Abstract
Like the Eukarya and Bacteria, the Archaea also perform N glycosylation. Using the haloarchaeon Haloferax volcanii as a model system, a series of Agl proteins involved in the archaeal version of this posttranslational modification has been identified. In the present study, the participation of HVO_1517 in N glycosylation was considered, given its homology to a known component of the eukaryal N-glycosylation pathway and because of the genomic proximity of HVO_1517 to agl genes encoding known elements of the H. volcanii N-glycosylation process. By combining the deletion of HVO_1517 with mass spectrometric analysis of both dolichol phosphate monosaccharide-charged carriers and the S-layer glycoprotein, evidence was obtained showing the participation of HVO_1517, renamed AglJ, in adding the first hexose of the N-linked pentasaccharide decorating this reporter glycoprotein. The deletion of aglJ, however, did not fully prevent the attachment of a hexose residue to the S-layer glycoprotein. Moreover, in the absence of AglJ, the level of only one of the three monosaccharide-charged dolichol phosphate carriers detected in the cell was reduced. Nonetheless, in cells lacking AglJ, no further sugar subunits were added to the remaining monosaccharide-charged dolichol phosphate carriers or to the monosaccharide-modified S-layer glycoprotein, pointing to the importance of the sugar added through the actions of AglJ for proper N glycosylation. Finally, while aglJ can be deleted, H. volcanii surface layer integrity is compromised in the absence of the encoded protein.
N glycosylation is a posttranslational modification of proteins in all three domains of life. However, in contrast to our relatively advanced description of the eukaryal and bacterial N-glycosylation pathways (12, 24, 26), many questions regarding the parallel process in the Archaea remain. With the identification of a series of agl (archaeal glycosylation) genes in the haloarchaeon Haloferax volcanii and the methanogens Methanococcus voltae and Methanococcus maripaludis, some insight into archaeal N glycosylation is, however, available (for a review, see references 6 and 28). For H. volcanii, AglB, AglD, AglE, AglF, AglG, AglI, AglM, and AglP were shown to participate in the assembly and attachment of a pentasaccharide to select Asn residues of the surface (S)-layer protein, a reporter of N glycosylation in this species (23). Specifically, AglG, AglI, AglE, and AglD are thought to be glycosyltransferases involved in adding the second, third, fourth, and fifth pentasaccharide subunits (2, 3, 29), respectively; AglF is a glucose-1-phosphate uridyltransferase (30); AglM is a UDP-glucose dehydrogenase (30); AglP is an S-adenosyl-l-methionine-dependent methyltransferase (17); and AglB is the oligosaccharyltransferase (2).
Despite these advances, proteins catalyzing several central steps in the H. volcanii N-glycosylation pathway have yet to be described. Accordingly, one such candidate, HVO_1517, encoding one of the five previously identified H. volcanii homologues of eukaryal dolichol phosphate mannosyltransferase 1 (Dpm1-C) (1), responsible for catalyzing the transfer of mannose from GDP-mannose to dolichol phosphate in the endoplasmic reticulum membrane (5), was considered here. Indeed, the genomic proximity of HVO_1517 to other genes involved in H. volcanii N glycosylation warrants such an analysis (27). Relying on mass spectrometry (MS) analysis of an HVO_1517 deletion strain, the contribution of the encoded protein, renamed AglJ, to N glycosylation is shown. The results demonstrate the participation of AglJ in adding the first hexose of the N-linked pentasaccharide decorating the H. volcanii S-layer glycoprotein, serving to generate a monosaccharide-modified dolichol phosphate carrier. Finally, this study reveals the importance of AglJ action for the proper assembly of the H. volcanii surface layer, formed from the S-layer glycoprotein.
MATERIALS AND METHODS
Strains and growth conditions.
H. volcanii parent strain WR536 (H53) and the isogenic strain deleted of HVO_1517 were grown in medium containing 3.4 M NaCl, 0.15 M MgSO4·7H2O, 1 mM MnCl2, 4 mM KCl, 3 mM CaCl2, 0.3% (wt/vol) yeast extract, 0.5% (wt/vol) tryptone, and 50 mM Tris-HCl (pH 7.2) at 40°C (19).
Deletion of HVO_1517.
The deletion of H. volcanii HVO_1517 was achieved by using a previously described approach (1, 4). To amplify approximately 500-bp-long regions flanking the coding sequence of HVO_1517, primers HVO_1517-5′upfor (gggctcgagGCTCGCGCAACTCATCAGAG [the genomic sequence is in capital letters]) and HVO_1517-5′uprev (cccaagcttGTCTCGATATGATTGTGCTG), directed against the upstream flanking region, and primers HVO_1517-3′downfor (gggggatccCGAACCGGGCCGTCGGGACTC) and HVO_1517-3′downrev (ccctctagaCGACCGTGGCACCGGGCGAGG), directed against the downstream flanking region, were employed. XhoI and HindIII sites were introduced by using primers HVO_1517-5′upfor and HVO_1517-5′uprev, respectively, while BamHI and XbaI sites were introduced by using primers HVO_1517-3′downfor and HVO_1517-3′downrev, respectively.
To confirm the deletion of HVO_1517 at the DNA level, PCR amplification was performed by using forward primers directed against an internal region of either HVO_1517 (HVO_1517-for [ATGCCCACCCCCGATGCCGTC]) or trpA (cccgaattcTTATGTGCGTTCCGGATGCG) together with a reverse primer against a region downstream of HVO_1517 (HVO_1517-5′downrev), respectively, yielding primer pairs a and b, or by using primers HVO_1517-for and HVO_1517-rev (TCACTCCAGTTCTTCGATTC), designed to amplify a section of the HVO_1517 coding region (primer pair c). Reverse transcription (RT)-PCR was performed as described previously (1), using primer pair c to test for HVO_1517 transcription so as to confirm the HVO_1517 deletion at the RNA level.
Isolation of the H. volcanii lipid fraction.
The total lipid contents from H. volcanii cells were extracted as follows. Cells were harvested (8,000 × g for 30 min at 4°C) and frozen at −20°C until extraction was performed. At that point, the pelleted cells were thawed, resuspended in double-distilled water (DDW) (1.33 ml DDW/g cells) and DNase (1.7 μg/ml; Sigma, St. Louis, MO), and stirred overnight at room temperature. Methanol and chloroform were added to the cell extract to yield a methanol-to-chloroform-to-cell extract ratio of 2:1:0.8. After stirring for 24 h at room temperature, the mixture was centrifuged (1,075 × g for 30 min at 4°C). The supernatant fractions were collected, combined, and filtered through glass wool. Chloroform and DDW were added to the filtrate to yield a chloroform-to-DDW-to-filtrate ratio of 1:1:3.8 in a separating funnel. After separation, the lower clear organic phase, containing the total lipid extract, was collected into a round-bottomed flask and evaporated in a rotary evaporator at 35°C. For analysis of the dolichol phosphate pool, the total lipid extracts were subjected to normal-phase liquid chromatography (LC)/mass spectrometry (MS) analysis without prefractionation.
LC/MS.
Normal-phase LC-electron spray ionization (ESI)/MS of lipids was performed by using an Agilent 1200 quaternary LC system coupled to a Q-Star XL quadrupole time-of-flight (TOF) tandem mass spectrometer (Applied Biosystems, Foster City, CA). An Ascentis Si high-performance liquid chromatography (HPLC) column (5 μm; 25 cm by 2.1 mm) was used. Mobile phase A consisted of chloroform-methanol-aqueous ammonium hydroxide (800:195:5, vol/vol/vol). Mobile phase B consisted of chloroform-methanol-water-aqueous ammonium hydroxide (600:340:50:5, vol/vol/vol/vol). Mobile phase C consisted of chloroform-methanol-water-aqueous ammonium hydroxide (450:450:95:5, vol/vol/vol/vol). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min, held at 100% mobile phase C for 3 min, and finally returned to 100% mobile phase A over 0.5 min and held at 100% mobile phase A for 5 min. The total LC flow rate was 300 μl/min. The postcolumn splitter diverted ∼10% of the LC flow to the ESI source of the Q-Star XL mass spectrometer, with MS settings as follows: ion spray of −4,500 V, curtain of 20 lb/in2, gas source 1 of 20 lb/in2, declustering potential of −55 V, and focusing potential of −150 V. Nitrogen was used as the collision gas for tandem MS (MS/MS) experiments. Data acquisition and analysis were performed by using the instrument's Analyst QS software.
Mass spectrometry analyses of the S-layer glycoprotein from cells of the H. volcanii parent strain and of H. volcanii ΔHVO_1517 cells were performed as described elsewhere previously (3).
Proteolytic digestion of the H. volcanii S layer.
S-layer resistance to the proteolysis of cells of the H. volcanii parent strain and of H. volcanii ΔHVO_1517 cells (1 ml) grown to the mid-exponential phase was tested upon the addition of proteinase K (1 mg/ml) for up to 3 h. Aliquots (100 μl) were removed at 30-min intervals during this window, and the proportion of intact S-layer glycoprotein remaining was verified by densitometry following SDS-PAGE and Coomassie staining at each time point. Densitometry was performed by using EZQuant-Gel v.2.1 software. Values are expressed as percentages of the band intensity measured prior to protease treatment.
Nucleotide sequence accession number.
The sequence of H. volcanii AglJ has been deposited into the EMBL/GenBank/DDBJ database and assigned accession number CAR66203.
RESULTS
Deletion of H. volcanii HVO_1517 affects the S-layer glycoprotein.
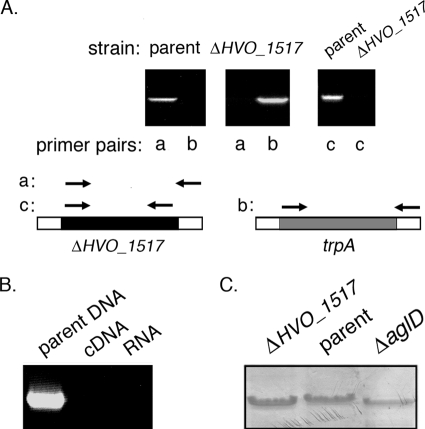
To begin assessing the involvement of H. volcanii HVO_1517 in N glycosylation, the gene was deleted from the genome as previously described (1, 4). In this so-called “pop-in/pop-out” approach, the genomic copy of the gene of interest in a strain auxotrophic for tryptophan is replaced by the H. volcanii tryptophan synthase-encoding trpA sequence. The successful replacement of HVO_1517 by trpA was first verified at the DNA level by PCR, using genomic DNA from the parent or the deletion strain as a template together with primer pair a (comprising a forward primer directed at a region within HVO_1517 and a reverse primer directed at a downstream region) (Fig. 1 A, left and middle) or primer pair b (comprising the same reverse primer together with a forward primer directed at a region within trpA) (Fig. 1A, left and middle). While the parent strain contains the HVO_1517 sequence (Fig. 1A, left), the gene was not detected in the deletion strain, having been replaced by the trpA sequence (Fig. 1A, middle). The absence of the HVO_1517 gene in the deletion strain was further indicated by the failure to obtain a PCR product using primers directed against the coding region of this sequence (Fig. 1A, right).
FIG. 1.
Deletion of HVO_1517 does not affect cell viability but enhances S-layer glycoprotein SDS-PAGE migration. (A, left and middle) PCR amplification was performed by using a forward primer directed at the 5′ HVO_1517 flanking region and a reverse primer directed at a sequence within the HVO_1517 coding region (yielding primer pair a) or using a forward primer directed at a sequence within the trpA sequence and the same reverse primer as that described above (yielding primer pair b), together with genomic DNA from cells of the parent strain (left) or from cells that had replaced the HVO_1517 gene with the trpA sequence (middle) as a template. (Right) PCR amplification was performed by using primers directed against the HVO_1517 coding region (i.e., primer pair c), together with genomic DNA from cells of the parent strain (parent) or the HVO_1517-deleted strain (ΔHVO_1517). Schematic diagrams showing the relative positions of the forward and reverse primers in each primer pair appear below the panels. Note that primer pairs a and b share the same reverse primer, while primer pairs a and c share the same forward primer. (B) RT-PCR was performed by using primers directed at HVO_1517 (primer pair c) and genomic DNA from parent strain cells or cDNA or RNA from HVO_1517-deleted cells as a template. (C) Deletion of H. volcanii HVO_1517 affects the apparent molecular weight of the S-layer glycoprotein. Equivalent aliquots of H. volcanii cells lacking HVO_1517 (ΔHVO_1517), cells of the parent strain (parent), or cells of the same strain lacking aglD (ΔaglD) were separated by 7.5% SDS-PAGE and Coomassie stained. Only the gel region containing the S-layer glycoprotein is shown.
The deletion of HVO_1517 was next confirmed at the RNA level by RT-PCR performed using primers directed to the HVO_1517 coding region. In these reactions, cDNA derived from the parent or the deletion strain served as a template. No HVO_1517-derived band could be detected using cDNA from the HVO_1517 deletion strain (Fig. 1B, middle). In control experiments, HVO_1517 was amplified by using genomic DNA from the parent strain as a template but not when RNA served as the template (Fig. 1B, left and right lanes, respectively).
To determine whether HVO_1517 is involved in protein glycosylation, the S-layer glycoprotein, a well-studied reporter of H. volcanii N glycosylation (1-3, 17, 18, 23, 29, 30), was characterized for cells lacking HVO_1517. Previous efforts (1, 29, 30) revealed that the deletion of agl genes encoding components of the H. volcanii N-glycosylation pathway often resulted in versions of the S-layer glycoprotein that migrate faster on SDS-PAGE gels than does the protein from parent strain cells, due to absent or perturbed N glycosylation in the mutant cells. The migration of the S-layer glycoprotein in cells deleted of HVO_1517 was enhanced relative to the SDS-PAGE migration of the S-layer glycoprotein from cells of the parent strain yet was comparable to that seen in cells lacking AglD (Fig. 1C). AglD was shown previously to be involved in the addition of the fifth sugar of the pentasaccharide N linked to the S-layer glycoprotein (2).
Deletion of HVO_1517 affects the composition of the glycan N linked to the S-layer glycoprotein.
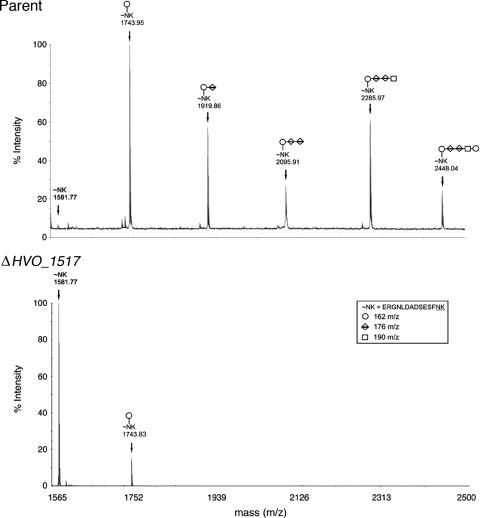
To determine whether HVO_1517 plays a role in N glycosylation, the S-layer glycoprotein from the HVO_1517 deletion mutant was examined by mass spectrometry. In cells of the parent strain, at least two S-layer glycoprotein sequons (i.e., Asn-X-Ser/Thr motifs, where X is any residue but Pro) are modified by a pentasaccharide consisting of two hexose residues, two hexuronic acid residues, and a methyl ester of a hexuronic acid. As previously shown (2, 3, 17, 29, 30), mass spectrometry analysis of an Asn-13-containing S-layer glycoprotein-derived tryptic peptide isolated from cells of the parent strain revealed the presence of the peptide modified by the pentasaccharide (m/z 2,448.04) (Fig. 2, top). In addition, other peptide peaks modified by monosaccharides (m/z 1,743.95), disaccharides (m/z 1,919.86), trisaccharides (m/z 2,095.91), and tetrasaccharides (m/z 2,285.97) were seen. In contrast, an examination of the Asn-13-linked glycan present on the same peptide derived from cells lacking HVO_1517 (Fig. 2, bottom) revealed the presence of only a minor peak corresponding to the monosaccharide-modified peptide (m/z 1,743.83) as well as a major peak corresponding to the nonmodified peptide (m/z 1,581.77). Peaks corresponding to di-, tri-, tetra-, and pentasaccharide-modified peptides were completely absent in this sample.
FIG. 2.
Matrix-assisted laser desorption ionization (MALDI)-TOF analysis of an Asn-13-containing H. volcanii S-layer glycoprotein-derived glycopeptide. The MALDI-TOF spectra of the Asn-13-containing tryptic peptides derived from the S-layer glycoprotein from cells of the parent strain (top) or of the HVO_1517-deleted strain (ΔHVO_1517) (bottom) are shown. The components of the glycopeptide-associated sugar residues, as well as the glycopeptide amino acid sequence, are shown in the inset box, while the glycan moieties decorating the peptide peaks are marked on the MALDI-TOF spectra accordingly.
Deletion of HVO_1517 is manifested at the monosaccharide-charged dolichol phosphate level.
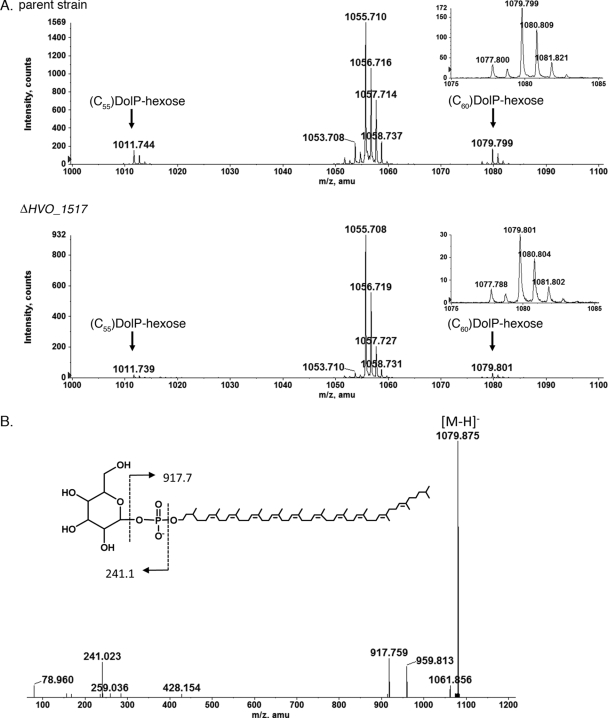
The isolation of the dolichol phosphate pool from the H. volcanii parent strain and from the same cells deleted of HVO_1517, followed by mass spectrometric analysis, provided support for HVO_1517 as adding the first hexose of the N-linked pentasaccharide decorating the S-layer glycoprotein. Although a detailed analysis of glycan-charged dolichol phosphates in H. volcanii will be addressed in a forthcoming study (Z. Guan et al., submitted for publication), it is shown here that the H. volcanii parent strain presents a mass spectrometry profile that includes ion peaks of m/z 1,011.744 {corresponding to the [M − H]− ion of hexose-(C55)dolichol phosphate} and m/z 1,079.799 {corresponding to the [M − H]− ion of hexose-(C60)dolichol phosphate; the calculated mass of the [M − H]− ion is 1,079.805} (Fig. 3 A, top). In addition, a major peak at m/z 1,055.7, corresponding to a previously described sulfoglycolipid (22), was also observed. When the same profile was considered for cells lacking HVO_1517 (Fig. 3A, bottom), the peaks corresponding to hexose-modified (C55/C60)dolichol phosphate were also detected, although at barely detectable levels and approximately 6-fold less than what was observed for the parent strain (Fig. 3A, compare top and bottom insets). Indeed, the decrease in the amount of hexose-modified (C55/C60)dolichol phosphate resulting from the deletion of HVO_1517 is also apparent when the relative heights of these peaks and that of the sulfoglycolipid are compared for each strain. In Fig. 3B, the MS/MS spectrum confirming the structure of hexose-(C60)dolichol phosphate is shown.
FIG. 3.
The absence of HVO_1517 affects the level of monosaccharide-modified dolichol phosphate. (A) Normal-phase LC-ESI/MS analysis in the negative-ion mode of the total lipid extracts from cells of the parent strain (parent) (top) and the HVO_1517-deleted strain (ΔHVO_1517) (bottom). amu, atomic mass units. The mass spectra were averaged from the spectra obtained between the 15.5- and 17.5-min retention times. The inset in each panel represents a 5-fold expansion of the hexose-(C60)dolichol phosphate region of the profile. (B) MS/MS verification of dolichylphosphate-hexose by collision-induced dissociation of its [M − H]− ion at m/z 1,079.9.
Given its role in N glycosylation, as demonstrated at the levels of both the dolichol carrier and the modified protein target, HVO_1517 was renamed aglJ (GenBank accession number CAR66203), in accordance with the nomenclature proposed previously by Chaban et al. (7).
AglJ is involved in modifying only one of three H. volcanii monosaccharide-charged dolichol phosphates.
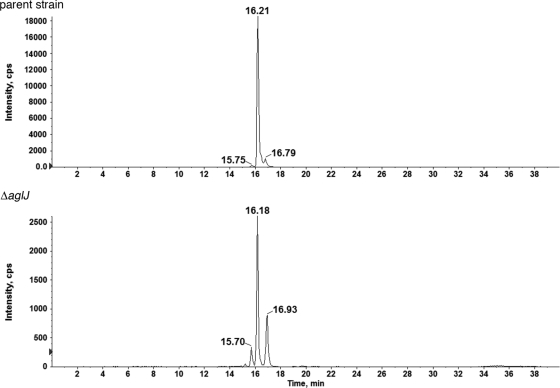
To better understand the origin of the minor monosaccharide-modified peaks associated with both the S-layer glycoprotein-derived peptide and the dolichol phosphate carrier in the absence of AglJ, the monosaccharide-charged (C60)dolichol phosphate peak was isolated from both the parent and deletion strains, and each one was examined in a single LC/MS run, over a 40-min span. As reflected in Fig. 4, the single m/z 1,079 ion peak from each strain could be resolved into three distinct species, distinguished by their slightly different LC retention times. When the relative levels of each peak from the parent strain were compared with their counterparts in the deletion strain, it was noted that although the first and third fractions were not affected by the absence of AglJ, the second peak was reduced from a value of over 19,000 intensity units to 2,600 intensity units in cells lacking AglJ, reflecting a 7-fold decrease in intensity (Fig. 4). This drop is comparable to the decrease in the intensity of both the monosaccharide-modified S-layer glycoprotein-derived peptide peak and the total monosaccharide-charged dolichol phosphate peak observed upon the deletion of aglJ (Fig. 2 and 3, respectively).
FIG. 4.
Only one of three monosaccharide-modified dolichol phosphates is affected by a lack of AglJ. Normal-phase LC-extracted ion chromatograms (EIC) of the dolichylphosphate-hexose [M − H]− ion at m/z 1,079.8 from the parent strain (top) and the ΔaglJ strain (bottom) are shown. The peaks at different retention times suggest the existence of three different dolichylphosphate-hexose species. Note the different scales used on the ordinates of the two graphs, highlighting that the 16.2-min peak is reduced 7.3-fold in the mutant compared with the parent strain, suggesting that AglJ is specific for the formation of this particular monosaccharide-modified dolichol phosphate species.
One other H. volcanii Dpm1 homologue generates monosaccharide-modified dolichol phosphate.
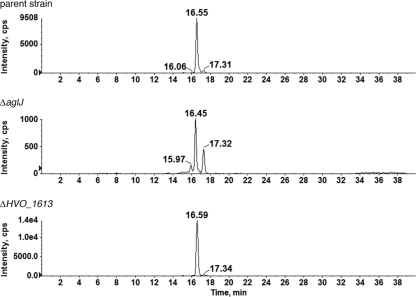
As H. volcanii was originally reported to contain five Dpm1 homologues (1), the putative ability of these sequences to generate monosaccharide-charged dolichol phosphate, including that minor species observed in the absence of AglJ, was next considered. Accordingly, the monosaccharide-charged (C60)dolichol phosphate peak pools from H. volcanii strains lacking either HVO_2601, first identified as Dpm1-A, or HVO_1613, first identified as Dpm1-D, were each examined in a single LC/MS run over a 40-min span. Whereas the deletion of HVO_2601 had no effect on the monosaccharide-charged (C60)dolichol phosphate profile (not shown), cells lacking HVO_1613 did not generate the small monosaccharide-charged dolichol phosphate species retained at the 16.06-min point in the parent strain (Fig. 5). The remaining two Dpm1 homologues were not considered in these experiments, since Dpm1-B was previously reannotated as AglE, shown to be responsible for adding pentasaccharide subunit 4 (3), while the final Dpm1 homologue, HVO_A0194, was originally shown to be transcribed only under heat shock conditions but not during log-phase growth in complete medium (1). As such, HVO_A0194 could not contribute to the monosaccharide charging of dolichol phosphate processing observed here. Hence, it can be concluded that HVO_1613 serves to add a monosaccharide to dolichol phosphate although not that subunit onto which additional sugars are added.
FIG. 5.
HVO_1613, a Dpm1 homologue, is responsible for generating one of three H. volcanii monosaccharide-modified dolichol phosphates. Normal-phase LC EICs of the dolichylphosphate-hexose [M − H]− ion at m/z 1,079.8 from the parent strain (top) and from ΔaglJ (middle) and ΔHVO_1613 cells (bottom) are shown. In the absence of HVO_1613, the first dolichylphosphate-hexose species (16.06 min) is absent.
In cells lacking AglJ, S-layer integrity is compromised.
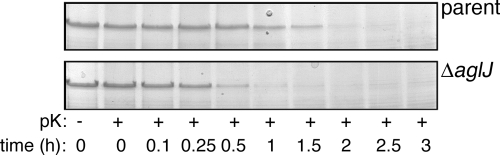
To assess whether the observed modification of the S-layer glycoprotein glycan in AglJ-lacking cells also affected the integrity of the S layer surrounding H. volcanii cells (thought to be composed solely of the S-layer glycoprotein [23]), parent strain cells and cells of the same strain deleted of aglJ were challenged with proteinase K for up to 3 h. The level of intact S-layer glycoprotein remaining at various time points within this window was revealed by densitometry following SDS-PAGE and Coomassie staining. The results show that substantially more full-length S-layer glycoprotein from the parent strain survived the proteinase K challenge than from cells lacking AglJ (Fig. 6). Indeed, densitometric quantitation of repeats of the experiment revealed that whereas almost 51% of the starting S-layer glycoprotein in parent strain cells survived 90 min of incubation with the protease, only 13% of the same protein remained after this interval in AglJ-lacking cells. It thus appears that the reduced N glycosylation experienced by the S-layer glycoprotein in the absence of AglJ compromises the proper assembly of the protein shell surrounding H. volcanii cells, as was previously observed in the case of cells lacking AglF, AglG, AglI, or AglM (29, 30).
FIG. 6.
The S layer surrounding H. volcanii cells is protease sensitive in cells deleted of aglJ. Shown are data for cells of the parent strain (parent) (top) or cells of the same strain lacking AglJ (ΔaglJ) or that were challenged with 1 mg/ml proteinase K (pK) at 37°C (bottom). Aliquots were removed immediately prior to incubation with the protease and at subsequent intervals up to 3 h and examined by 7.5% SDS-PAGE.
DISCUSSION
In the present study, mass spectrometry analysis at both the glycopeptide and dolichol phosphate carrier levels assigned AglJ a role in adding the first, as-yet-unidentified hexose to the pentasaccharide decorating the H. volcanii S-layer glycoprotein. However, in contrast to previous studies addressing the other predicted glycosyltransferases participating in H. volcanii N glycosylation (i.e., AglD, AglE, AglG, and AglI), where the deletion of the encoding gene led to the appearance of N-linked glycans totally lacking the sugar subunit added by the glycosyltransferase in question (2, 3, 29), small amounts of hexose-modified dolichol phosphate and S-layer glycoprotein-derived peptide were observed for cells lacking AglJ.
This observation can be explained upon a detailed analysis of the monosaccharide-charged dolichol phosphate pool in the parent and aglJ deletion strains. Such an analysis revealed the existence of three distinct monosaccharide-modified lipid carriers, reminiscent of a previous study reporting that radiochemical amounts of glucose- and galactose-charged dolichol phosphate could be detected in H. volcanii (14). Of the three monosaccharide-modified lipid carriers identified in the present study, the deletion of AglJ affected only one species. The other two minor peaks unaffected by the absence of AglJ would thus apparently be the products of different glycosyltransferases. Indeed, it was shown that HVO_1613 is responsible for generating one of these minor monosaccharide-charged dolichol phosphate species. The same is likely true for the minor peak observed for the deletion strain at the position normally occupied by the AglJ-processed peak, which could appear due to the actions of another glycosyltransferase inefficiently filling the void left in the absence of AglJ. Alternatively, this minor monosaccharide-modified dolichol phosphate may be naturally present but is unmasked only now, in the absence of an AglJ-catalyzed glycosylation of dolichol phosphate, meaning that four different monosaccharide-modified dolichol phosphate pools would exist in H. volcanii, namely, those generated by the glycosyltransferases AglJ, HVO_1613, and two others, which are currently unidentified. The results confirm that HV_2601, previously identified as a Dpm1 homologue (1), does not serve such a role. It is also conceivable that AglJ does not act alone in adding the first hexose of the N-linked pentasaccharide such that the relevant monosaccharide-modified lipid carrier and protein target observed for cells lacking AglJ reflect the residual contribution of a second protein involved in adding this first pentasaccharide sugar subunit.
Regardless of the agent(s) responsible for generating the monosaccharide-charged dolichol phosphate species observed for the aglJ deletion strain, no additional saccharide subunits are added to that minor monosaccharide-modified dolichol phosphate carrier seen at the position of the AglJ-processed peak in this mutant (or, for that matter, to any of the other minor populations of monosaccharide-modified carriers). Moreover, no additional saccharide subunits are bound to the monosaccharide N linked to the S-layer glycoprotein in the aglJ deletion strain. Thus, it seems unlikely that the monosaccharide added to the lipid carrier found at the same position as the AglJ-processed lipid carrier in the deletion strain represents an alternative linking sugar of a pentasaccharide variant decorating the S-layer glycoprotein. Likewise, the other minor monosaccharide-modified dolichol phosphates do not appear to participate in generating the pentasaccharide N linked to the S-layer glycoprotein. Moreover, given that sugar subunits 2 and 3 of the S-layer-linked pentasaccharide are hexuronic acids (2) while subunit 4 is a methyl ester of hexuronic acid (17), it is also unlikely that the hexose-charged dolichol phosphates generated through the actions of glycosyltransferases other than AglJ serve as lipid carriers for those sugars ultimately found at pentasaccharide position 2, 3, or 4. As such, it appears that the two hexuronic acid and the methyl ester of hexuronic acid components of the N-linked pentasaccharide are derived from soluble activated species sequentially added onto an AglJ-processed, monosaccharide-charged dolichol phosphate carrier. Still, the possibility remains that the hexose found at pentasaccharide position 5 is derived from one of the non-AglJ-dependent monosaccharide-charged dolichol phosphates. AglD, previously shown to be involved in adding the final pentasaccharide subunit (2), could fulfill such a role. Finally, the minor amount of monosaccharide-modified dolichol phosphate carriers generated through the actions of enzymes other than AglJ could participate in the biosynthesis of other glycoconjugates, such as glycolipids. Indeed, the biogenesis of H. volcanii glycolipids, a process of which little is known, was previously shown not to involve any of the Agl glycosyltransferases (20).
Previous results have shown that in cells deleted of aglG, encoding the predicted glycosyltransferase responsible for adding the second sugar of the pentasaccharide N linked to the H. volcanii S-layer glycoprotein, significant amounts of monosaccharide-modified protein were generated (29). As such, the as-yet-unidentified archaeal flippase(s) responsible for delivering the glycan-charged dolichol phosphate carrier across the plasma membrane as well as AglB, the archaeal oligosaccharyltransferase responsible for transferring the “flipped” dolichol phosphate-bound glycan to the protein target, are apparently able to recognize even the AglJ-processed, monosaccharide-modified substrate. Likewise, in the present study, the minor amounts of the monosaccharide-modified S-layer glycoprotein observed for the ΔaglJ strain point to the abilities of the H. volcanii flippase(s) and AglB to deliver even smaller amounts of non-AglJ-processed monosaccharides from their dolichol phosphate carriers to target proteins. Moreover, the finding that the monosaccharide-modified S-layer glycoprotein was observed for the ΔaglJ strain, likely bearing an alternative sugar from that found on the monosaccharide-modified S-layer glycoprotein population seen in native cells, is indicative of the relaxed specificity of H. volcanii AglB for the linking sugar, similar to what was seen previously with bacterial oligosaccharyltransferases (9-11). Indeed, such a relaxed specificity of the archaeal oligosaccharyltransferase was previously suggested in light of reports of the Halobacterium salinarum S-layer glycoprotein being modified by two different N-linked glycans, each bearing a unique linking sugar, despite the seeming presence of a single AglB protein in this species (15, 16).
In conclusion, AglJ can now be added to the growing list of H. volcanii components shown to contribute to protein N glycosylation in this haloarchaeon that includes AglB, AglD, AglE, AglF, AglG, AglI, AglM, and AglP (2, 3, 12, 29, 30), all of which (with the exception of AglD) are encoded by a single agl gene cluster (27). These findings, along with similar efforts addressing other members of the Archaea, such as Methanococcus voltae (7, 8, 21), Methanococcus maripaludis (25), and Pyrococcus furiosus (13), are contributing to a better understanding of archaeal N glycosylation.
Acknowledgments
Plasmid pTA131 and H. volcanii WR536 (H53) cells were kindly provided by Moshe Mevarech (Tel Aviv University). J.E. is supported by the Israel Science Foundation (grant 30/07) and the U.S. Army Research Office (grant W911NF-07-1-0260).
The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by Lipid Maps large-scale collaborative grant GM-069338 from the NIH. A.D. is supported by the Biotechnology and Biological Sciences Research Council (grants BBF0083091 and BBC5196701). L.K. is the recipient of a Negev-Zin Associates scholarship. V.V.V. is supported by Dstl.
Footnotes
Published ahead of print on 27 August 2010.
REFERENCES
- 1.Abu-Qarn, M., and J. Eichler. 2006. Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol. Microbiol. 61:511-525. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Qarn, M., S. Yurist-Doutsch, A. Giordano, A. Trauner, H. R. Morris, P. Hitchen, O. Medalia, A. Dell, and J. Eichler. 2007. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 374:1224-1236. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Qarn, M., A. Giordano, F. Battaglia, A. Trauner, P. Hitchen, H. R. Morris, A. Dell, and J. Eichler. 2008. Identification of AglE, a second glycosyltransferase involved in N-glycosylation of the Haloferax volcanii S-layer glycoprotein. J. Bacteriol. 190:3140-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allers, T., H. P. Ngo, M. Mevarech, and R. G. Lloyd. 2004. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 70:943-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burda, P., and M. Aebi. 1999. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta 1426:239-257. [DOI] [PubMed] [Google Scholar]
- 6.Calo, D., L. Kaminski, and J. Eichler. 2010. Protein glycosylation in Archaea: sweet and extreme. Glycobiology 20:1065-1076. [DOI] [PubMed] [Google Scholar]
- 7.Chaban, B., S. Voisin, J. Kelly, S. M. Logan, and K. F. Jarrell. 2006. Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea. Mol. Microbiol. 61:259-268. [DOI] [PubMed] [Google Scholar]
- 8.Chaban, B., S. M. Logan, J. F. Kelly, and K. F. Jarrell. 2009. AglC and AglK are involved in biosynthesis and attachment of diacetylated glucuronic acid to the N-glycan in Methanococcus voltae. J. Bacteriol. 191:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiGiandomenico, A., M. J. Matewish, A. Bisaillon, J. R. Stehle, J. S. Lam, and P. Castric. 2002. Glycosylation of Pseudomonas aeruginosa 1244 pilin: glycan substrate specificity. Mol. Microbiol. 46:519-530. [DOI] [PubMed] [Google Scholar]
- 10.Faridmoayer, A., M. A. Fentabil, D. C. Mills, J. S. Klassen, and M. F. Feldman. 2007. Functional characterization of bacterial oligosaccharyltransferases involved in O-linked protein glycosylation. J. Bacteriol. 189:8088-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faridmoayer, A., M. A. Fentabil, M. F. Haurat, W. Yi, R. Woodward, P. G. Wang, and M. F. Feldman. 2008. Extreme substrate promiscuity of the Neisseria oligosaccharyltransferase involved in protein O-glycosylation. J. Biol. Chem. 283:34596-34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73:1019-1049. [DOI] [PubMed] [Google Scholar]
- 13.Igura, M., N. Maita, J. Kamishikiryo, M. Yamada, T. Obita, K. Maenaka, and D. Kohda. 2008. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 27:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuntz, C., J. Sonnenbichler, I. Sonnenbichler, M. Sumper, and R. Zeitler. 1997. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology 7:897-904. [DOI] [PubMed] [Google Scholar]
- 15.Lechner, J., and F. Wieland. 1989. Structure and biosynthesis of prokaryotic glycoproteins. Annu. Rev. Biochem. 58:173-194. [DOI] [PubMed] [Google Scholar]
- 16.Magidovich, H., and J. Eichler. 2009. Glycosyltransferases and oligosaccharyltransferases in Archaea: putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol. Lett. 300:122-130. [DOI] [PubMed] [Google Scholar]
- 17.Magidovich, H., S. Yurist-Doutsch, Z. Konrad, V. V. Ventura, A. Dell, P. G. Hitchen, and J. Eichler. 2010. AglP is a S-adenosyl-L-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. Mol. Microbiol. 76:190-199. [DOI] [PubMed] [Google Scholar]
- 18.Mengele, R., and M. Sumper. 1992. Drastic differences in glycosylation of related S-layer glycoproteins from moderate and extreme halophiles. J. Biol. Chem. 267:8182-8185. [PubMed] [Google Scholar]
- 19.Mevarech, M., and R. Werczberger. 1985. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 162:461-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naparstek, S., E. Vinagradov, and J. Eichler. 2010. Different glycosyltransferases are involved in lipid glycosylation and protein N-glycosylation in the halophilic archaeon Haloferax volcanii. Arch. Microbiol. 192:581-584. [DOI] [PubMed] [Google Scholar]
- 21.Shams-Eldin, H., B. Chaban, S. Niehus, R. T. Schwarz, and K. F. Jarrell. 2008. Identification of the archaeal alg7 gene homolog (N-acetylglucosamine-1-phosphate transferase) of the N-linked glycosylation system by cross-domain complementation in yeast. J. Bacteriol. 190:2217-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprott, G. D., S. Larocque, N. Cadotte, C. J. Dicaire, M. McGee, and J. R. Brisson. 2003. Novel polar lipids of halophilic eubacterium Planococcus H8 and archaeon Haloferax volcanii. Biochim. Biophys. Acta 1633:179-188. [DOI] [PubMed] [Google Scholar]
- 23.Sumper, M., E. Berg, R. Mengele, and I. Strobel. 1990. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J. Bacteriol. 172:7111-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szymanski, C. M., and B. W. Wren. 2005. Protein glycosylation in bacterial mucosal pathogens. Nat. Rev. Microbiol. 3:225-237. [DOI] [PubMed] [Google Scholar]
- 25.VanDyke, D. J., J. Wu, S. M. Logan, J. F. Kelly, S. Mizuno, S. I. Aizawa, and K. F. Jarrell. 2009. Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. 72:633-644. [DOI] [PubMed] [Google Scholar]
- 26.Weerepana, E., and B. Imperiali. 2006. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology 16:91R-101R. [DOI] [PubMed] [Google Scholar]
- 27.Yurist-Doutsch, S., and J. Eichler. 2009. Manual annotation, transcriptional analysis and protein expression studies reveal novel genes in the agl cluster responsible for N-glycosylation in the halophilic archaeon Haloferax volcanii. J. Bacteriol. 191:3068-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yurist-Doutsch, S., B. Chaban, D. VanDyke, K. F. Jarrell, and J. Eichler. 2008. Sweet to the extreme: protein glycosylation in Archaea. Mol. Microbiol. 68:1079-1084. [DOI] [PubMed] [Google Scholar]
- 29.Yurist-Doutsch, S., M. Abu-Qarn, F. Battaglia, H. R. Morris, P. G. Hitchen, A. Dell, and J. Eichler. 2008. aglF, aglG and aglI, novel members of a gene cluster involved in the N-glycosylation of the Haloferax volcanii S-layer glycoprotein. Mol. Microbiol. 69:1234-1245. [DOI] [PubMed] [Google Scholar]
- 30.Yurist-Doutsch, S., H. Magidovich, V. V. Ventura, P. G. Hitchen, A. Dell, and J. Eichler. 2010. N-glycosylation in Archaea: on the coordinated actions of Haloferax volcanii AglF and AglM. Mol. Microbiol. 74:1047-1058. [DOI] [PubMed] [Google Scholar]