Abstract
Orientations of the seven invertible polysaccharide biosynthesis locus promoters of Bacteroides fragilis were determined from bacteria grown in vitro, from feces of monoassociated and complex colonized mice, and from B. fragilis-induced murine abscesses. Bacteria grown in vivo have greater variability in orientation of polysaccharide locus promoters than culture-grown organisms.
Intestinal Bacteroidales species are prominent members of the gut microbiota but can become opportunistic pathogens following spillage of intestinal contents into the abdominal cavity, with bacteria frequently isolated from blood and intra-abdominal abscesses (8, 16). The synthesis of a large number of capsular polysaccharides (PSs) that undergo phase variation is a relatively conserved property within intestinal Bacteroidales species (3). The model intestinal Bacteroidales species used to study PS synthesis and regulation is Bacteroides fragilis. The prototype strain NCTC9343 synthesizes eight distinct capsular polysaccharides, designated PSA to PSH (7). Previous studies have shown that PS production is necessary for gut colonization (2, 11). In addition, antigen-specific immune responses to PSA can stimulate immune system development in germ-free mice and prevent intestinal inflammation in animal models of colitis (12, 13). Furthermore, PSA production is required for B. fragilis to initiate abscess formation in experimental models (5, 18).
Genes encoding the enzymes necessary for the synthesis of the individual PSs are organized as distinct operons, each with a single upstream promoter, referred to here as PS locus promoters. Seven of the eight PS locus promoters are flanked by 19- to 25-bp inverted-repeat sequences (IRs) (7). The DNA segments between the IRs range in size from 168 to 193 bp and undergo reversible inversions (7). A serine family site-specific recombinase, Mpi, mediates the inversions of each of the seven promoter regions, thereby placing them in the correct (on) or incorrect (off) orientation for transcription of the downstream polysaccharide biosynthesis genes (6).
Despite the importance of PS synthesis for intestinal colonization and abscess formation, little is known about PS locus promoter orientations in vivo. This study builds upon our current knowledge of PS regulation by examining the orientations of all seven invertible PS locus promoters from bacteria grown in culture and from in vivo sites representing symbiosis and disease.
A previously described PCR digestion technique was used to quantify the percentage of B. fragilis bacteria that had each PS locus promoter oriented on or off (7) (Fig. 1A; also see Table S1 and Fig. S1A in the supplemental material). Chromosomal DNA was isolated from B. fragilis grown under various conditions, and PCRs were performed using primers that annealed outside the IRs flanking each invertible promoter region such that there was no bias in the PCR for either promoter orientation (see Fig. S1B in the supplemental material). Each PCR product was digested with a restriction enzyme that cut asymmetrically between the IRs, resulting in four differently sized fragments, two from a promoter in the on orientation and two from a promoter in the off orientation (Fig. 1A; also see Fig. S1A). The percentage of bacteria that had a PS locus promoter in the on orientation was calculated by measuring the intensity of each of the resulting DNA fragments following ethidium bromide staining in agarose gels and dividing the intensity of the fragments from the promoters in the on orientation by the intensity of the fragments from the promoters in both the on and off orientations using Image J software (available at rsbweb.nih.gov/ij/).
FIG. 1.
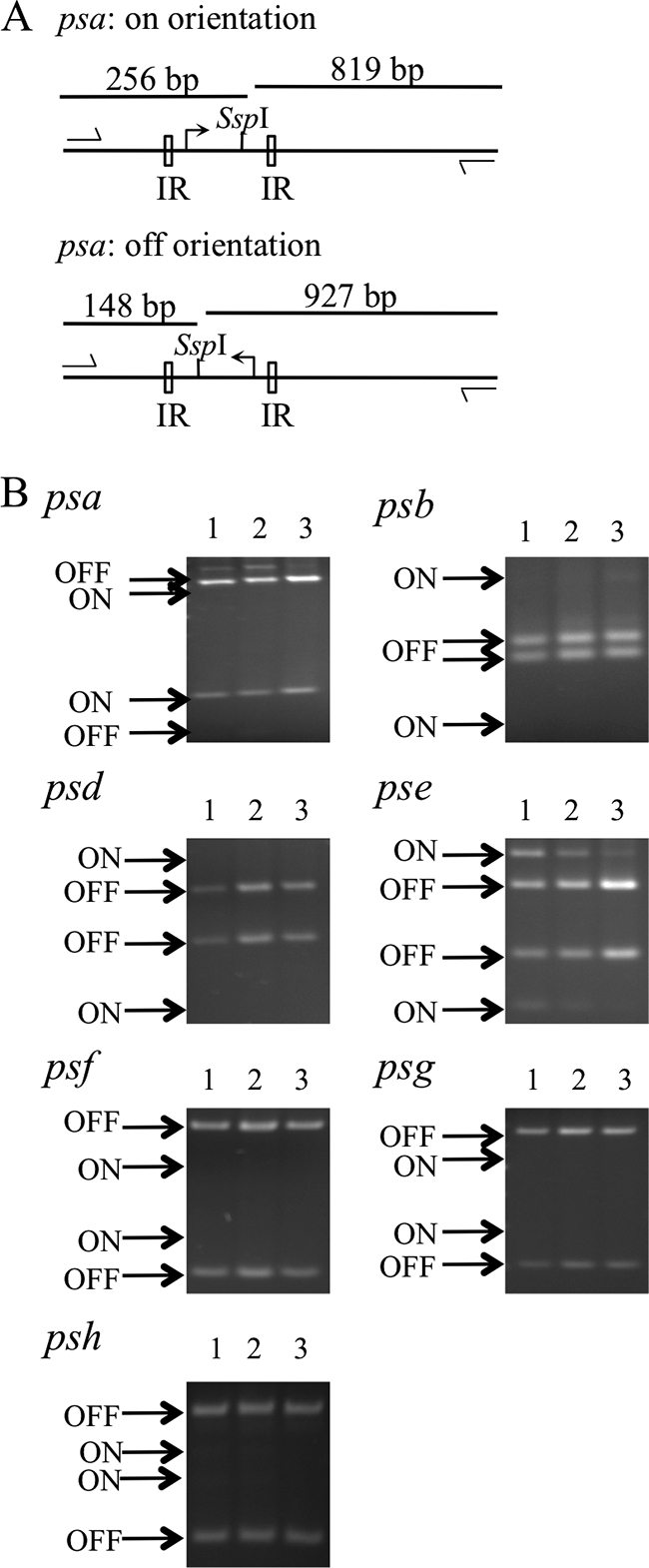
PS locus promoter orientations from B. fragilis grown in vitro. (A) Schematic diagram of the PCR digestion method used to determine psa promoter orientation. All PCR programs had an initial incubation of 2 min at 94°C, followed by 30 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 90 s and a final 5-min incubation at 72°C. The DNA fragments were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining. (B) Ethidium bromide-stained agarose gels demonstrating the fragments resulting from PCR digestion analysis of bacteria from three independent broth cultures. Each lane represents the data from one independent broth culture.
To study B. fragilis PS locus promoter orientations independent of host factors and other microbial species, PS locus promoter orientations from three independent exponential-phase broth cultures were calculated. While the psa promoter was in the on orientation in greater than 80% of the bacteria from each of the broth cultures, none of other PS locus promoters, with the exception of the pse promoter, were observed in the on orientation in greater than 8% of bacteria (Fig. 1 and 2).
FIG. 2.
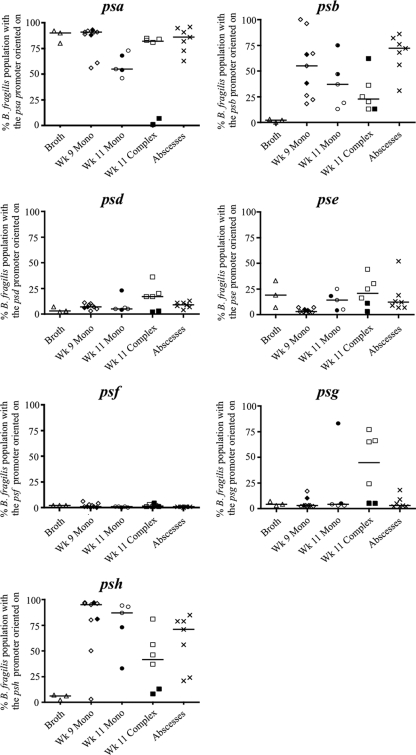
B. fragilis PS locus promoter orientations from bacteria grown in broth cultures (triangles), from fecal samples taken from monocolonized mice at 9 weeks of age (wk 9 mono, diamonds), from fecal samples taken 2 weeks later from the same mice that remained in the gnotobiotic isolator (wk 11 mono, circles) or that were removed from the isolator at 9 weeks and acquired a complex flora (wk 11 complex, squares), and from murine intraperitoneal abscesses (X's). Each data point represents one independent broth culture, mouse, or abscess. Pertaining to the fecal samples, open symbols indicate that the mice were from litter A and filled symbols indicate that the mice were from litter B. Fecal samples from the mice of litter B that remained in the isolator for the duration of the experiment were not analyzed at the first time point. The horizontal bar represents the median.
To study the effects of host factors on PS locus promoter orientations during intestinal colonization, we examined bacteria from fresh fecal samples of 9-week-old Swiss-Webster gnotobiotic mice that were monocolonized with B. fragilis from birth via fecal-oral transmission from the monocolonized mother. In this model, monocolonization with B. fragilis is stably maintained over time at a level of approximately 3 × 1010 CFU/g feces (4). Samples were collected from nine male mice from two different litters (see Fig. S2 in the supplemental material) and examined individually. Bacterial DNA was isolated using a QiaAmp DNA stool minikit (Qiagen). Similarly to the broth cultures, the psa promoter was in the on orientation in a majority of B. fragilis bacteria from the feces of each of the monocolonized mice (Fig. 2). The psb and psh promoter orientations were variable between different monocolonized mice; in some mice, the psb and psh promoters were on in greater than 90% of bacteria, whereas in other mice, less than 25% of the bacteria had these promoters oriented on (Fig. 2; also see Fig. S3 in the supplemental material). In all cases, the percentages of bacteria with these two PS locus promoters oriented on were greater than those of bacteria grown in vitro. Analysis of the other four PS locus promoters revealed consistent trends between all mice of both litters, with a very small percentage of the bacteria with these promoters oriented on (Fig. 2; also see Fig. S3). In comparison to the in vitro-grown bacteria, which demonstrate very little variability in the percentage of the population with a particular PS locus promoter oriented on, i.e., tight clustering between samples, analysis of bacteria 9 weeks after monoassociation demonstrated more variability in PS locus promoter orientations. We applied Levene's test of variance homogeneity (9) and rejected the null hypothesis of equivalent variances between in vitro-grown and monocolonizing bacteria and obtained a significant value for the PSB (P = 0.026) promoter, with all other promoters except the PSE promoter markedly more variable for bacteria from monocolonized mice.
The experiments in monocolonized mice provided insights into the influence of host factors on PS locus promoter orientations. However, as no other microbes are present, it is a simplified model of the intestinal environment. To examine the effects of a complex microbiota on PS locus promoter orientations, the mice in the two litters, here referred to as litter A and litter B, were split immediately following the promoter orientation analysis described above. Half of the mice of each litter were removed from the isolator and placed in a cage with an 8- to 10-week-old specific-pathogen-free (SPF) Swiss-Webster mouse. After 24 h, the SPF mouse was removed from the cage. Fecal samples were collected from the mice 2 weeks after they had been moved into the SPF facility and from monocolonized littermates that remained in the gnotobiotic isolator for the duration of the experiment (see Fig. S2 in the supplemental material).
The greatest differences were observed when comparing promoter orientations from B. fragilis from the monocolonized and complex colonized mice from the two litters (Fig. 2; also see Fig. S4 in the supplemental material). Within litter A, a larger percentage of B. fragilis bacteria from the complex colonized mice than from the monocolonized mice had the psa, psd, and psg promoters in the on orientation. The largest increase was observed in the psg promoter, which was in the on orientation in only 3.3% ± 0.5% of the bacteria from the monocolonized mice but in 40.5% ± 32.8% of those from the complex colonized mice of litter A. In contrast, within litter B, there was a decrease in the percentage of the bacteria with the psa and psh promoters in the on orientation and no increase in the psd and psg promoters in the complex colonized mice relative to the levels in the two monocolonized mice. Furthermore, with the exception of the psb promoter in one of the two mice, no other PS locus promoters were in the on orientation in greater than 11% of the bacteria from either of the two complex colonized mice of litter B (Fig. 2; also see Fig. S4 in the supplemental material). To assess differential clustering of promoter orientations between monocolonized and complex colonized states, we used extended generalized estimating equations (20) to estimate the intralitter correlation coefficients for the proportion of PS locus promoters in the on orientation in each of the two states. There was no evidence of PS locus promoter clustering by litter from bacteria of monocolonized mice (intralitter correlation of −0.036, P = 0.67). However, PS locus promoter orientations from bacteria from the complex microbiota demonstrated high intralitter clustering (correlation of 0.65, P = 0.022).
These data suggest that the presence of a complex microbiota influences B. fragilis PS locus promoter orientations. The significant differences observed between the two litters under complex colonization may be due to differences in composition of the microbiota. The complex microbiotas were acquired by cohabitation with SPF mice. The two litters of mice were born 45 days apart and were housed with unrelated SPF mice. A study has shown that kinship is a contributing factor in the composition of the mouse microbiota and that unrelated mice of the same strain and housed in the same animal facility have significant compositional differences in their microbiotas at the genus level (10). Hence, there may have been significant differences in the microbiotas acquired by these two litters of mice, which may have altered host-B. fragilis or microbe-B. fragilis interactions in a way that affected PS locus promoter orientations.
As B. fragilis is both an intestinal symbiont and an opportunistic pathogen, we sought to determine if PS locus promoter orientations are different for bacteria in symbiotic and pathogenic states. Therefore, analyses were performed with B. fragilis from murine intraperitoneal abscesses. The abscess model was performed similarly to a model previously described (19). Briefly, 6- to 8-week-old male C57BL/6J mice from Jackson Laboratory (Bar Harbor, ME) were injected intraperitoneally with B. fragilis (1 × 108 CFU/mouse) mixed with sterile rat cecal contents (1:1, vol/vol, 0.2 ml total volume), which mimics the spillage of colonic contents into the peritoneal cavity (14, 17). Seven days later, intra-abdominal abscesses were excised and processed for bacterial DNA isolation. One abscess was collected from each of seven mice. Five abscesses were isolated from the liver and two from the mesentery.
The three PS locus promoters that were oriented on in the greatest percentage of bacteria from abscesses were the psa, psb, and psh promoters, the same promoters that were most frequently oriented on from bacteria in the feces of monocolonized mice (Fig. 2; also see Fig. S5 in the supplemental material). Both the abscess and monocolonization models are devoid of other microbes in the compartment studied and reflect the interaction of B. fragilis alone with the host. Clinical abscesses, however, are usually polymicrobial. It is possible that, within abscesses, the PS locus promoter orientations would vary if other bacteria were present. PSA has been demonstrated to have a role in the induction of intra-abdominal abscesses in this model system (5, 18). This is the first study to demonstrate that the psa promoter is actually oriented on in a majority of the bacteria in abscesses, supporting that this abscess-inducing polysaccharide may be relevant to the disease process.
Overall, there are relatively consistent patterns of PS locus promoter orientations between different growth environments, although there is more PS locus promoter orientation diversity between mice in the polymicrobial environment. The psa promoter is the only PS locus promoter that is oriented on in the majority of bacteria from all sites analyzed. In the in vitro-grown bacteria, only two PS locus promoters, the psa and pse promoters, are present in the on orientation in more than 5% of the bacteria. Bacteria present in various in vivo environments tended to have other PS locus promoters, most frequently the psb and psh promoters, present in the on orientation.
The inversions of each of the seven PS locus promoters are mediated by the same DNA invertase, Mpi, which binds the IRs that flank each of the promoter regions. The PS locus promoters that are most frequently observed in the on orientation and demonstrate the most variability in terms of orientation in the environments examined, the psa, psb, pse, and psh promoters, are flanked by identical IRs (see Fig. S6 in the supplemental material). The IRs surrounding the psd, psf, and psg promoters share the same 10-bp consensus sequence but differ from each other and the other four IRs outside this region (7). In addition, DNA sequences extending beyond the IRs are likely also recognized by the recombinase (15). Differences in these IRs and sequences in the surrounding regions may influence the binding affinity of Mpi and lead to certain promoters, such as the psd, psf, and psg promoters, oriented off in most of the bacteria.
The experiments outlined in this paper provide evidence that there are as-yet-undefined factors that influence PS locus promoter orientations and, hence, PS synthesis in B. fragilis. Potential factors influencing PS locus promoter orientation include environmental cues or selection against bacteria synthesizing a particular PS. PS synthesis is controlled by multiple levels of regulation, with on orientation of a PS locus promoter being a necessary, yet incomplete requirement for expression of the respective polysaccharide (1). Future investigations are necessary to reveal factors that influence PS locus promoter orientations in different in vivo environments.
Supplementary Material
Acknowledgments
We thank M. J. Coyne for PCR digestion assay design and R. Cohen for work with the abscess model.
This work was supported by National Institutes of Health/NIAID grant AI081843 and training grant T32AI007061.
Footnotes
Published ahead of print on 20 August 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Chatzidaki-Livanis, M., K. G. Weinacht, and L. E. Comstock. 2010. Trans locus inhibitors limit concomitant polysaccharide synthesis in the human gut symbiont Bacteroides fragilis. Proc. Natl. Acad. Sci. U. S. A. 107:11976-11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyne, M. J., M. Chatzidaki-Livanis, L. C. Paoletti, and L. E. Comstock. 2008. Role of glycan synthesis in colonization of the mammalian gut by the bacterial symbiont Bacteroides fragilis. Proc. Natl. Acad. Sci. U. S. A. 105:13099-13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyne, M. J., and L. E. Comstock. 2008. Niche-specific features of the intestinal Bacteroidales. J. Bacteriol. 190:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyne, M. J., B. Reinap, M. M. Lee, and L. E. Comstock. 2005. Human symbionts use a host-like pathway for surface fucosylation. Science 307:1778-1781. [DOI] [PubMed] [Google Scholar]
- 5.Coyne, M. J., A. O. Tzianabos, B. C. Mallory, V. J. Carey, D. L. Kasper, and L. E. Comstock. 2001. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect. Immun. 69:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyne, M. J., K. G. Weinacht, C. M. Krinos, and L. E. Comstock. 2003. Mpi recombinase globally modulates the surface architecture of a human commensal bacterium. Proc. Natl. Acad. Sci. U. S. A. 100:10446-10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krinos, C. M., M. J. Coyne, K. G. Weinacht, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414:555-558. [DOI] [PubMed] [Google Scholar]
- 8.Lassmann, B., D. R. Gustafson, C. M. Wood, and J. E. Rosenblatt. 2007. Reemergence of anaerobic bacteremia. Clin. Infect. Dis. 44:895-900. [DOI] [PubMed] [Google Scholar]
- 9.Levene, H. 1960. Robust tests for equality of variances, p. 278-292. In I. Olkin (ed.), Contributions to probability and statistics: essays in honor of Harold Hotelling. Stanford University Press, Palo Alto, CA.
- 10.Ley, R. E., F. Backhed, P. Turnbaugh, C. A. Lozupone, R. D. Knight, and J. I. Gordon. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 102:11070-11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, C. H., S. M. Lee, J. M. Vanlare, D. L. Kasper, and S. K. Mazmanian. 2008. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc. Natl. Acad. Sci. U. S. A. 105:3951-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazmanian, S. K., C. H. Liu, A. O. Tzianabos, and D. L. Kasper. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107-118. [DOI] [PubMed] [Google Scholar]
- 13.Mazmanian, S. K., J. L. Round, and D. L. Kasper. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453:620-625. [DOI] [PubMed] [Google Scholar]
- 14.Nulsen, M. F., J. J. Finlay-Jones, J. M. Skinner, and P. J. McDonald. 1983. Intra-abdominal abscess formation in mice: quantitative studies on bacteria and abscess-potentiating agents. Br. J. Exp. Pathol. 64:345-353. [PMC free article] [PubMed] [Google Scholar]
- 15.Patrick, S., J. Parkhill, L. J. McCoy, N. Lennard, M. J. Larkin, M. Collins, M. Sczaniecka, and G. Blakely. 2003. Multiple inverted DNA repeats of Bacteroides fragilis that control polysaccharide antigenic variation are similar to the hin region inverted repeats of Salmonella typhimurium. Microbiology 149:915-924. [DOI] [PubMed] [Google Scholar]
- 16.Polk, B. F., and D. L. Kasper. 1977. Bacteroides fragilis subspecies in clinical isolates. Ann. Intern. Med. 86:569-571. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro, M. E., A. B. Onderdonk, D. L. Kasper, and R. W. Finberg. 1982. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J. Exp. Med. 155:1188-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzianabos, A. O., A. B. Onderdonk, R. S. Smith, and D. L. Kasper. 1994. Structure-function relationships for polysaccharide-induced intra-abdominal abscesses. Infect. Immun. 62:3590-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzianabos, A. O., P. R. Russell, A. B. Onderdonk, F. C. Gibson III, C. Cywes, M. Chan, R. W. Finberg, and D. L. Kasper. 1999. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J. Immunol. 163:893-897. [PubMed] [Google Scholar]
- 20.Yan, J., and J. Fine. 2004. Estimating equations for association structures. Stat. Med. 23:859-880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.