 56 °C water bath for 16 h. The proteinase K solution consisted of 1 mg/mL proteinase K, 0.01 mg/mL pepstatin A, and 0.185 mg/mL iodoacetamide, in a tris-EDTA buffer made by dissolving 6.055 mg/mL tris(hydroxymethyl aminomethane) and 0.372 mg/mL EDTA with pH adjusted to 7.6. DNA and matrix components were extracted via three repetitions of a freeze, thaw, and sonication cycle, where samples were frozen for 30 min at −80 °C, thawed for 30 min at room temperature, and sonicated for 30 min in order to allow DNA and matrix components into the solution.
Total collagen content was determined by quantifying hydroxyproline using the colorimetric hydroxyproline assay and hydroxyproline standards as previously described [11]. Absorbance was measured on a plate reader (BioTek PowerWave, Winooski, VT). Resulting hydroxyproline measurements in μg were converted to collagen contents for each sample following a 1:10 ratio of hydroxyproline to collagen [19].
Glycosaminoglycan content was determined by quantifying GAG using the colorimetric dimethylmethylene blue assay (Sigma-Aldrich, St. Louis, MO) and chondroitin sulfate standards as previously described [11]. Absorbance was measured on a plate reader (BioTek PowerWave, Winooski, VT). Resulting GAG measurements in μg were determined for each sample. For glycosaminoglycan synthetic activity, the resulting GAG amounts were normalized to the amount of DNA for each sample.
Cellularity was determined by quantifying double-stranded DNA using the fluorometric PicoGreen assay (Invitrogen, Carlsbad, CA) and DNA standards as previously described [11]. Fluorescence was measured on a plate reader (BioTek FL x800, Winooski, VT). Resulting DNA measurements in μg were converted to cell numbers by correlating to DNA extracted from a known number of MSCs.
Scanning electron microscopy
Samples for scanning electron microscopy were fixed in 2.5% glutaraldehyde, dehydrated through a graded series of ethanol from 70% to 100%, air-dried overnight then mounted on aluminum stubs to visualize the top surface. Samples were sputter coated with gold for 1 min prior to imaging via SEM (FEI Quanta 400, Hillsboro, OR).
Real-time reverse transcription polymerase chain reaction
Samples for real-time reverse transcription polymerase chain reaction were stored in RNAlater (Qiagen, Valencia, CA) at −20 °C to stabilize and protect RNA. After all samples were collected, total RNA was extracted using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions as previously described [20]. Constructs were placed in lysis buffer to lyse cells. After gentle mixing to allow RNA into the solution, the lysate was transferred to a QIAshredder column (Qiagen, Valencia, CA) for homogenization. An equal volume of 70% ethanol was added to the lysate and the mixture was transferred to an RNeasy mini spin column (Qiagen, Valencia, CA) where RNA was isolated and purified according to the manufacturer's animal cell protocol, with additional washes as previously described to improve the purity of total RNA [21]. Reverse transcription was then carried out to synthesize cDNA from purified RNA samples using Oligo(dT) primers (Promega, San Luis Obispo, CA) and SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). Finally, cDNA was subjected to real-time PCR (Applied Biosystems 7300 Real-Time PCR System, Foster City, CA) to quantify the gene expression of aggrecan, collagen type II, and collagen type I.
Results were analyzed using the 2−ΔΔCt method to determine relative changes in target gene expression as compared to untreated controls [22]. Target gene expression was first normalized to the expression of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) then converted to a fold ratio as compared to the baseline expression of that target gene measured in MSC controls taken directly after expansion just prior to seeding onto scaffolds. The sequences of primers used in this analysis are as follows: GAPDH: 5'-TCACCATCTTCCAGGAGCGA-3', 5'-CACAATGCCGAAGTGGTCGT-3'; aggrecan: 5'-GCTACGGAGACAAGGATGAGTTC-3', 5'-CGTAAAAGACCTCACCCTCCAT-3'; collagen type II: 5'-AACACTGCCAACGTCCAGAT-3', 5'-CTGCAGCACGGTATAGGTGA-3'; collagen type I: 5'-ATGGATGAGGAAACTGGCAACT-3', 5'-GCCATCGACAAGAACAGTGTAAGT-3'.
Statistical analysis
Both characterization and chondrogenic differentiation studies were performed each with two separate and independent experiments. Although the trends were similar between experimental runs, the data presented here for both characterization and chondrogenic differentiation studies are derived from one experiment to mitigate potential differences between cell harvests. For each experiment, cells from six rabbits were pooled together in effort to reduce variation between individual animals [18].
Characterization results for the glycosaminoglycan and collagen contents of cartilaginous extracellular matrix within PCL/ECM constructs generated in flow perfusion culture, and subsequent PCL/ECM composite scaffolds obtained following decellularization, are reported as mean ± standard deviation for n = 3. A Student's t-test at a significance level of 5% was performed to determine whether the decellularization procedure (Construct vs. Scaffold) had a significant effect on glycosaminoglycan and collagen contents.
Chondrogenic differentiation in static culture was assessed through biochemical assays to evaluate cellularity and glycosaminoglycan content and synthetic activity with n = 3 and quantitative gene expression of aggrecan, collagen type II, and collagen type I with n = 4. Results are reported as mean ± standard deviation. A three-factor ANOVA was first performed to determine significant global effects or interactions among scaffold group (PCL and PCL/ECM), growth factor treatment (−TGF and +TGF), and culture time (9, 15, and 21 days). Multiple pairwise comparisons were then made using the Tukey procedure to determine significant differences. All statistical analyses were performed at a significance level of 5%.
Results
Cartilaginous matrix characterization
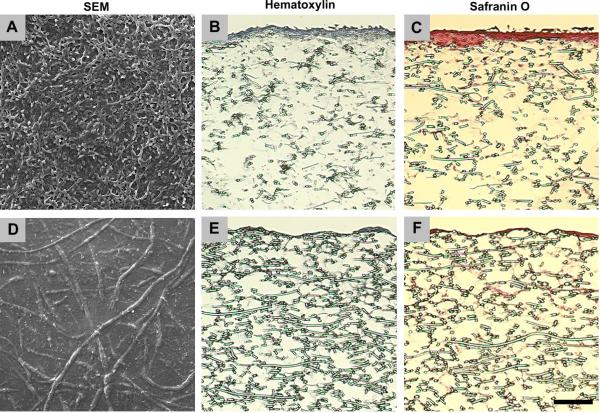
PCL/ECM constructs and PCL/ECM composite scaffolds were visualized to assess overall morphological appearance in response to the decellularization procedure. Bovine chondrocytes cultured on electrospun microfiber scaffolds under dynamic conditions in the flow perfusion bioreactor for 9 days to generate PCL/ECM constructs, are most prevalent at the top surface in scanning electron micrographs shown in Fig. 2A, with some cells present within the construct seen through hematoxylin staining in Fig. 2B. Cartilaginous matrix is predominantly localized to the chondrocytes with a sparse distribution of Safranin O staining evident within the construct in Fig. 2C. Following the decellularization procedure to yield PCL/ECM composite scaffolds, which included three cycles of freeze and thaw, air-dry overnight, and sterilization via ethylene oxide exposure, chondrocytes are no longer apparent at the top surface in scanning electron micrographs shown in Fig. 2D, as well as within the scaffold as seen through hematoxylin staining in Fig. 2E. A layer of cartilaginous matrix remains visible at the top surface of the scaffold in scanning electron micrographs shown in Fig. 2D, while retaining a similar distribution within the scaffold as shown through Safranin O staining in Fig. 2F.
Figure 2.
Morphology of PCL/ECM constructs generated through flow perfusion culture of bovine chondrocytes (A–C) and PCL/ECM composite scaffolds obtained following decellularization (D–F). Images show scanning electron micrographs illustrating surface characteristics (A & D), histological sections stained with hematoxylin to visualize cells (B & E), and histological sections stained with Safranin O to visualize cartilaginous matrix (C & F). The scale bar represents 100 μm for all images.
Cartilaginous matrix composition in terms of glycosaminoglycan and collagen contents for PCL/ECM constructs and PCL/ECM composite scaffolds are shown in Fig. 3. Although there is no significant difference in the collagen content between constructs and scaffolds, constructs contain more glycosaminoglycan than scaffolds, indicating a reduction in glycosaminoglycan content in response to the decellularization procedure.
Figure 3.
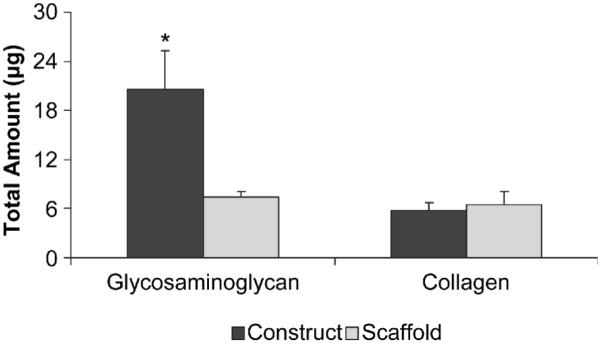
Composition of PCL/ECM constructs generated through flow perfusion culture of bovine chondrocytes and PCL/ECM composite scaffolds obtained following decellularization. Plots show glycosaminoglycan and collagen contents as mean ± standard deviation for n = 3 from one experiment, although similar trends were observed in two independent experiments. Significant difference (p < 0.05) between treatments is noted with (*).
Cellularity and glycosaminoglycan
PCL/ECM composite scaffolds along with plain PCL control scaffolds were seeded with rabbit MSCs and cultured under static conditions for 9, 15, and 21 days in serum-free medium with or without the addition of TGF-β1 to evaluate the chondrogenic properties of PCL/ECM composite scaffolds. Table 1 summarizes the global effect of each experimental factor on the biochemical results. TGF-β1 treatment had a significant effect on the glycosaminoglycan synthetic activity (GAG/DNA) of MSCs differentiating along the chondrogenic lineage but only on PCL/ECM composite scaffolds. The presence of cartilaginous extracellular matrix on electrospun microfiber scaffolds had a significant effect on cellularity, glycosaminoglycan content (GAG), and GAG/DNA.
Table 1.
Global effect of experimental factors on biochemical results
| Factor Comparison | Cellularity | GAG | GAG/DNA |
|---|---|---|---|
| −TGF-β1 vs. +TGF-β1 | NS | NS | p < 0.05 |
| PCL vs. PCL/ECM | p < 0.05 | p < 0.05 | p < 0.05 |
| Day 9 vs. Day 15 | p < 0.05 | p < 0.05 | NS |
| Day 9 vs. Day 21 | p < 0.05 | p < 0.05 | NS |
| Day 15 vs. Day 21 | p < 0.05 | NS | NS |
Significance levels were determined using a three-factor ANOVA and the Tukey procedure. Not significant is abbreviated as NS.
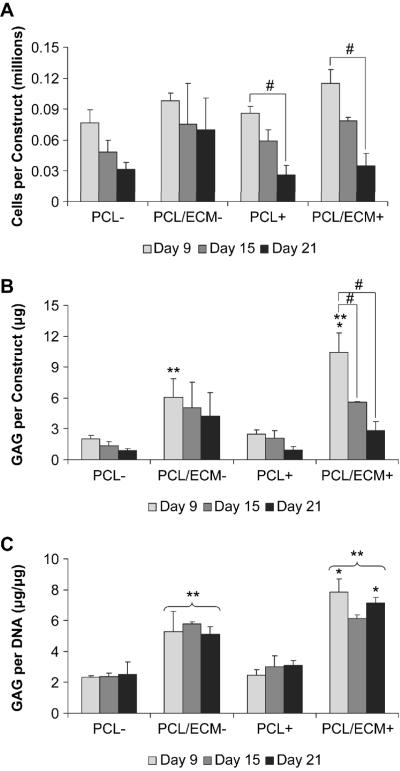
Cellularity results showed that cell numbers were not statistically different between treatment groups at each time point, whether cells were cultured with or without TGF-β1 on PCL scaffolds or PCL/ECM composite scaffolds, as shown in Fig. 4A. Cellularity remained constant over time without TGF-β1 exposure, while all constructs treated with TGF-β1 exhibited a decrease in cellularity from 9 to 21 days of culture. Glycosaminoglycan content remained constant over time for PCL constructs and was not statistically different between PCL constructs cultured with or without TGF-β1, as shown in Fig. 4B. PCL/ECM constructs contained more GAG than PCL constructs at 9 days, with PCL/ECM constructs cultured with TGF-β1 containing the most GAG at 9 days. Though PCL/ECM composite scaffolds started with an initial amount of GAG (7.45 ± 0.59 μg) in addition to the amount of GAG inherent for the seeded cells (2.77 ± 0.73 μg), a reduction in GAG was observed sooner at 9 days for cultures without TGF-β1 and later at 15 days for cultures with TGF-β1. Glycosaminoglycan synthetic activity remained constant over time for all treatment groups, as shown in Fig 4C. Interestingly, although cells cultured on PCL scaffolds did not exhibit higher GAG/DNA in response to TGF-β1 exposure, those cultured on PCL/ECM composite scaffolds and treated TGF-β1 did however demonstrate higher GAG/DNA at 9 and 21 days.
Figure 4.
Biochemical results for MSCs cultured on plain polymer scaffolds (PCL) and composite scaffolds (PCL/ECM) either with (+) or without (−) the addition of TGF-β1. Plots show cellularity (A), glycosaminoglycan content (B), and glycosaminoglycan synthetic activity (C), as mean ± standard deviation for n = 3 from one experiment, although similar trends were observed in two independent experiments. Within a specific treatment group, significant difference (p < 0.05) between time points is noted with (#). At a specific time point for each scaffold group, significant difference (p < 0.05) compared to −TGF-β1 controls is noted with (*). At a specific time point for each growth factor treatment group, significant difference (p < 0.05) compared to PCL controls is noted with (**).
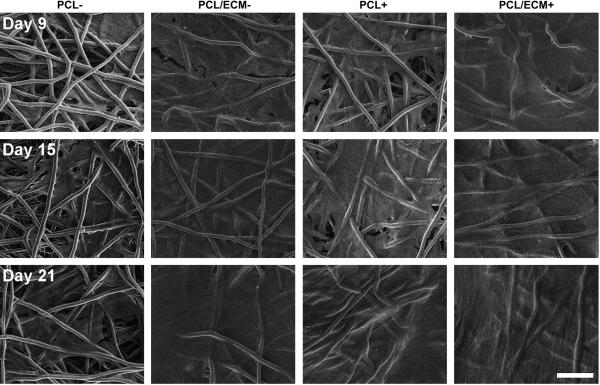
Scanning electron micrographs were taken of the top surface of constructs to visualize the overall morphology throughout the culture period, as shown in Fig. 5. Though PCL/ECM composite scaffolds started with an initial cartilaginous matrix while PCL scaffolds did not, MSCs did not visibly accumulate extracellular matrix over time on either scaffold. In contrast to constructs with chondrocytes where rounded cell bodies were well distinguished, MSCs were flat and spread forming a smooth coat over the construct surface. Those constructs treated with TGF-β1 appeared to develop a striated texture with a rippled appearance after 21 days of culture.
Figure 5.
Representative scanning electron micrographs of the top surface of plain polymer scaffolds (PCL) and composite scaffolds (PCL/ECM) seeded with MSCs and cultured either with (+) or without (−) the addition of TGF-β1. Three rows of images are shown for constructs after 9 days of culture, 15 days of culture, and 21 days of culture. The scale bar represents 100 μm for all images.
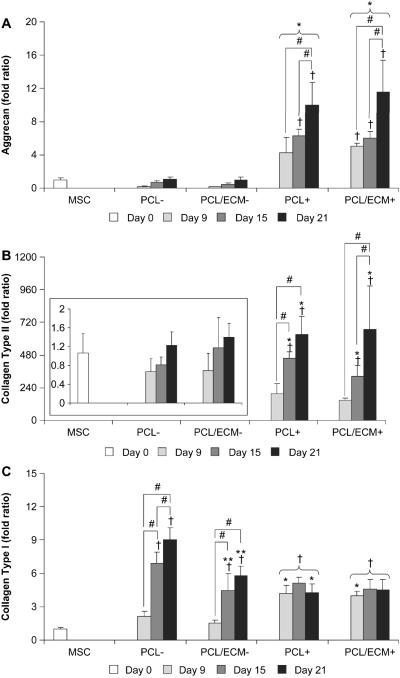
Quantitative gene expression
Aggrecan, collagen type II, and collagen type I gene expression was measured by real-time reverse transcription polymerase chain reaction to assess the chondrogenic properties PCL/ECM composite scaffolds. Table 2 summarizes the global effect of each experimental factor on the quantitative gene expression results. TGF-β1 had a significant effect on the expression of aggrecan, collagen type II, and collagen type I. The presence of cartilaginous extracellular matrix on electrospun microfiber scaffolds had a significant effect on the expression of collagen type I.
Table 2.
Global effect of experimental factors on quantitative gene expression results
| Factor Comparison | Aggrecan | Collagen Type II | Collagen Type I |
|---|---|---|---|
| −TGF-β1 vs. +TGF-β1 | p < 0.05 | p < 0.05 | p < 0.05 |
| PCL vs. PCL/ECM | NS | NS | p < 0.05 |
| Day 0 vs. Day 9 | p < 0.05 | NS | p < 0.05 |
| Day 0 vs. Day 15 | p < 0.05 | p < 0.05 | p < 0.05 |
| Day 0 vs. Day 21 | p < 0.05 | p < 0.05 | p < 0.05 |
| Day 9 vs. Day 15 | NS | p < 0.05 | p < 0.05 |
| Day 9 vs. Day 21 | p < 0.05 | p < 0.05 | p < 0.05 |
| Day 15 vs. Day 21 | p < 0.05 | p < 0.05 | NS |
Significance levels were determined using a three-factor ANOVA and the Tukey procedure. Not significant is abbreviated as NS.
Aggrecan gene expression was significantly higher for cells cultured with TGF-β1 and exhibited an increasing trend over time, as shown in Fig. 6A. In cultures without TGF-β1, aggrecan expression was not statistically different than MSCs at day 0 and remained constant from 9 to 21 days of culture, with no statistical difference between cells cultured on PCL scaffolds or PCL/ECM composite scaffolds. In cultures with TGF-β1 however, statistical differences in aggrecan expression compared to MSCs at day 0 were detected beginning at 9 days for PCL/ECM composite scaffolds and later at 15 days for PCL scaffolds. While cells cultured with TGF-β1 exhibited the highest levels of aggrecan expression at 21 days, there was no statistical difference between cells cultured on PCL scaffolds (10.0 ± 2.7 fold) or PCL/ECM composite scaffolds (11.6 ± 3.8 fold).
Figure 6.
Quantitative gene expression results for MSCs cultured on plain polymer scaffolds (PCL) and composite scaffolds (PCL/ECM) either with (+) or without (−) the addition of TGF-β1. Plots show aggrecan expression (A), collagen type II expression with the inset on a rescaled axis (B), and collagen type I expression (C). Data are presented as fold ratio after being normalized to the expression of GAPDH. Fold ratios are shown as mean ± standard deviation for n = 4 from one experiment, although similar trends were observed in two independent experiments. Significant difference (p < 0.05) compared to the gene expression of MSCs at day 0 is noted with (†). Within a specific treatment group, significant difference (p < 0.05) between time points is noted with (#). At a specific time point for each scaffold group, significant difference (p < 0.05) compared to −TGF-β1 controls is noted with (*). At a specific time point for each growth factor treatment group, significant difference (p < 0.05) compared to PCL controls is noted with (**).
Similar to aggrecan gene expression, collagen type II gene expression was significantly higher for cells cultured with TGF-β1 and exhibited an increasing trend over time, as shown in Fig. 6B. In cultures without TGF-β1, collagen type II expression was not statistically different than MSCs at day 0 and remained constant from 9 to 21 days of culture, with no statistical difference between cells cultured on PCL scaffolds or PCL/ECM composite scaffolds. In cultures with TGF-β1, statistical differences in collagen type II expression compared to MSCs at day 0 were detected beginning at 15 days for both PCL scaffolds and PCL/ECM composite scaffolds. While cells cultured with TGF-β1 exhibited the highest levels of collagen type II expression at 21 days, there was no statistical difference between cells cultured on PCL scaffolds (629.3 ± 135.6 fold) or PCL/ECM composite scaffolds (668.4 ± 317.7 fold).
In contrast to aggrecan and collagen type II gene expression, collagen type I gene expression was significantly lower for cells cultured with TGF-β1 and remained constant over time, with no statistical difference between cells cultured on PCL scaffolds or PCL/ECM composite scaffolds, as shown in Fig. 6C. In cultures without TGF-β1, collagen type I expression increased over time and was the highest for cells cultured on PCL scaffolds at 15 and 21 days (6.9 ± 1.0 fold and 9.0 ± 1.1 fold), while cells cultured on PCL/ECM composite scaffolds on the other hand, demonstrated significantly lower collagen type I expression (4.5 ± 1.5 fold and 5.8 ± 0.8 fold). Furthermore, the level of collagen type I expression for cells cultured on PCL/ECM composite scaffolds was comparable to the expression observed with TGF-β1 treatment.
Discussion
The objective of this study was to fabricate PCL/ECM composite scaffolds consisting of electrospun microfibers coated with cartilaginous extracellular matrix, and evaluate their ability to support the chondrogenic differentiation of MSCs in vitro. This study was designed to investigate the fabrication of PCL/ECM composite scaffolds through dynamic culture of bovine chondrocytes in a flow perfusion bioreactor, and to determine how the decellularization procedure affects matrix morphology and composition. PCL/ECM composite scaffolds where evaluated for their ability to support the chondrogenic differentiation of rabbit MSCs in vitro induced by TGF-β1 exposure, and to determine whether the presence of cartilaginous matrix would further enhance this differentiation response by providing cells with a more biological microenvironment compared to plain PCL scaffolds.
Culturing chondrocytes under direct flow perfusion conditions providing a low level of fluid shear stress has been shown to stimulate proliferation and accumulation of glycosaminoglycan and collagen [23–26]. Thus in this study, dynamic culture in a flow perfusion bioreactor was employed to generate PCL/ECM constructs. With our electrospun microfiber scaffolds and the chosen flow rate, we observed that bovine chondrocytes deposited cartilaginous extracellular matrix predominately localized to their pericellular space with a sparse distribution of matrix throughout the thickness of the constructs. Although with our present scaffold geometry and culture parameters, cartilaginous matrix was not very well distributed throughout the depth of our constructs, the optimal combination of seeding density, flow rate, and pore size may be further investigated to balance cell retention and matrix distribution throughout the constructs.
From scanning electron micrographs and histological sections, it appears that chondrocytes may have proliferated quickly to occlude the surface porosity of the electrospun microfiber scaffolds, and thus resulted in a large amount of cartilaginous matrix at the surface of the constructs, consisting of glycosaminoglycan and collagen. In decellularizing PCL/ECM constructs to yield PCL/ECM composite scaffolds, we observed a decrease in glycosaminoglycan content with collagen content unaffected. Safranin O staining revealed that the reduction in glycosaminoglycan is likely associated with the chondrocytes removed from the surface of the constructs via the decellularization procedure. However, since cartilaginous matrix was visibly present in PCL/ECM composite scaffolds, and due to the amount of glycosaminoglycan and collagen detected through biochemical assays, we sought to evaluate PCL/ECM composite scaffolds for their ability to support the chondrogenic differentiation of MSCs in vitro.
PCL/ECM composite scaffolds along with plain PCL control scaffolds were seeded with rabbit MSCs and cultured in serum-free medium either with our without the addition of TGF-β1. Serum-free culture was applied in this study in order to strictly investigate the effects of physical matrix interactions and biochemical signaling on chondrogenic differentiation. Although serum-free culture is beneficial for studying chondrogenic differentiation in a controlled manner in vitro, serum deprivation has been shown to affect cell attachment and inhibit proliferation [27]. As such, we observed a decrease in cellularity for cells cultured on both PCL scaffolds and PCL/ECM composite scaffolds driven toward chondrogenic differentiation through TGF-β1 exposure.
Our results demonstrated that while MSCs did not accumulate a detectable amount of glycosaminoglycan over time, culturing MSCs on both PCL scaffolds and PCL/ECM composite scaffolds with TGF-β1 significantly enhanced chondrogenic differentiation, as seen in the up-regulation of aggrecan and collagen type II gene expression over time relative to the baseline expression of MSCs at day 0. This differentiation response is further supported by the minimal collagen type I expression throughout the 21 days of culture, where collagen type I expression indicates pre-chondrogenic undifferentiated MSCs or a fibroblastic phenotype [28]. As previously shown with electrospun PCL nanofiber scaffolds (average fiber size 500 to 700 nm) [8, 29], we prove here that microfiber scaffolds (average fiber size 10 μm) also support MSC chondrogenesis induced by TGF-β1. Additionally, we demonstrate that PCL/ECM composite scaffolds, containing cartilaginous matrix generated by chondrocytes, are also capable of supporting the chondrogenic differentiation of MSCs in vitro.
While the presence of cartilaginous matrix did not seem to enhance chondrogenic gene expression beyond the levels seen with TGF-β1 exposure, it did however promote an up-regulation in aggrecan expression sooner than plain scaffolds without cartilaginous matrix. Furthermore, cells cultured on composite scaffolds containing cartilaginous matrix exhibited significantly lower collagen type I expression comparable to the minimal levels seen with TGF-β1 treatment. Therefore, the presence of cartilaginous matrix alone without the addition of growth factors may provide biological signals to reduce the fibroblastic phenotype of differentiating MSCs as marked in the collagen type I expression levels observed for cells cultured on PCL/ECM composite scaffolds.
The up-regulation in aggrecan gene expression in response to TGF-β1 treatment did not translate to an increase in glycosaminoglycan content in the constructs. Given that both GAG content and GAG/DNA levels remained constant over time, it is likely that the soluble proteoglycans produced by differentiating cells were not incorporated into the constructs but rather released into the medium, which has been reported in other studies with both chondrocytes and stem cells cultured on polymer scaffolds [30, 31]. Thus, the absence of glycosaminoglycan accumulation may be attributed to the controlled in vitro culture conditions and regular medium changes. Although different outcomes may be likely under physiological conditions in vivo, assessment of chondrogenesis in vivo was beyond the scope of this present study.
PCL/ECM composite scaffolds contained an initial cartilaginous matrix deposited by chondrocytes in flow perfusion culture. Since the cartilaginous matrix was not crosslinked or physically conjugated to the polymer scaffolds, we observed a reduction in GAG content particularly within the first two weeks of culture, where proteoglycans may be leaching into the aqueous environment; similar to what has been observed with cartilage explants in culture [32, 33]. Interestingly, we found that PCL/ECM constructs cultured with TGF-β1 retained a higher amount of GAG in the first week of culture than those cultured without TGF-β1. Thus, the combination of cartilaginous matrix and growth factor treatment promotes the retention of GAG either originally present in PCL/ECM composite scaffolds or produced by the cultured cells. Furthermore, we discovered that only when cells where cultured in the presence of cartilaginous matrix in PCL/ECM composite scaffolds, did TGF-β1 treatment result in higher GAG/DNA. Therefore, it appears that cartilaginous matrix facilitates chondrogenesis by enhancing the effects of TGF-β1 in vitro.
The cartilaginous matrix deposited by chondrocytes in fabricating PCL/ECM composite scaffolds proved to be too dense for MSCs to remodel and penetrate under static culture conditions, as confirmed through histology (data not shown). While specific cell-matrix interactions are important in regulating the initial attachment of MSCs, the lack of cell penetration through the dense layer of cartilaginous matrix at the surface of PCL/ECM composite scaffolds limits their spatial contact with extracellular matrix proteins to essentially two-dimensions. Thus in this study, although a complex set of matrix molecules were presented, the full potential of these biological signals to guide chondrogenic differentiation might not be experienced by cells in three-dimensions.
Though the porous nature of sponge-like scaffolds fabricated using native cartilage components facilitate cell seeding, hence promoting three-dimensional interactions [5, 6], the precise mechanisms leading to chondrogenesis are unclear. That is, since the entire scaffold structure is comprised of matrix proteins, it is difficult to distinguish whether MSC chondrogenic differentiation is simply due to maintaining cells in a three-dimensional geometry, or whether chondrogenesis is particularly attributed to specific cell-matrix interactions with biological signals in the matrix. Alternatively, the composite nature of our PCL/ECM scaffolds, with a porous fiber mesh structure as the base material, makes it possible to examine the underlying mechanisms of chondrogenesis; as in this study where we observed that the presence of cartilaginous matrix in PCL/ECM composite scaffolds in fact augmented the effect of growth factor treatment otherwise not seen for plain PCL controls. By tailoring scaffold properties through controllable electrospinning parameters, together with adjusting the morphology and composition of cartilaginous matrix in varying the conditions of chondrocyte seeding and culture, we may be able to engineer PCL/ECM composite scaffolds with sufficient porosity to support chondrogenesis. Maintaining adequate porosity to facilitate subsequent cell seeding and infiltration would allow us to investigate MSC chondrogenic differentiation in a purely structural three-dimensional environment (PCL), or in an instructive cartilaginous microenvironment containing complex arrays of biological signals (PCL/ECM), to gain a better understanding of the mechanisms regulating chondrogenesis.
In this study we utilized a xenogenic source of chondrocytes to generate cartilaginous matrix in fabricating PCL/ECM composite scaffolds. While limited research has been done to explore the potential inductive properties of xenogenic cartilaginous matrix, xenogenic osteochondral grafts decellularized through a photooxidation technique have been shown to repair cartilage defects with no adverse immune response [34]. Also, acellular bovine cartilage matrix molded through freeze-drying and crosslinked via ultraviolet irradiation, showed good biocompatibility with rabbit MSCs and no cytotoxic effects in both direct contact and extraction assays [35]. Chondrocytes can be stimulated to deposit large amounts of cartilaginous matrix under engineered culture conditions as demonstrated with the fabrication of PCL/ECM composite scaffolds in this study. Due to the limitations and drawbacks with autogenic or allogenic chondrocyte harvest, xenogenic chondrocytes are a potentially clinically applicable cell source in generating acellular cartilaginous scaffolds to guide cartilage repair, provided that the decellularization procedure effectively removes cellular components [36].
Conclusions
In this work, we fabricated PCL/ECM composite scaffolds consisting of electrospun microfibers coated with cartilaginous extracellular matrix, and evaluated their ability to support the chondrogenic differentiation of MSCs in vitro. PCL/ECM composite scaffolds supported chondrogenic differentiation induced by TGF-β1 exposure, as evidenced in the up-regulation of aggrecan and collagen type II gene expression. The presence of xenogenic cartilaginous matrix alone reduced collagen type I gene expression to levels comparable to those observed with TGF-β1 treatment. Cartilaginous matrix further enhanced the effects of growth factor treatment as evidenced in the higher glycosaminoglycan synthetic activity for cells cultured on PCL/ECM composite scaffolds with TGF-β1, whereas TGF-β1 treatment alone did not translate to higher GAG/DNA levels for cells cultured on plain PCL scaffolds. The present study demonstrated the fabrication of polymer/extracellular matrix composite scaffolds using a flow perfusion bioreactor to incorporate biological signals in a synthetic scaffolding system for cartilage tissue engineering applications.
Acknowledgements
This work has been supported by the National Institutes of Health (R01 AR57083). We thank Dr. Galen I. Papkov for assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Simon TM, Jackson DW. Articular cartilage: injury pathways and treatment options. Sports Med Arthrosc. 2006;14(3):146–154. doi: 10.1097/00132585-200609000-00006. [DOI] [PubMed] [Google Scholar]
- [2].Keeling JJ, Gwinn DE, McGuigan FX. A comparison of open versus arthroscopic harvesting of osteochondral autografts. Knee. 2009;16(6):458–462. doi: 10.1016/j.knee.2009.02.010. [DOI] [PubMed] [Google Scholar]
- [3].Brittberg M. Autologous chondrocyte implantation--technique and long-term follow-up. Injury. 2008;39(Suppl 1):S40–49. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- [4].Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23(2):288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- [5].Farrell E, O'Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, et al. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12(3):459–468. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- [6].Cheng NC, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15(2):231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67(4):1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- [8].Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26(6):599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [9].Gomes ME, Bossano CM, Johnston CM, Reis RL, Mikos AG. In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12(1):177–188. doi: 10.1089/ten.2006.12.177. [DOI] [PubMed] [Google Scholar]
- [10].Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103(8):2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liao J, Guo X, Nelson D, Kurtis Kasper F, Mikos AG. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta Biomater. 2010;6(7):2386–2393. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- [13].Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9(3):549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- [14].Hu JC, Athanasiou KA. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials. 2005;26(14):2001–2012. doi: 10.1016/j.biomaterials.2004.06.038. [DOI] [PubMed] [Google Scholar]
- [15].Medalie DA, Eming SA, Collins ME, Tompkins RG, Yarmush ML, Morgan JR. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. Transplantation. 1997;64(3):454–465. doi: 10.1097/00007890-199708150-00015. [DOI] [PubMed] [Google Scholar]
- [16].Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26(9):971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [17].Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, et al. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8(2):333–347. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- [18].Solchaga LA, Johnstone B, Yoo JU, Goldberg VM, Caplan AI. High variability in rabbit bone marrow-derived mesenchymal cell preparations. Cell Transplant. 1999;8(5):511–519. doi: 10.1177/096368979900800506. [DOI] [PubMed] [Google Scholar]
- [19].Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- [20].Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29(18):2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- [22].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [23].Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- [24].Pazzano D, Mercier KA, Moran JM, Fong SS, DiBiasio DD, Rulfs JX, et al. Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnol Prog. 2000;16(5):893–896. doi: 10.1021/bp000082v. [DOI] [PubMed] [Google Scholar]
- [25].Freyria AM, Yang Y, Chajra H, Rousseau CF, Ronziere MC, Herbage D, et al. Optimization of dynamic culture conditions: effects on biosynthetic activities of chondrocytes grown in collagen sponges. Tissue Eng. 2005;11(5–6):674–684. doi: 10.1089/ten.2005.11.674. [DOI] [PubMed] [Google Scholar]
- [26].Raimondi MT, Moretti M, Cioffi M, Giordano C, Boschetti F, Lagana K, et al. The effect of hydrodynamic shear on 3D engineered chondrocyte systems subject to direct perfusion. Biorheology. 2006;43(3–4):215–222. [PubMed] [Google Scholar]
- [27].Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22(7):1152–1167. doi: 10.1634/stemcells.2004-0062. [DOI] [PubMed] [Google Scholar]
- [28].Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- [29].Wise JK, Yarin AL, Megaridis CM, Cho M. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage. Tissue Eng Part A. 2009;15(4):913–921. doi: 10.1089/ten.tea.2008.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mahmood TA, Shastri VP, van Blitterswijk CA, Langer R, Riesle J. Tissue engineering of bovine articular cartilage within porous poly(ether ester) copolymer scaffolds with different structures. Tissue Eng. 2005;11(7–8):1244–1253. doi: 10.1089/ten.2005.11.1244. [DOI] [PubMed] [Google Scholar]
- [31].Wang L, Seshareddy K, Weiss ML, Detamore MS. Effect of initial seeding density on human umbilical cord mesenchymal stromal cells for fibrocartilage tissue engineering. Tissue Eng Part A. 2009;15(5):1009–1017. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Campbell MA, Handley CJ, Hascall VC, Campbell RA, Lowther DA. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch Biochem Biophys. 1984;234(1):275–289. doi: 10.1016/0003-9861(84)90350-3. [DOI] [PubMed] [Google Scholar]
- [33].Bolis S, Handley CJ, Comper WD. Passive loss of proteoglycan from articular cartilage explants. Biochim Biophys Acta. 1989;993(2–3):157–167. doi: 10.1016/0304-4165(89)90158-x. [DOI] [PubMed] [Google Scholar]
- [34].von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing--an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11(4):265–277. doi: 10.1016/s1063-4584(03)00006-2. [DOI] [PubMed] [Google Scholar]
- [35].Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, et al. Fabrication and characterization of a novel acellular cartilage matrix scaffold for tissue engineering. Tissue Eng Part C Methods. 2009 doi: 10.1089/ten.TEC.2009.0444. doi:10.1089/ten.tec.2009.0444. [DOI] [PubMed] [Google Scholar]
- [36].Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30(22):3749–3756. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]