Abstract
In Vibrio alginolyticus, the flagellar motor can rotate at a remarkably high speed, ca. three to four times faster than the Escherichia coli or Salmonella motor. Here, we found a Vibrio-specific protein, FlgT, in the purified flagellar basal body fraction. Defects of FlgT resulted in partial Fla− and Mot− phenotypes, suggesting that FlgT is involved in formation of the flagellar structure and generating flagellar rotation. Electron microscopic observation of the basal body of ΔflgT cells revealed a smaller LP ring structure compared to the wild type, and most of the T ring was lost. His6-tagged FlgT could be coisolated with MotY, the T-ring component, suggesting that FlgT may interact with the T ring composed of MotX and MotY. From these lines of evidence, we conclude that FlgT associates with the basal body and is responsible to form an outer ring of the LP ring, named the H ring, which can be distinguished from the LP ring formed by FlgH and FlgI. Vibrio-specific structures, e.g., the T ring and H ring might contribute the more robust motor structure compared to that of E. coli and Salmonella.
The bacterial flagellar motor is a rotary nanomotor, which converts the electrochemical potential difference of the coupling ion (H+ or Na+) into rotational energy. Escherichia coli and Salmonella spp. have H+-driven motors, and Vibrio alginolyticus has Na+-driven motors. The rotation speed of the Vibrio motor is remarkably fast, 1,100 Hz on average and up to 1,700 Hz maximum, which is more than four times faster than that of the E. coli motor (24, 27).
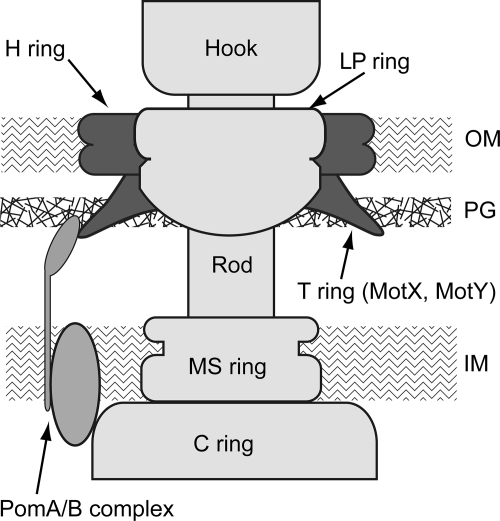
The flagellum is coordinately and hierarchically constructed from more than 30 related proteins and is composed of rotor, stator, universal joint (hook), and helical filament (22, 43). The rotor part (also called the basal body) contains several rings and a drive shaft, which are named the L, P, MS, and C rings and the rod (1, 14). The L, P, MS, and C rings are thought to be located in positions corresponding to the outer membrane, peptidoglycan layer, cytoplasmic membrane, and cytoplasm, respectively (Fig. 1). Because the LP ring is thought to be a bushing for rotation of the rod, the LP ring seems not to rotate. Analyses of the basal body components of Salmonella were carried out in detail, thereby identifying all of the gene products that are responsible for the substructures. The L, P and MS rings are composed of FlgH, FlgI, and FliF, respectively, while the C ring is composed of three different proteins, FliG, FliM, and FliN, and the rod is composed of FlgB, FlgC, FlgF, and FlgG (14, 17, 18, 39, 44).
FIG. 1.
Model of the flagellar basal body in Vibrio. The H ring and the T ring are shown in dark gray. The LP ring and the other basal body parts are shown in light gray. The PomA/B complex is shown in the medium gray. OM, outer membrane; PG, peptidoglycan layer; IM, inner membrane.
The stator part is responsible for torque generation. The torque generation unit of the stator is composed of MotA and MotB in E. coli or PomA and PomB in Vibrio spp. and is a hexamer of four A subunits and two B subunits. They assemble around the rotor and transfer the coupling ions (H+ in E. coli and Na+ in Vibrio) across the membrane due to the electrochemical potential (2, 4, 11, 15, 37, 38, 40, 41). MotX and MotY are species-specific (e.g., Vibrio and Shewanella spp.) stator proteins, and defects in these proteins result in a mot phenotype in which flagellar morphogenesis is normal but the flagella cannot rotate (21, 30, 31, 33, 36). Pseudomonas spp. have only MotY but not MotX; MotY is required for flagellar rotation (12). In Vibrio alginolyticus it has been shown that MotX and MotY are produced as precursor proteins with signal sequences and are translocated to the periplasmic space by a general secretion pathway (35). MotX and MotY form a ring structure called the T ring in addition to the LP ring (Fig. 1). The N-terminal domain of MotY has been suggested to directly associate with the basal body, probably the P ring and MotX (23, 42), and MotX has been suggested to interact with PomB (34). Based on these lines of evidence, the T ring was proposed to be involved in the incorporation and/or stabilization of the PomA/B complex into the motor and provide a connection between the rotor and PomA/B in Vibrio (42).
When flagellar basal bodies were purified from various species, the basic structures were similar but the details were different. When we compared the structures from Vibrio cells and E. coli cells, the Vibrio LP rings were bigger than those of E. coli (42). We speculated that additional proteins were present in the Vibrio LP rings. In the present study, we recognized a novel ring structure on the basal body of V. alginolyticus, and it was composed of the product of a recently identified motility gene, flgT. It was reported in that in Vibrio cholerae FlgT is somehow involved in motility and flagellar formation (9, 29). Furthermore, V. cholerae strains with defects in FlgT develop outer membrane blebbing and release the flagellum into the medium, suggesting that FlgT is involved in anchoring the flagellar base on the cell surface (29). We found that FlgT is necessary to form an outer ring of the LP ring, named the H ring (for holding ring of the flagellar base on the cell surface). The H ring is thought to be involved in assembly of MotX and MotY to the basal body.
MATERIALS AND METHODS
Bacterial strains, plasmids and growth condition.
Bacterial strains and plasmids used in the present study are listed in Table 1. V. alginolyticus was cultured in VC medium (0.5% [wt/vol] Bacto tryptone, 0.5% [wt/vol] yeast extract, 0.4% [wt/vol] K2HPO4, 3% [wt/vol] NaCl, 0.2% [wt/vol] glucose) or in VPG500 medium (1% [wt/vol] Bacto tryptone, 0.4% [wt/vol] K2HPO4, 500 mM NaCl, 0.5% [wt/vol] glycerol) at 30°C. E. coli was cultured in LB broth (1% [wt/vol] Bacto tryptone, 0.5% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl). In the second selection to isolate mutants, V. alginolyticus was cultured at 30°C in plates containing sucrose (1% [wt/vol] polypeptone, 30 mM NaCl, 55 mM KCl, 10% [wt/vol] sucrose, 1.25% [wt/vol] agar). Chloramphenicol was added to final concentrations of 2.5 μg/ml for V. alginolyticus and 25 μg/ml for E. coli. Kanamycin was added to final concentrations of 100 μg/ml for V. alginolyticus and 50 μg/ml for E. coli. Ampicillin was added to a final concentration of 50 μg/ml for E. coli.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| V. alginolyticus | ||
| VIO5 | VIK4 laf (Rifr Pof+ Laf−) | 36 |
| NMB191 | VIO5 pomAB (Mot−) | 49 |
| KK148 | VIO5 flhG (multi-Pof+) | 25 |
| TH3 | KK148 ΔmotX ΔmotY | 42 |
| TH6 | VIO5 ΔflgT | This study |
| TH7 | KK148 ΔflgT | This study |
| YM14 | YM4 rpoN (Pof− Laf−) | 19a |
| E. coli | ||
| SM10λpir | thi thr leu tonA lacY supE recA::RP4−2-Tc::Mu Km λpirRK6 | 32 |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 λ− Δ(lac-proAB) (F′ traD36 proAB lacIqlacZΔM15) | 48 |
| Plasmids | ||
| pSU41 | PlaclacZa; Kmr | 3 |
| pGEM-T | Cloning vector to TA cloning method; Ampr | Promega |
| pET3a | Expression vector; Ampr | Novagen |
| pKY704 | Suicide vector; Cmr | 46 |
| pHFS401 | sacB in pSU41 | 42 |
| pKJ502 | motY (SalI-XbaI) in pSU41 | 35 |
| pTH103 | flgT in pGEM-T | This study |
| pTH104 | flgT (BamHI-SacI) in pSU41 | This study |
| pTH105 | flgT-His6 (BamHI-SacI) in pSU41 | This study |
| pTH106 | flgT (BamHI-SacI) in pKJ502 | This study |
| pTH107 | flgT-His6 (BamHI-SacI) in pKJ502 | This study |
| pTH108 | flgT-His6 (NdeI-BamHI) in pET3a | This study |
| pTH109 | Deletion fragment of flgT in pGEM-T | This study |
| pTH110 | Deletion fragment of flgT (SacI-SacI) and sacB (XbaI-XbaI) in pKY704 | This study |
Rifr, rifampin resistant; Pof+, normal polar flagellar formation; Laf−, defective in lateral flagellar formation; Mot−, nonmotile; multi-Pof+, multiple polar flagellar formation; Ampr, ampicillin resistant; Kmr, kanamycin resistant; Cmr, chloramphenicol resistant; Plac, lac promoter.
Swarming assay.
VPG500 semisolid agar (1% [wt/vol] Bacto tryptone, 0.4% [wt/vol] K2HPO4, 500 mM NaCl, 0.5% [wt/vol] glycerol, 0.25% [wt/vol] Bacto agar) was used for motility assays of V. alginolyticus. A 1-μl aliquot of an overnight culture was spotted onto VPG500 semisolid agar, followed by incubation at 30°C for the desired time.
Immunoblotting.
The samples were suspended with sodium dodecyl sulfate (SDS) loading buffer and boiled at 95°C for 5 min, and SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting were performed as described previously (49). Antisera against PomA (PomA1312), PomB (PomB93), MotX (MotXB0080), MotY (MotYB0079), and FliF (FliFB0424) were prepared previously (47, 49; R. Ogawa et al., unpublished data). Antisera against FlgT (VA15390B0472) were prepared (see below), and a His probe (Santa Cruz) was purchased. Horseradish peroxidase-linked goat anti-rabbit IgG (Santa Cruz) was used as the secondary antibody.
Introduction of plasmids into V. alginolyticus.
Transformations were carried out by electroporation as described previously (19).
Isolation of flagellar basal bodies.
The isolation of the flagellar basal bodies was carried out as described previously with several modifications (42). EDTA for forming spheroplasts was modified to a final concentration of 5 mM and, in the following step, MgSO4 was added to a final concentration of 10 mM.
Electron microscopy.
The isolated flagellar structures were negatively stained with 2% uranyl acetate and observed with a JEM-2010 electron microscope (JEOL, Japan).
High-intensity dark-field microscopy.
Flagella were observed by using a dark-field microscope (Olympus model BHT) equipped with a 100-W mercury lamp (Ushio USH-102). Images was recorded by using a charge-coupled device camera (Sony model SSC-M370) and a DVD video recorder (Panasonic model DMR-E100H).
flgT gene cloning, plasmid construction, and disruption.
The flgT gene and flanking regions (−541 to +1660) were amplified from purified chromosomal DNA from strain VIO5 by PCR and then subcloned in pGEM-T (Promega), and the resultant plasmid was named pTH103. The flgT gene and upstream sequence (−30 to −1) with a BamHI site at the 5′ end and SacI site at the 3′ end was cloned in pSU41 or pKJ502, and the resultant plasmids were named pTH104 and pTH106, respectively. The DNA sequence of a hexahistidine tag was attached by site-directed mutagenesis (Stratagene), and the resultant plasmids were named pTH105 and pTH107, respectively. For purification of FlgT proteins, the flgT-His6 gene with an NdeI site at the 5′ end and a BamHI site at the 3′ end was cloned in pET3a (Novagen), and the resultant plasmid was named pTH108. The flgT deletion mutant was generated by homologous recombination using a suicide vector as described previously (42). The sequence upstream of the flgT coding region (−541 to −1) with a SacI site at the 5′ end and the downstream sequence (+1135 to +1144) at the 3′ end, and the downstream sequence of the flgT coding region (+1135 to +1660) with upstream sequence (−10 to −1) at the 5′ end and a SacI site at the 3′ end were amplified by PCR. Their PCR products were used as primer and template in the following PCR. A deletion fragment was amplified by PCR and subcloned into pGEM-T, and the resultant plasmid was named pTH109. The flgT deletion fragment was inserted into the SacI site of pKY704, followed by insertion of the sacB gene, which was obtained from pHFS401, in the XbaI site, and the resultant plasmid was named pTH110. pTH110 was used to transform E. coli SM10λpir. The deletion allele was introduced into VIO5 or KK148 by a conjugation-based method. The resultant strains were named TH6 and TH7, respectively.
Determination of N-terminal amino acid sequence.
The proteins were separated by SDS-PAGE and transferred electrophoretically to a polyvinylidene difluoride membrane (Millipore) and stained with Coomassie blue R250. The MotX, MotY, and FlgI bands were excised. The N-terminal amino acid sequences were determined by Aproscience (Tokushima, Japan), using the Edman degradation method.
Antibody raised against FlgT.
BL21(DE3)/pLysS cells harboring pTH108 were cultured in LB broth at 30°C, and FlgT proteins were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at early exponential phase. Cells were collected by centrifugation, resuspended in buffer A (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 25% [wt/vol] sucrose), incubated on ice for 10 min, diluted with 2 volumes of buffer B (100 mM NaCl, 1.5 mM sodium-EDTA [pH 8]), and incubated on ice for 20 min. The supernatant was recovered by centrifugation (10,000 × g for 20 min), MgCl2 and imidazole were added to final concentrations of 2 and 20 mM, respectively, and then the periplasmic fraction was recovered by ultracentrifugation (100,000 × g for 60 min). The periplasmic fraction was applied to HisTrap FF (GE Healthcare), and the bound proteins were eluted with a 20 to 500 mM linear gradient of imidazole (using the buffers 20 mM Tris-HCl [pH 7.5] and 100 mM NaCl containing 20 or 500 mM imidazole). FlgT-His6 was purified with a HiTrap Q column (GE Healthcare) and Superdex 200HR 10/30 (GE Healthcare) as necessary. Purified FlgT was separated by SDS-PAGE, stained with Coomassie blue R250, and excised. Rabbit anti-FlgT antibody was produced by Biogate (Tokushima, Japan).
Coelution assay to detect the interaction between FlgT and MotY.
The coelution assay was carried out by the purification protocol described above with slight modifications. YM14 cells, which are sigma54− mutant (rpoN mutant) cells that do not express the flagellar genes, harboring pTH106 or pTH107 were cultured in VPG500 broth at 30°C. The pH of Tris buffer was changed from 7.5 to 8.0.
RESULTS
Identification of a novel component in the Vibrio basal body.
We purified the wild-type hook-basal bodies from the flhG mutant (KK148), which produces multiple polar flagella (25). The fractions of purified hook-basal bodies were analyzed by SDS-PAGE and stained (Fig. 2). We obtained a similar band profile, and the most intense band of 50 kDa was the hook protein, FlgE, as previously reported (42). To confirm the basal body components, we determined the N-terminal amino acid sequences of some of the protein bands, which appeared to be MotX, MotY and FlgI (Fig. 2). The sequences of the 25- and 30-kDa bands were NVADV and VMGKR, which correspond to the sequences of MotX and MotY without the putative N-terminal signal sequences, respectively. The 38-kDa band contained two sequences, ARIKD and SWYEV, which correspond to the sequences of FlgI without the signal sequence and a hypothetical gene product (VA15390 of V. alginolyticus strain 12G01). This result suggests that the hypothetical gene product, VA15390, is a component of the Vibrio basal body. A homolog of VA15390 has been identified as a gene required for motility in V. cholerae and was named FlgT (9). FlgT homologs from various species were aligned, and the secondary structure of FlgT from V. alginolyticus strain VIO5 was predicted by using PSIPRED (8) (Fig. 3). FlgT homologs are likely to exist in only Vibrio, Shewanella and related species, which are known to possess MotX and MotY. The deduced flgT products have a signal sequence for secretion and the amino acid sequence of FlgT detected from the basal body fraction had lost the signal sequence. This suggests that FlgT is cleaved between Ala23 and Ser24 and is translocated into the periplasmic space. FlgT was predicted to have an α/β mixed structure in the N-terminal half and be rich in β-strand structures in the C-terminal half. There are two conserved cysteine residues that might form a disulfide bond for protein stabilization.
FIG. 2.
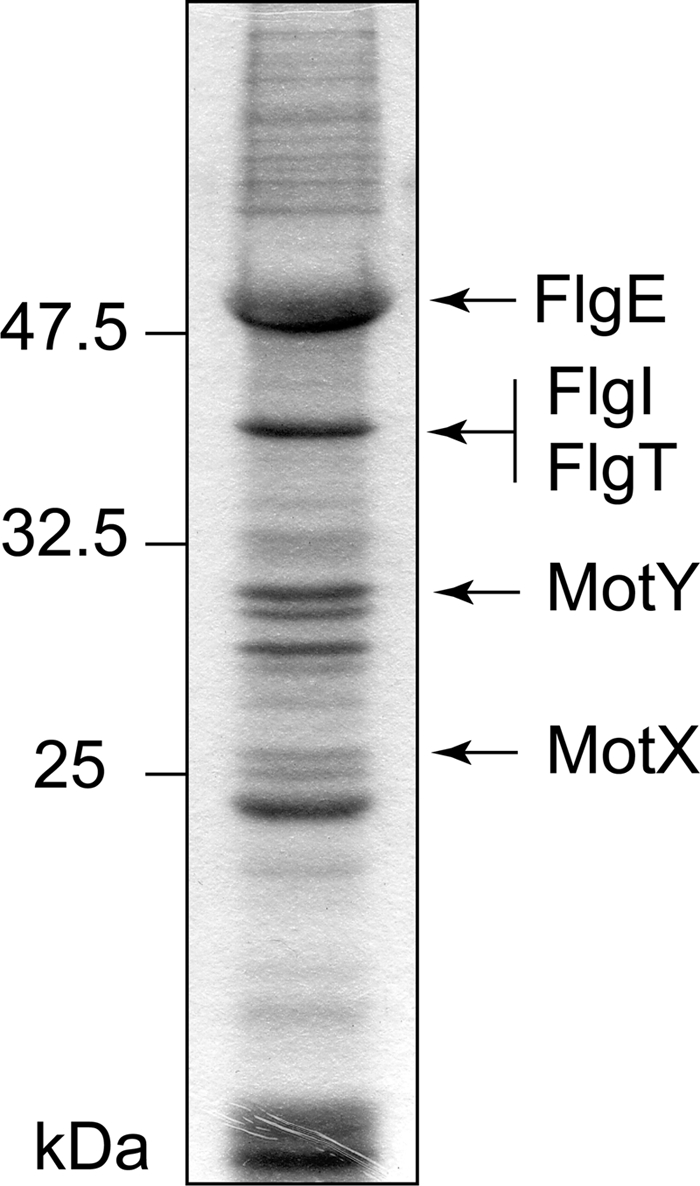
Identification of the proteins containing the purified hook-basal body fraction. The proteins of the hook-basal body from KK148 were separated by SDS-PAGE. The N-terminal amino acid sequences of the putative MotX, MotY or FlgI bands were determined by Edman degradation.
FIG. 3.
Alignment of the amino acid sequences of V. alginolyticus strain VIO5 FlgT and homologous proteins from various species. The sequence alignment was generated with Genetyx software (Genetyx Corp.). Abbreviations: VaFlgT, FlgT of Vibrio alginolyticus strain VIO5; VP0767, hypothetical protein of Vibrio parahaemolyticus RIMD 2210633; VC2208, FlgT of Vibrio cholerae El Tor N16961; Il1154, hypothetical protein of Idiomarina loihiensis L2TR; SO_3258, hypothetical protein of Shewanella oneidensis MR-1. White letters in black boxes indicate residues that are identical in all sequences. Letters in gray boxes show residues that matched in at least three of the five sequences. The arrowhead shows the site of signal sequence cleavage. Asterisks show the conserved cysteine residues. Green boxes indicate β-strands predicted by PSIPRED, and orange wavy lines indicate predicted α-helixes.
Motility of ΔflgT cells.
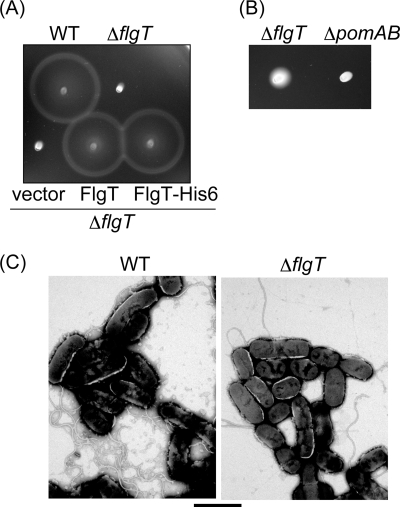
To examine the role of FlgT in the Vibrio flagellar system, we deleted the flgT gene and examined the motility of the ΔflgT cells based on swimming ability in semisolid agar and in liquid medium (Fig. 4 and data not shown). The ΔflgT cells scarcely showed any ability to swim in 4 h (Fig. 4A) and slightly expanded in semisolid agar by 9 h (Fig. 4B) compared to strain NMB191 (Mot−, ΔpomAB). Although most of the ΔflgT cells did not have a flagellum (Fla−), only a small fraction had a flagellum (Fla+) (Fig. 4C). We did not observe released flagella with attached basal bodies as reported previously for V. cholerae (29). This might suggest that the strength of the membrane between the two species is not the same. When we observed flagella using high-intensity dark-field microscopy, cells of wild type (VIO5) were more than 90% flagellate, and all of the flagellate cells were motile; on the other hand, the flgT mutant cells were ca. 30% flagellate, and only ca. 10% of the flagellate cells were motile but very slow. Therefore, FlgT seems to partially (not critically) contribute to both flagellar formation and rotation.
FIG. 4.
Motility of ΔflgT cells in semisolid agar. (A) Cells were inoculated into VPG500 semisolid agar and incubated at 30°C for 4 h. WT, VIO5; ΔflgT, TH6; vector, pSU41; FlgT, pTH104; FlgT-His6, pTH105. (B) Cells were inoculated into VPG500 semisolid agar and incubated at 30°C for 9 h. ΔflgT, TH6; ΔpomAB, NMB191. (C) Cells of VIO5 (WT) and TH6 (ΔflgT) grown in panel A were negatively stained with 2% potassium phosphotungstate and observed with a JEM-2010 electron microscope (JEOL, Japan). Bar, 2 μm.
Basal body structure purified from the ΔflgT cells.
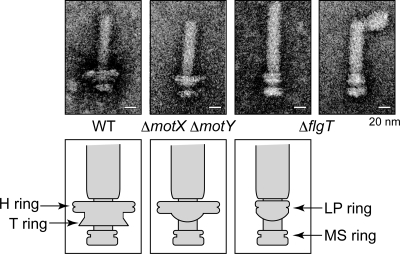
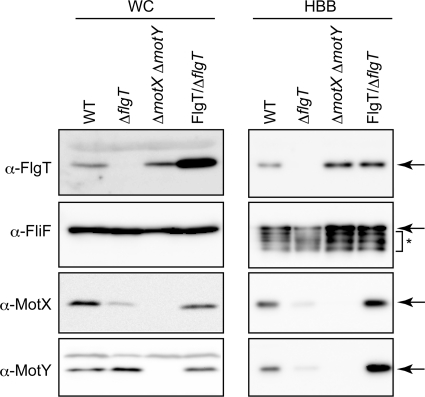
Because FlgT seemed to be a component of the basal body, we speculated that FlgT forms a substructure in the basal body. To investigate this possibility, we purified the basal bodies from the ΔflgT cells of a multiple-flagellated strain (KK148) and observed them by electron microscopy. The basal bodies of the ΔflgT cells had smaller LP rings than those of wild type, and the T ring beneath the LP ring was lost, suggesting that FlgT is a component protein of the ring structure outside of the LP ring (Fig. 5). Therefore, the ring that has been referred to as the Vibrio LP ring could be separated into a ring that is lost in the flgT mutant and the conventional LP ring formed by FlgH and FlgI. The new ring was named the H ring (Fig. 1). Next, to examine whether depletion of FlgT affects the other components of the basal body (MotX, MotY, and FliF), we analyzed whole-cell lysates or basal body fractions by immunoblotting using anti-MotX, MotY, FliF, or FlgT antibodies. In whole-cell lysates, MotY and FliF were expressed at similar levels regardless of the presence or absence of FlgT, but MotX was decreased in the absence of FlgT (Fig. 6). In the basal body fraction, FliF in the ΔflgT cells was detected at lower levels than in the wild-type cells. We measured the band densities of FliF from the wild-type and the ΔflgT cells. The intensity of the band from the ΔflgT cells was reduced to 18 or 51% compared to the intensity of wild type in two independent experiments. FliF in the basal body fraction was detected as smeared bands, suggesting that the N- or C-terminal region of FliF was degraded during the purification step. On the other hand, MotX and MotY were barely detectable in ΔflgT cells. These results suggest that the H ring is involved in basal body formation and is necessary to assemble the T ring composed of MotX and MotY (Fig. 6).
FIG. 5.
Electron microscopy images of the hook-basal bodies purified from V. alginolyticus. The hook-basal bodies were negatively stained with 2% (wt/vol) uranyl acetate and observed with a JEM-2010 electron microscope (JEOL, Japan). Bar, 20 nm. WT, KK148; ΔmotX ΔmotY, TH3; ΔflgT, TH7. The diagrams of the flagellar basal bodies isolated from the various strains are shown below the pictures.
FIG. 6.
Detection of FlgT, FliF, MotX, and MotY in the purified hook-basal body fraction. Whole-cell lysate (WC) and the purified hook-basal bodies (HBB) of V. alginolyticus were subjected to SDS-PAGE, followed by immunoblotting with anti-FlgT, FliF, MotX, and MotY antibodies. Asterisks show degraded products of FliF proteins in the basal body. WT, KK148; ΔflgT, TH7; ΔmotX ΔmotY, TH3; FlgT/ΔflgT, pTH104/TH7.
Interaction between FlgT and MotY.
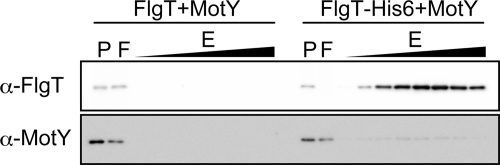
We speculated that FlgT interacts directly with MotX or MotY, which has been suggested to directly interact with the basal body (23). To determine whether this interaction occurs, a hexahistidine tag-fused FlgT and MotY were produced in sigma54− mutant (mutant rpoN) cells that do not produce the other flagellar components (Fig. 7). As for MotX, we cannot purify it as a soluble protein (34, 35), and thus we could not test for interaction between MotX and FlgT. After the periplasmic fraction was applied to a HisTrap column and washed, the bound proteins were eluted with imidazole. Although most of the MotY protein was detected in the flowthrough fraction, MotY was also coeluted with FlgT-His6 but not with plain FlgT. The elution profile of MotY corresponded to that of FlgT-His6 eluted by the liner gradient of imidazole. The present results support a direct interaction of MotY with FlgT. Under these experimental conditions, only FlgT and MotY were produced, so the interaction between them may be weak, and other flagellar proteins might be necessary for more stable interaction to form the ring structures.
FIG. 7.
Coelution assay of MotY with FlgT-His6. FlgT or FlgT-His6 proteins with MotY proteins were expressed from pTH106 or pTH107 in strain YM14 (rpoN mutant). The samples were subjected to SDS-PAGE, followed by immunoblotting with anti-FlgT and MotY antibodies. P, periplasmic fraction; F, flowthrough fraction; E, eluted fraction.
DISCUSSION
A hypothetical gene, flgT, was identified in V. cholerae, and flgT mutant cells were very rarely flagellated (9). FlgT was predicted to have structural homology to TolB, a protein involved in maintaining outer membrane integrity. Since the sheath of the V. cholerae flagellum appears to be derived from the cell's outer membrane, it has been speculated that FlgT may play a role in flagellar sheath formation (9). Taylor and coworkers (29) reported that the flagellar base of V. cholerae is released into the culture medium or is associated with membrane blebs in a flgT deletion mutant, and these researchers speculated that FlgT interacts with different components of the basal body. Transcriptional analysis of the flgT mutant has previously been carried out in V. cholerae (9), and it was determined that the mutant was specifically stalled at the class III/IV assembly checkpoint, which is controlled by FlgM, the anti-sigma factor. In the present study we observed that the number of complete basal bodies decreased significantly in the flgT mutant and that the amount of MotX, whose gene belongs to the class IV transcriptional hierarchy (20), was decreased in whole-cell lysates. Our results are consistent with the previous speculation that FlgT affected not only anchoring of the flagellum base on the membrane but also the construction of the rod/hook structure (29).
In the present study, we showed that FlgT is isolated with the basal body fraction of V. alginolyticus and that FlgT of V. alginolyticus is involved in flagella formation and rotation similar to V. cholerae. We found that FlgT is necessary to form a new ring structure, named the H ring (for holding ring of the flagellar base on the cell surface), which has been recognized as the outermost part of the LP rings (Fig. 1). Therefore, the ring of Vibrio looks obviously bigger than that of E. coli. These observations might imply that the H ring reinforces the association of the flagellar base with the outer membrane or peptidoglycan layer in Vibrio spp. It is possible that the H ring is composed of other flagellar proteins in addition to FlgT. Because FlgT is a periplasmic protein, a region corresponding to the outer membrane in the H ring may be formed by outer membrane proteins, such as FlgO and FlgP, which are somehow involved in the motility of V. cholerae (28). The flgO and flgP genes are located in the region next to the flgT gene on genome, and it has been reported that flagella in a flgO or flgP mutant are shorter in length than wild-type flagella (28). Further work needs to be done to identify all of the components contained in the purified basal body fraction.
MotX and MotY were poorly associated with the basal body of the ΔflgT mutant. We demonstrated that FlgT interacts directly with MotY by coelution assays. These results suggest that FlgT is a primary target for assembly of MotY into the basal body and the H ring is a scaffold for forming the T ring structure. On the other hand, a component other than FlgT, e.g., the P ring component FlgI, might be a second target for assembly of MotX and MotY to the basal body because very small amounts of MotX and MotY are associated with the basal body in the ΔflgT mutant. If the T ring, which is composed of MotX and MotY, is formed in the basal body of ΔflgT, the stators can be assembled around the rotor. Consequently, a small fraction of the ΔflgT cells seems to be able to swim.
Using the program, Phyre, it was predicted that FlgT has structural homology with the N-terminal domain of TolB in the Tol-Pal system, which is a supramolecular complex required for outer membrane integrity and resistance to antibiotics (9). TolB has an N-terminal mixed α/β domain and a C-terminal six-bladed β-propeller domain (5, 26). The α/β domain of TolB interacts with the periplasmic domain of TolA, which is a monotropic cytoplasmic membrane protein (6, 13, 45). The β-propeller domain of TolB interacts with the peptidoglycan-binding (PGB) domain of Pal, which is an outer membrane lipoprotein (6, 7). Furthermore, TolB is capable of interacting with OmpA and Lpp, which are outer membrane proteins (10). Therefore, TolB is thought to act as the “network hub” for the Tol-Pal complex (5). We have shown that the PGB region of Pal is interchangeable with the PGB region of MotB in E. coli (16). We suggested that FlgT interacts directly with MotY and the LP ring of the basal body in the present study and we have shown that MotX interacts with MotY and PomB (34). Based on this information, we speculate that FlgT, as well as MotY or MotX, also serves as a “network hub” to connect between the outer membrane LP ring of the basal body and the PomA/B complex.
FlgT, MotX, and MotY are specific flagellar components in Vibrio, Shewanella, and related species. Why are they present in only these species? The flagellar motor of V. alginolyticus can achieve remarkably fast rotation. To allow such rapid rotation, FlgT might be required to hold the flagellar base on the cell surface and as a scaffold to form the T ring. The H ring might reinforce the bushing robustness, thereby, might protect against physical breaking of the basal body. MotX interacts with PomB and thereby is involved in incorporation and stabilization of the PomAB complex. These specific components might contribute a robust motor structure compared to that of E. coli and Salmonella.
Acknowledgments
We thank Toshiaki Goto for technical advice regarding electron microscopy and also Noriko Nishioka for technical help to take electron micrographs.
This study was supported in part by grants-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan; the Japan Science and Technology Corp. (to M.H. and S.K.); and the Japan Society for the Promotion of Science (to H.T.).
Footnotes
Published ahead of print on 20 August 2010.
REFERENCES
- 1.Aizawa, S., G. E. Dean, C. J. Jones, R. M. Macnab, and S. Yamaguchi. 1985. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J. Bacteriol. 161:836-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, Y., S. Kojima, H. Kato, N. Nishioka, I. Kawagishi, and M. Homma. 1997. Putative channel components for the fast-rotating sodium-driven flagellar motor of a marine bacterium. J. Bacteriol. 179:5104-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartolome, B., Y. Jubete, E. Martínez, and F. de la Cruz. 1991. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. [DOI] [PubMed] [Google Scholar]
- 4.Blair, D. F., and H. C. Berg. 1990. The MotA protein of Escherichia coli is a proton-conducting component of the flagellar motor. Cell 60:439-449. [DOI] [PubMed] [Google Scholar]
- 5.Bonsor, D. A., I. Grishkovskaya, E. J. Dodson, and C. Kleanthous. 2007. Molecular mimicry enables competitive recruitment by a natively disordered protein. J. Am. Chem. Soc. 129:4800-4807. [DOI] [PubMed] [Google Scholar]
- 6.Bonsor, D. A., O. Hecht, M. Vankemmelbeke, A. Sharma, A. M. Krachler, N. G. Housden, K. J. Lilly, R. James, G. R. Moore, and C. Kleanthous. 2009. Allosteric beta-propeller signaling in TolB and its manipulation by translocating colicins. EMBO J. 28:2846-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouveret, E., R. Derouiche, A. Rigal, R. Lloubes, C. Lazdunski, and H. Benedetti. 1995. Peptidoglycan-associated lipoprotein-TolB interaction. A possible key to explaining the formation of contact sites between the inner and outer membranes of Escherichia coli. J. Biol. Chem. 270:11071-11077. [DOI] [PubMed] [Google Scholar]
- 8.Bryson, K., L. J. McGuffin, R. L. Marsden, J. J. Ward, J. S. Sodhi, and D. T. Jones. 2005. Protein structure prediction servers at University College London. Nucleic Acids Res. 33:W36-W38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, D. E., J. M. Urbach, and J. J. Mekalanos. 2008. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 105:8736-8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clavel, T., P. Germon, A. Vianney, R. Portalier, and J. C. Lazzaroni. 1998. TolB protein of Escherichia coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp, and OmpA. Mol. Microbiol. 29:359-367. [DOI] [PubMed] [Google Scholar]
- 11.Dean, G. D., R. M. Macnab, J. Stader, P. Matsumura, and C. Burks. 1984. Gene sequence and predicted amino acid sequence of the motA protein, a membrane-associated protein required for flagellar rotation in Escherichia coli. J. Bacteriol. 159:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle, T. B., A. C. Hawkins, and L. L. McCarter. 2004. The complex flagellar torque generator of Pseudomonas aeruginosa. J. Bacteriol. 186:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubuisson, J. F., A. Vianney, and J. C. Lazzaroni. 2002. Mutational analysis of the TolA C-terminal domain of Escherichia coli and genetic evidence for an interaction between TolA and TolB. J. Bacteriol. 184:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis, N. R., G. E. Sosinsky, D. Thomas, and D. J. Derosier. 1994. Isolation, characterization, and structure of bacterial flagellar motors containing the switch complex. J. Mol. Biol. 235:1261-1270. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka, H., T. Yakushi, A. Kusumoto, and M. Homma. 2005. Assembly of motor proteins, PomA and PomB, in the Na+-driven stator of the flagellar motor. J. Mol. Biol. 351:707-717. [DOI] [PubMed] [Google Scholar]
- 16.Hizukuri, Y., J. F. Morton, T. Yakushi, S. Kojima, and M. Homma. 2009. The peptidoglycan-binding (PGB) domain of the Escherichia coli pal protein can also function as the PGB domain in E. coli flagellar motor protein MotB. J. Biochem. 146:219-229. [DOI] [PubMed] [Google Scholar]
- 17.Homma, M., S.-I. Aizawa, G. E. Dean, and R. M. Macnab. 1987. Identification of the M-ring protein of the flagellar motor of Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 84:7483-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma, M., K. Kutsukake, M. Hasebe, T. Iino, and R. M. Macnab. 1990. FlgB, FlgC, FlgF, and FlgG a family of structurally related proteins in the flagellar basal body of Salmonella typhimurium. J. Mol. Biol. 211:465-477. [DOI] [PubMed] [Google Scholar]
- 19.Kawagishi, I., I. Okunishi, M. Homma, and Y. Imae. 1994. Removal of the periplasmic DNase before electroporation enhances efficiency of transformation in a marine bacterium Vibrio alginolyticus. Microbiology 140:2355-2361. [Google Scholar]
- 19a.Kawagishi, I., M. Nakada, N. Nishioka, and M. Homma. 1997. Cloning of a Vibrio alginolyticus rpoN gene that is required for polar flagellar formation. J. Bacteriol. 179:6851-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, Y. K., and L. L. McCarter. 2000. Analysis of the polar flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 182:3693-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koerdt, A., A. Paulick, M. Mock, K. Jost, and K. M. Thormann. 2009. MotX and MotY are required for flagellar rotation in Shewanella oneidensis MR-1. J. Bacteriol. 191:5085-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima, S., and D. F. Blair. 2004. The bacterial flagellar motor: structure and function of a complex molecular machine. Int. Rev. Cytol. 233:93-134. [DOI] [PubMed] [Google Scholar]
- 23.Kojima, S., A. Shinohara, H. Terashima, T. Yakushi, M. Sakuma, M. Homma, K. Namba, and K. Imada. 2008. Insights into the stator assembly of the Vibrio flagellar motor from the crystal structure of MotY. Proc. Natl. Acad. Sci. U. S. A. 105:7696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo, S., Y. Magariyama, and S.-I. Aizawa. 1990. Abrupt changes in flagellar rotation observed by laser dark-field microscopy. Nature 346:677-680. [DOI] [PubMed] [Google Scholar]
- 25.Kusumoto, A., K. Kamisaka, T. Yakushi, H. Terashima, A. Shinohara, and M. Homma. 2006. Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. J. Biochem. 139:113-121. [DOI] [PubMed] [Google Scholar]
- 26.Loftus, S. R., D. Walker, M. J. Mate, D. A. Bonsor, R. James, G. R. Moore, and C. Kleanthous. 2006. Competitive recruitment of the periplasmic translocation portal TolB by a natively disordered domain of colicin E9. Proc. Natl. Acad. Sci. U. S. A. 103:12353-12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magariyama, Y., S. Sugiyama, K. Muramoto, Y. Maekawa, I. Kawagishi, Y. Imae, and S. Kudo. 1994. Very fast flagellar rotation. Nature 381:752. [DOI] [PubMed] [Google Scholar]
- 28.Martinez, R. M., M. N. Dharmasena, T. J. Kirn, and R. K. Taylor. 2009. Characterization of two outer membrane proteins, FlgO and FlgP, that influence Vibrio cholerae motility. J. Bacteriol. 191:5669-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, R. M., B. A. Jude, T. J. Kirn, K. Skorupski, and R. K. Taylor. 2010. Role of FlgT in anchoring the flagellum of Vibrio cholerae. J. Bacteriol. 192:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarter, L. L. 1994. MotX, the channel component of the sodium-type flagellar motor. J. Bacteriol. 176:5988-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarter, L. L. 1994. MotY, a component of the sodium-type flagellar motor. J. Bacteriol. 176:4219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okabe, M., T. Yakushi, Y. Asai, and M. Homma. 2001. Cloning and characterization of motX, a Vibrio alginolyticus sodium-driven flagellar motor gene. J. Biochem. 130:879-884. [DOI] [PubMed] [Google Scholar]
- 34.Okabe, M., T. Yakushi, and M. Homma. 2005. Interactions of MotX with MotY and with the PomA/PomB sodium ion channel complex of the Vibrio alginolyticus polar flagellum. J. Biol. Chem. 280:25659-25664. [DOI] [PubMed] [Google Scholar]
- 35.Okabe, M., T. Yakushi, M. Kojima, and M. Homma. 2002. MotX and MotY, specific components of the sodium-driven flagellar motor, colocalize to the outer membrane in Vibrio alginolyticus. Mol. Microbiol. 46:125-134. [DOI] [PubMed] [Google Scholar]
- 36.Okunishi, I., I. Kawagishi, and M. Homma. 1996. Cloning and characterization of motY, a gene coding for a component of the sodium-driven flagellar motor in Vibrio alginolyticus. J. Bacteriol. 178:2409-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato, K., and M. Homma. 2000. Functional reconstitution of the Na+-driven polar flagellar motor component of Vibrio alginolyticus. J. Biol. Chem. 275:5718-5722. [DOI] [PubMed] [Google Scholar]
- 38.Sato, K., and M. Homma. 2000. Multimeric structure of PomA, the Na+-driven polar flagellar motor component of Vibrio alginolyticus. J. Biol. Chem. 275:20223-20228. [DOI] [PubMed] [Google Scholar]
- 39.Schoenhals, G. J., and R. M. Macnab. 1996. Physiological and biochemical analyses of FlgH, a lipoprotein forming the outer membrane L ring of the flagellar basal body of Salmonella typhimurium. J. Bacteriol. 178:4200-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stader, J., P. Matsumura, D. Vacante, G. E. Dean, and R. M. Macnab. 1986. Nucleotide sequence of the Escherichia coli MotB gene and site-limited incorporation of its product into the cytoplasmic membrane. J. Bacteriol. 166:244-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stolz, B., and H. C. Berg. 1991. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J. Bacteriol. 173:7033-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terashima, H., H. Fukuoka, T. Yakushi, S. Kojima, and M. Homma. 2006. The Vibrio motor proteins, MotX and MotY, are associated with the basal body of Na-driven flagella and required for stator formation. Mol. Microbiol. 62:1170-1180. [DOI] [PubMed] [Google Scholar]
- 43.Terashima, H., S. Kojima, and M. Homma. 2008. Flagellar motility in bacteria structure and function of flagellar motor. Int. Rev. Cell Mol. Biol. 270:39-85. [DOI] [PubMed] [Google Scholar]
- 44.Ueno, T., K. Oosawa, and S. I. Aizawa. 1992. M-ring, S-ring and proximal rod of the flagellar basal body of Salmonella-Typhimurium are composed of subunits of a single protein, FliF. J. Mol. Biol. 227:672-677. [DOI] [PubMed] [Google Scholar]
- 45.Walburger, A., C. Lazdunski, and Y. Corda. 2002. The Tol/Pal system function requires an interaction between the C-terminal domain of TolA and the N-terminal domain of TolB. Mol. Microbiol. 44:695-708. [DOI] [PubMed] [Google Scholar]
- 46.Xu, M., K. Yamamoto, T. Honda, and X. Ming. 1994. Construction and characterization of an isogenic mutant of Vibrio parahaemolyticus having a deletion in the thermostable direct hemolysin-related hemolysin gene (trh). J. Bacteriol. 176:4757-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagasaki, J., M. Okabe, R. Kurebayashi, T. Yakushi, and M. Homma. 2006. Roles of the intramolecular disulfide bridge in MotX and MotY, the specific proteins for sodium-driven motors in Vibrio spp. J. Bacteriol. 188:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 49.Yorimitsu, T., K. Sato, Y. Asai, I. Kawagishi, and M. Homma. 1999. Functional interaction between PomA and PomB, the Na+-driven flagellar motor components of Vibrio alginolyticus. J. Bacteriol. 181:5103-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]