Abstract
To obtain insight into the in vivo dynamics of RNA polymerase (RNAP) on the Bacillus subtilis genome, we analyzed the distribution of the σA and β′ subunits of RNAP and the NusA elongation factor on the genome in exponentially growing cells using chromatin affinity precipitation coupled with gene chip mapping (ChAP-chip). In contrast to Escherichia coli RNAP, which often accumulates at the promoter-proximal region, B. subtilis RΝΑP is evenly distributed from the promoter to the coding sequences. This finding suggests that, in general, B. subtilis RNAP recruited to the promoter promptly translocates away from the promoter to form the elongation complex and proceeds without intragenic transcription attenuation. We detected RNAP accumulation in the promoter-proximal regions of some genes, most of which can be identified as transcription attenuation systems in the leader region. Our findings suggest that the differences in RNAP behavior between E. coli and B. subtilis during initiation and elongation steps might result in distinct strategies for postinitiation control of transcription. The E. coli mechanism involves trapping at the promoter and promoter-proximal pausing of RNAP in addition to transcription attenuation, whereas transcription attenuation in leader sequences is mainly employed in B. subtilis.
Biochemical studies on RNA polymerase (RNAP) show that transcriptional initiation involves three steps (20): (i) recruitment of RNAP to the promoter via recognition of consensus elements within the promoter sequence to form a closed complex; (ii) isomerization of the closed complex to an open complex, with opening of double-stranded DNA; and (iii) promoter clearance, coupled with formation of the elongation complex (EC) and initiation of transcriptional elongation. The efficiency of transcriptional initiation depends on the rate of each step. While recruitment of RNAP to promoters is the main target for control of gene expression, transitions to the open complex and the subsequent elongation complex are also proposed regulatory targets (29).
Recently, in vivo trafficking of RNAP during transcriptional initiation has been explored for several organisms using chromatin immunoprecipitation coupled with the gene chip mapping method (ChIP-chip). ChIP-chip analyses of RNAP distribution on the Escherichia coli genome reveal typically higher RNAP association at promoter regions than within coding sequences (21, 26). Initially, it was proposed that promoter-proximal peaks reflected RNAPs trapped at promoters, designated “poised RNAP,” since about 23% of RNAP peaks at promoters are not associated with transcripts (26). Consistent with this hypothesis, RNAP trapping at the promoter has been reported for a number of E. coli genes. The GalR repressor suppresses the galP1 promoter by trapping RNAP in an intermediate state between the closed and open complexes (3). Moreover, the ArgP activator bound to lysine restrains RNAP at the argO promoter in a step following open complex formation that precedes productive transcription, and the complex is altered to a transcriptionally active state upon arginine addition (18). In vivo and in vitro footprinting analyses additionally suggest that 10 to 20% of transcriptional complexes in E. coli stall at the promoter or promoter-proximal regions, depending on the −10 and/or −10-like sequences (11). However, Mooney et al. (21) proposed that promoter-proximal RNAP peaks result from transcriptional attenuation rather than the presence of poised RNAP at the promoter regions, since the average RNAP peak (β′ subunit peak) is offset from the average σ70 peak in the direction of transcription and coincides with that of NusA, which associates with the elongating complex (21).
Promoter-proximal RNAP peaks are common for human and Drosophila genes, where they appear to be correlated with developmentally regulated rather than housekeeping genes (10, 22, 34). In Saccharomyces cerevisiae, RNAPs are located upstream of several hundred genes prior to their transcriptional activation during the stationary phase, possibly allowing immediate adaptation to changes in environmental conditions (25).
Here, we report in vivo RNAP trafficking in the Gram-positive bacterium Bacillus subtilis. The major vegetative RNAP holoenzyme of B. subtilis is EσA, the counterpart of Eσ70 in E. coli. Both RNAPs have the same core subunit composition, comprising ββ′α2ω, and holoenzymes recognize the same consensus sequences at the −35 (TTGACA) and −10 (TATAAT) regions of the promoter. However, biochemical analyses show characteristic differences in their activities. E. coli RNAP forms a stable open complex, evident from the protection of DNA downstream of the promoter region from DNase I digestion upon binding of RNAP and formation of a heparin-resistant RNAP-promoter complex (23, 30). In contrast, B. subtilis RNAP is unable to protect DNA downstream of the promoter, indicating lower activity of this polymerase in forming a stable open complex than E. coli RNAP (1, 6, 23, 27, 30). Interestingly, chromatin affinity precipitation (ChAP)-chip analysis, a modified ChIP-chip method developed in our laboratory (16), showed that B. subtilis RNAP is distributed evenly from the promoter to the coding sequences in the majority of transcriptional units (TUs). We detected some promoter-proximal peaks of RNAP, which were mostly attributed to transcription attenuation mechanisms. Accordingly, we conclude that RNAP mainly initiates transcription without trapping in a poised or paused state at the promoter or promoter-proximal sites and proceeds without intragenic transcription attenuation in B. subtilis cells.
These findings suggest that the differences in RNAP behavior during initiation and elongation steps in E. coli and B. subtilis might result in different strategies for the postinitiation control of transcription. The E. coli mechanism involves trapping at the promoter region and promoter-proximal pausing of RNAP in addition to transcription attenuation, whereas transcription attenuation in leader sequences is mainly observed in B. subtilis.
MATERIALS AND METHODS
Construction of B. subtilis strains.
To construct B. subtilis strains expressing σA, β′, and NusA fused to histidine tags, 500-bp fragments encompassing the 3′ portions of the corresponding genes, except the stop codons, were amplified from B. subtilis 168 genomic DNA by PCR using the primer sets rpoC.f-rpoC.r, sigA.f-sigA.r, and nusA.f-nusA.r, respectively (see Table S3 in the supplemental material), and cloned between the HindIII or EcoRI and XhoI sites of pMUTinHis (15). The resultant plasmids were integrated into the B. subtilis genome via single crossing-over to obtain strains designated 168rpoCHis, 168sigAHis, and 168nusAHis, respectively.
ChAP-chip analysis.
The 168rpoCHis, 168sigAHis, and 168nusAHis stains were cultivated in LB at 37°C under aerobic conditions and harvested at log phase (optical density at 600 nm [OD600] = ∼0.4). Protein-DNA cross-linking with formaldehyde, purification of protein-DNA complexes with the Ni column, and mapping of copurified DNA fragments using a custom Affymetrix tiling chip were performed as described previously (15). Protein binding signals were analyzed and visualized using the software package In Silico Molecular Cloning, array edition (In Silico Biology). Protein binding signal intensities were estimated by dividing the signal intensities of DNA in the affinity-purified fraction (ChAP DNA) by control DNA intensity (enrichment factor) as previously described (32). The whole-cell extract fraction before purification of σA (for σA and β′ ChAP-chip) or NusA (for NusA ChAP-chip) was used as control DNA for ChAP-chip analyses. Distribution of protein binding signals along the genome coordinate in 2 independent ChAP-chip analyses for each protein is shown in Fig. S1 in the supplemental material.
Transcriptome analysis.
Total RNA was purified from wild-type cells cultured under conditions similar to those employed for ChAP-chip experiments. Synthesis and terminal labeling of cDNA, hybridization to the tiling chip, and data processing, including normalization of hybridization intensities of cDNA by those of genomic DNA, were performed according to previous protocols (16). The distribution of transcription signals along the genome coordinate was visualized with the In Silico Molecular Cloning program, array edition (In Silico Biology).
Extraction of TUs.
We extracted transcriptional units (TUs) using the average transcription signal intensities of each probe from three independent experiments. To eliminate the effects of artificial spikes and inefficient hybridization signals, sliding-window methods were usually applied for transcriptome analysis using a tiling chip, whereby the average values of contiguous probes in a window are determined to extract transcriptionally active regions with signals above a threshold value. However, symmetrical sliding-window methods tend to produce biased estimates of the starting points and endpoints of transcribed regions depending on the signal levels compared to background signals (13). To precisely determine the initiation and termination sites of transcripts, we designed a novel procedure applying asymmetrical sliding windows. In addition to the extraction of transcriptionally active regions using a 550-bp symmetrical sliding window, we applied two asymmetrical sliding windows, one comprising the regions 150 bp upstream and 400 bp downstream from each probe and the other comprising the regions 400 bp upstream and 150 bp downstream. In cases where the signal intensities of more than half of the probes in the upstream and downstream windows were higher than the threshold value, the probe was judged “transcription positive.” We set the threshold value as 0.5 in these analyses (see Fig. S5A in the supplemental material). In addition, if the distance between neighboring TUs was determined to be less than 150 bp (corresponding to a maximum of two probes in the coding regions and 6 probes in the intergenic regions), the TUs were concatenated. Finally, we combined the positive probes determined from the 3 windows, and 2,073 TUs (defined as a region containing positive probes determined by at least one window) were obtained.
To evaluate the general behavior of the B. subtilis RNAP, we extracted TUs suitable for the analysis using criteria similar to those adopted for E. coli studies (21, 26). If TUs have multiple promoters, transcriptional signal intensities tend to increase stepwise at initiation sites. These TUs were removed by extraction using a higher threshold value of 0.75. In total, 1,258 TUs displaying coincident transcription start sites (TSSs) determined using both thresholds were selected (see Fig. S5B in the supplemental material). Next, we extracted 741 TUs of more than 200 bp. Among these, we extracted 344 TUs located more than 500 bp from neighboring TUs to avoid overlapping protein binding and transcription signals. Finally, we visually selected 180 TUs with clear single TSSs, designated “high-quality TUs” in this study (see Table S1 in the supplemental material).
Detection of σA binding sites.
The σA binding peaks were computationally detected by searching more than 2 consecutive probes in noncoding regions with σA binding signals higher than the threshold value (determined as 4.0 for experiment 1 and 2.0 for experiment 2), depending on their background levels (see Fig. S4 in the supplemental material). Rough spacing of probes in the coding regions on our tiling chip did not allow precise detection of the σA binding sites in the coding region. Next, we selected σA binding sites whose center points of peaks were located within 100 bp (covered by maximally 8 probes) in two experiments, excluding promoters of rRNA and tRNA operons. The average median position was assigned as the binding position of σA, yielding 571 sites.
Estimation of the maximum enrichment position of the protein binding profile.
We estimated positions where σA, β′, and NusA binding signals reach the maximum level (peak) in each of the 180 high-quality TUs based on difference of average values between upstream and downstream windows. We set a pair of 200-bp sliding windows and calculated differences in average probe intensities between the upstream window and the downstream window. The window was moved at 50-bp intervals from bp −200 to bp +600 of the TSS. The position where the difference between the values for the up- and downstream windows changes from positive to negative was computationally detected as a peak. When multiple peaks were detected, the most upstream position was defined as the peak position.
Extraction of genes for calculation of the traveling ratio (TR).
Genes immediately downstream of the 571 σA binding sites were initially extracted. However, genes located divergently from the single σA binding sites were excluded from further analysis, since it was difficult to establish the precise relationship between σA binding and regulated genes. Promoters of rRNA and tRNA operons were additionally excluded. Genes for which β′ binding signals from upstream TUs overlapped in the promoter regions were removed via visual inspection, leading to a final selection of 416 genes (see Table S2 in the supplemental material).
Microarray data accession number.
Raw data (CEL format) from the ChAP-chip experiments described here have been deposited in the ArrayExpress database under accession number E-MEXP-2649.
RESULTS
ChAP-chip analysis of RNAP distribution on the B. subtilis genome.
σA, β′, and NusA fused with histidine tags were employed for ChAP-chip analysis. For this purpose, the coding sequence of the His tag was fused to the 3′ end of each gene at the authentic locus on the B. subtilis genome. Under the growth conditions used (LB at 37°C under aerobic conditions), the resultant strains apparently grow normally and the expression levels of His-tagged σA, β′, and NusA were similar to those of untagged proteins in wild-type cells (data not shown). Purification of protein-DNA complexes with a Ni column, mapping of copurified DNA fragments using a custom Affymetrix tiling chip, and quantitative analysis and visualization of protein-binding signals were performed as described previously (16). In parallel, transcripts were mapped in wild-type cells grown under similar conditions using the tiling chip. Two biologically independent analyses were performed for each ChAP-chip experiment, and typical examples of the distribution of protein binding and transcription signals are shown in Fig. 1. The complete data set is presented in Fig. S1 in the supplemental material.
FIG. 1.
Typical distribution of σA and β′ subunits of RNAP and NusA on the B. subtilis genome, compared with the transcription profile. Transcriptional signals (A) and binding signals of the σA (B) and β′ (C) subunits of RNAP and NusA (D) at the odhAB region in two independent experiments are presented. The arrangement of genes (thick arrows) and terminators (arrows with open circles) is shown schematically at the bottom. Transcriptional signals (A) and σA (B), β′ (C), and NusA (D) binding signals for each probe are indicated by vertical bars at appropriate genomic coordinates. The signal intensity for the initiation complex (IC) of RNAP is represented by the mean of signals within the 100-bp region centered on the respective σA-binding site (gray thick line), and that for the elongation complex (EC) of RNAP is represented by the mean of signals within the first gene following the σA-binding site. EC and IC signal intensities of RNAP of the odhAB TU in two experiments are indicated (C).
In B. subtilis, 75% of the predicted genes are densely arranged in the same direction (17) and many genes are transcribed from multiple promoters, as judged by Northern analysis (BSORF; http://bacillus.genome.ad.jp/), resulting in frequent overlapping of TUs. Thus, σA binding peaks were often detected inside computationally detected TUs, and signals from neighboring TUs often overlapped. However, many of TSSs clearly distinguishable from the neighboring TU are associated with σA binding signals (Fig. 1; see Fig. S1 in the supplemental material). TSSs without a σA binding signal might correspond to promoters transcribed by alternative σ factors. Detailed analysis of the correlation of σA binding peaks and promoter sequences is under way.
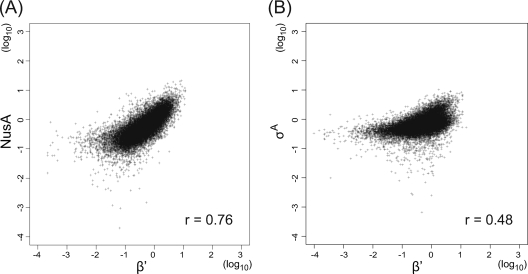
As expected, RNAP (represented by β′) binding signals are observed along the transcribed regions (Fig. 1; see Fig. S1 in the supplemental material). NusA binding signals are also additionally detected in most of the transcribed regions. Indeed, a scatter plot of the RNAP and NusA signals of each probe in the coding regions demonstrated their high genome-wide correlation (r = 0.76) (Fig. 2 A), strongly suggesting that NusA is a general transcription factor included in elongation complexes of RNAP, as reported for E. coli NusA (7, 8, 21). In E. coli, although signal intensity is low, a positive correlation of σ70 binding signals with those of RNAP in coding regions has been reported (21). We also observed a similar statistically significant correlation (r = 0.48) (Fig. 2B), suggesting interaction between σA and the elongation complex of RNAP and/or increased nonspecific binding of σA-containing RNAP in transcribed regions, as previously suggested (21).
FIG. 2.
Genome-wide correlation between RNAP and NusA or σA binding signals. Log scale scatter plots of signal intensities of RNAP (represented by β′) and NusA (A) and σA (B) binding signals for each 25-mer probe in coding regions are shown. Correlation coefficients (r) of signals of two proteins are also indicated.
Visualization of general RNAP trafficking profile in B. subtilis.
Interestingly and noticeably, in contrast to E. coli RNAP, which often accumulates at promoter-proximal regions in vivo (21, 26), B. subtilis RNAP appeared evenly distributed from the promoter to the coding regions (Fig. 1; see Fig. S1 in the supplemental material). To evaluate the general behavior of RNAP from initiation to elongation steps on the B. subtilis genome, we searched TUs suitable for the analysis of the distribution of σA, β′, and NusA on individual TUs, as described in Materials and Methods. As a result, we selected 180 TUs (high-quality TUs) (see Table S1 in the supplemental material) that are clearly separated from neighboring TUs, are associated with a single TSS, and have enough length (>200 bp) for the analysis of elongating RNAP.
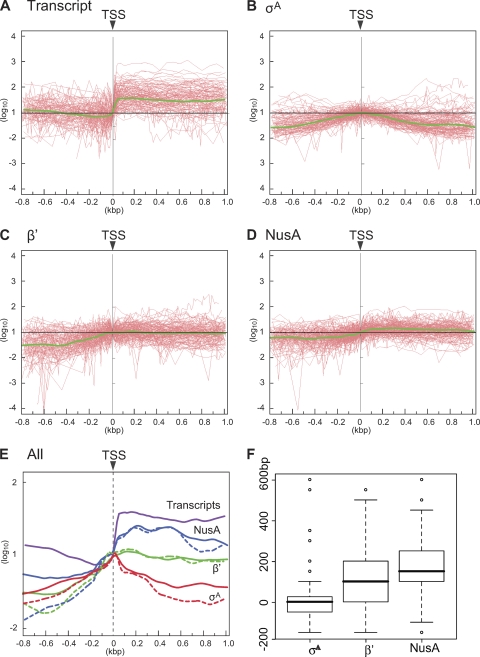
Then, the relative ratios of protein binding and transcription signal intensities to those at TSS were calculated for probes located from bp −800 to bp +1000 relative to TSS in each of 180 TUs and plotted as a function of distance (bp) from TSS (Fig. 3 A to D, red lines). Finally, as the relative positions of the probes in each TU were different, we calculated the average profiles of transcriptional and σA, β′, and NusA binding signal intensities from promoters to coding regions using the locally weighted scatter plot smoothing (LOWESS) method (5); these profiles were plotted (green lines in Fig. 3A to D) and overlaid (Fig. 3E). Average profiles of protein binding signals were evaluated using data from two independent experiments, and the results from one experiment are presented in Fig. 3B, C, and D. In Fig. 3E, we also included average profiles obtained from other data sets (see Fig. S2 in the supplemental material) to demonstrate the reproducibility of the results.
FIG. 3.
Average profiles of transcriptional and σA, β′, and NusA binding signals on 180 high-quality TUs. Relative signal intensities of the transcripts (A) and σA (B), β′ (C), and NusA (D) binding to those at TSSs (y axis) are plotted as a function of distance from TSSs (x = 0) and connected by red lines for selected 180 TUs. The average profiles are calculated using the LOWESS smoothing method and are presented as green lines (A to D). (E) Average profiles are merged after adjusting values at the TSSs to a log10 of 1. Solid and dashed lines indicate average profiles of data sets presented in this figure and in Fig. S2 in the supplemental material, respectively. (F) Box-and-whisker plots showing the distributions of peak positions of σA, β′, and NusA binding, determined by using duplicated data. The upper and lower limits of the boxes indicate the 75th and 25th percentiles, respectively, and the boldface lines across the boxes indicate median values. Outliers with values more than 1.5 times the interquartile range of the 75th percentile or less than 1.5 times the interquartile range of the 25th percentile are shown as open circles.
The average σA profile reveals a symmetric distribution centered at TSS, indicating that σA is rapidly released from the RNAP core after promoter clearance (Fig. 3A), similar to what is found for E. coli (21, 26). A significant level of the average signal of the β′ subunit is observed at the TSS, and the β′ signal reaches its maximum level immediately downstream of TSS, with relatively constant binding signals in the downstream transcribed regions (Fig. 3C). These results confirmed an even distribution of RNAP from the promoter to the coding regions, at least in the high-quality TUs, in B. subtilis. In contrast, Reppas et al. (26) reported that, in E. coli, the median ratio of the β′ signal 800 bp downstream of the promoter relative to the peak value at the promoter was 0.43 for 59 high-quality TUs similar to ours, and Mooney and coworkers (21) classified 29 TUs as having promoter-proximal peaks among 42 high-quality and highly transcribed TUs. Constant distribution was also observed for NusA (Fig. 3D). However, the average NusA binding signal appeared to reach a plateau downstream of the position where β′ binding reaches the maximum level, consistent with the exchange of σA with NusA on the RNAP core after promoter clearance. Resolution of our ChAP-chip data does not exclude the possibility of NusA binding to RNAP before σA release. However, an in vivo pulldown assay of σA and NusA in B. subtilis cells showed that σA was not copurified with NusA and vice versa, strongly suggesting that NusA is an exclusive component of the elongation complex of RNAP through exchange with σA bound to the initiation complex (IC) (our unpublished data).
Additionally, we verified that average profiles obtained by the LOWESS smoothing method indeed reflect characteristics of selected TUs by analyzing data sets with another method. We estimated positions where σA, β′, and NusA binding signals reach the maximum level (peak) in each of the 180 high-quality TUs as described in Materials and Methods. The distribution of peak positions of σA, β′, and NusA in 180 TUs is presented in Fig. 3F. The median values of the peak position of σA, β′, and NusA are 0, +100, and +150 bp from TSS, respectively, and statistical evaluation of these results revealed that there are significant differences between estimated maximal enrichment points of σA and NusA (P = 2.186e−14), σA and β′ (P = 1.423e−08), and β′ and NusA (P = 0.026), supporting statistically significant differences of average binding profiles of σA, β′, and NusA.
Characterization of RNAP distribution based on the TR.
For selection of high-quality TUs analyzed in the previous section, we adopted the criterion that contiguous signals of transcription and β′ binding are distributed in sequences of greater than 200 bp. This specification would eliminate short transcripts associated with RNAP paused at the promoter or promoter-proximal sites or transcriptional termination by attenuation signals. Next, we attempted to characterize the transition of RNAP occupancy from the promoter to the coding region by calculating the relative ratio of RNAP occupancy (traveling ratio [TR]) (26) at 571 automatically detected σA binding sites as described in Materials and Methods. We then selected 416 genes (see Table S2 in the supplemental material) located immediately downstream of the σA binding site with a β′ binding signal in the promoter region that did not overlap with those of neighboring TUs. Genes located divergently from the single σA binding site were excluded, since the precise relationships between σA binding and genes regulated were difficult to establish.
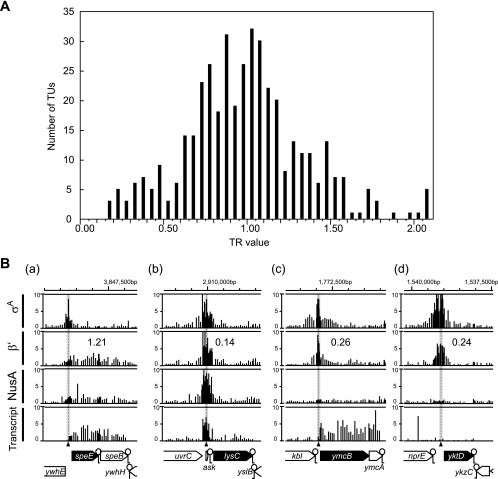
We calculated the average β′ binding signal intensities of probes in the 100-bp region centered at the σA binding site (IC signal intensity) and the coding region (EC signal intensity) (Fig. 1). The TR value for each gene, obtained by dividing EC signal intensity by IC signal intensity (see Table S2 in the supplemental material) is presented as a histogram in Fig. 4 A. If RNAP is distributed evenly from a promoter to a coding region, TR values are expected to give a normal distribution, with the average value near 1.0. In fact, the distribution of TR values is apparently similar to the normal distribution, with an average of 0.85 (Fig. 4A). We estimated the false discovery rate (FDR; possibility of existence of genes that do not actually belong to the normal distribution centered at a TR of 1.0). The q value of the FDR was determined for each gene by a one sided t test (α = 0.05) with the null hypothesis of a log2 TR of ≤0, followed by the Benjamini-Hochberg (BH) method (2) (see Table S2 in the supplemental material). The results did not suggest the existence of a significant number of genes with significantly low TR values. These results again demonstrate that the majority of RNAPs are relatively evenly distributed from the promoter to coding regions in B. subtilis.
FIG. 4.
Distribution of the TR values of RNAP. (A) TR values of RNAP on 416 selected genes (averages of two independent experiments) are presented as a histogram at 0.05 intervals of TR values (x axis). (B) Typical distribution patterns of transcript, σA, β′, and NusA are shown as in Fig. 1. (a) Even distribution of RNAP; (b) promoter-proximal peak of RNAP at the riboswitch sequence; (c) promoter-proximal peak of RNAP with weak and constant NusA distribution but high transcription; (d) promoter-proximal peak of RNAP without NusA and transcript association. The genome coordinates (bases) and gene arrangements of each region are indicated at the top and bottom of each panel, respectively.
However, we visually found a limited number of low-TR genes in which RNAP accumulates at the promoter or promoter-proximal regions, as discussed below.
Characterization of the promoter-proximal peaks of RNAP.
Next, we focused on 40 genes with TR values of ≤0.5 in two independent experiments (Table 1; see Fig. S3 in the supplemental material). Typical examples of β′ peaks at the promoter or promoter-proximal regions, together with distribution of σA, NusA, and transcripts, are shown in Fig. 4B, b to d. The β′ peaks overlapped with the NusA signals, except in the cases of 8 genes (thdF, yerA, yusL, yktD, ylxS, ymcB, yfjO, and mutS), indicating that the majority of promoter or promoter-proximal RNAP peaks contain elongating RNAPs.
TABLE 1.
Transcriptional profile of genes whose TR values are ≤0.5a
| Gene (n = 40) | TR of β′ in expt: |
NusA accumulationb in expt: |
Transcriptc | Leader transcriptd | Binding factor or existence of terminatore | Figure no.f | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |||||
| Known attenuators (n = 25) | ||||||||
| ptsG | 0.48 | 0.38 | + | + | + | + | GlcT | 36 |
| glpD | 0.15 | 0.17 | + | + | + | + | GlpP | 6 |
| expZ (vmlR) | 0.24 | 0.21 | + | + | + | + | Unknown | 12 |
| proB | 0.28 | 0.28 | + | + | + | + | Unknown | 15 |
| valS | 0.23 | 0.25 | + | + | + | + | Unknown | 10 |
| ydaO | 0.35 | 0.42 | + | + | + | + | Unknown | 25 |
| yvbW | 0.31 | 0.22 | + | + | + | + | Unknown | 22 |
| mgtE (ykoK) | 0.50 | 0.47 | + | + | + | + | Mg2+ | 37 |
| yusC | 0.32 | 0.34 | + | + | + | + | SAM | 24 |
| lysC | 0.14 | 0.18 | + | + | − | + | Lysine | 3 |
| mtnW (ykrW) | 0.32 | 0.37 | + | + | − | + | SAM | 23 |
| tenA | 0.38 | 0.27 | + | + | − | + | TPP | 28 |
| thiC | 0.16 | 0.21 | + | + | − | + | TPP | 7 |
| ykkC | 0.26 | 0.45 | + | + | − | + | Unknown | 15 |
| ykoY | 0.45 | 0.37 | + | + | − | + | Unknown | 35 |
| trpE | 0.38 | 0.44 | + | + | − | NC | TRAP | 27 |
| glpF | 0.41 | 0.24 | + | + | + | NC | GlpP | 6 |
| bglP | 0.27 | 0.27 | + | + | + | NC | LicT | 18 |
| queC (ykvJ) | 0.24 | 0.40 | + | + | + | NC | preQ1 | 12 |
| thrS | 0.36 | 0.42 | + | + | + | NC | tRNA (Thr) | 26 |
| tyrS | 0.42 | 0.44 | + | + | + | NC | tRNA (Tyr) | 32 |
| alaS | 0.29 | 0.33 | + | + | + | NC | Unknown | 19 |
| glyQ | 0.26 | 0.37 | + | + | + | NC | Unknown | 14 |
| hisS | 0.45 | 0.49 | + | + | + | NC | Unknown | 17 |
| ileS | 0.30 | 0.47 | + | + | + | NC | Unknown | 21 |
| Candidates in this study (n = 15) | ||||||||
| pheS | 0.40 | 0.37 | + | + | + | + | + | 30 |
| yyaE | 0.44 | 0.41 | + | + | + | + | + | 33 |
| ndhF | 0.11 | 0.10 | + | + | − | NC | + | 2 |
| ybgE | 0.19 | 0.19 | + | + | − | NC | + | 8 |
| prkA | 0.14 | 0.17 | + | + | − | NC | ND | 4 |
| ypbR | 0.10 | 0.12 | + | + | + | NC | + | 1 |
| yrhG | 0.15 | 0.14 | + | + | + | NC | + | 5 |
| thdF | 0.45 | 0.39 | − | − | + | NC | ND | 34 |
| yerA | 0.30 | 0.30 | − | − | + | NC | ND | 20 |
| yusL | 0.22 | 0.25 | Low | Low | − | NC | + | 9 |
| yktD | 0.24 | 0.35 | Low | Low | − | NC | ND | 13 |
| ylxS | 0.40 | 0.41 | Low | − | + | NC | + | 31 |
| ymcB | 0.26 | 0.25 | Low | Low | + | NC | ND | 16 |
| yfjO | 0.39 | 0.48 | Low | Low | + | NC | ND | 29 |
| mutS | 0.42 | 0.46 | Low | Low | + | NC | ND | 16 |
Only genes for which NusA binding was detected by our algorithm are shown. The full list of attenuators is shown in Table S3 in the supplemental data.
The NusA peak was judged by visual inspection. +, accumulation at promoter-proximal site is detected (n = 32 for experiments 1 and 2); low, NusA signal is weak (n = 6 for experiment 1 and 5 for experiment 2); −, NusA is distributed constantly from promoter to coding region (n = 2 for experiment 1 and 3 for experiment 2).
+, transcript was detected at the coding region (n = 28); −, transcript not detected (n = 12).
+, transcript detected (n = 17); NC, not clear (n = 23).
For known attenuators, the binding factor is indicated. TPP, thiamine pyrophosphate; SAM, S-adenosylmethionine; TRAP, trp RNA-binding attenuation protein; preQ1, 7-aminomethyl-7-deazaguanine; unknown, not reported. For new candidate attenuators. the existence of a terminator (+) was predicted by GENETYX-MAC (GENETYX Corporation). ND, not detected.
Figure number in Fig. S3 in the supplemental data.
B. subtilis often uses transcriptional attenuation for regulation of gene expression at the postinitiation step (19). These transcription attenuation sequences are located between the promoters and coding sequences, and transcribed RNA molecules form two distinct structures for either termination before the coding region or read-through toward the coding region, depending on association of protein, tRNA, and metabolites (riboswitch). In B. subtilis, 59 attenuation sequences have been reported, and the behavior of RNAP at these regions is summarized in Table S3 in the supplemental material. Twenty-five genes presented in Table S3 in the supplemental material were excluded from this study, since σA binding at the promoter regions was undetected (18 genes; yvrC, ribD, ypaA, cysH, metE, metI, yitJ, yxjG, yxjH, ykoF, ylmB, yueJ, ktrA, hutH, bglS, pyrP, sacB, and sacX) or the σA peak was shared by divergent genes (7 genes; gcvT, metK, yoaD, yxkD, yybP, sacP, and yqjO). In the remaining 34 genes, 25 genes showed TR values of ≤0.5 in two independent experiments (Table 1, known attenuators). In addition, visual inspection of 6 genes (pbuE, xpt, purE, mtnK, yczA, and serS) showed clear accumulation of RNAP at the promoter-proximal regions (average TR values of duplicate experiments are 0.53 to 0.82) (see Table S3 in the supplemental material). Thus, most genes regulated by attenuation sequences (31 of 34 genes, 91%) displayed a promoter-proximal peak of RNAP. The remaining 3 genes (glmS, pbuG, and yxjA) displayed a constant distribution of β′ binding and transcription signals, implying that transcriptional attenuation in these cases is not significant under our experimental conditions. It should be also noted that, although σA binding was not assigned to ribD in our algorithm because of the existence of a small open reading frame (ORF), ypuE, upstream of it (see Table S3 in the supplemental material), the TU also showed significant accumulation of RNAP at the promoter-proximal region (see Fig. S1 in the supplemental material).
In vitro experiments indicate that RNAP pauses at the leader regions of trpE (protein-mediated attenuation), glyQS (tRNA-mediated attenuation), and ribD (riboswitch), probably to provide sufficient time for proper folding of the RNA molecule and effective ligand binding (9, 31, 33). Since this pause occurs regardless of the binding of ligands to attenuation structures, RNAP accumulation at the leader region should be observed, even without transcription termination. We detected short leader transcripts, possibly resulting from pausing and/or premature termination of transcription, for 15 genes. The failure to detect leader transcripts may be attributed to several reasons. The transcripts are very unstable, as reported for S-adenosylmethionine (SAM)-dependent riboswitches (28) and/or too short to act as templates for cDNA synthesis during the preparation of hybridization probes for transcript mapping. It is additionally possible that completed transcripts accumulating in the cytoplasm mask the signals of ongoing transcription.
While the remaining 15 genes listed as “candidates” in Table 1 are not known to be regulated by attenuators, we identified possible ρ-independent terminator sequences upstream of the coding regions for 8 genes (pheS, yyaE, ndhF, ybgE, ypbR, yrhG, yusL, and ylxS), which may act as part of an unknown attenuation system. In fact, short leader transcripts were detected for two of the sequences. The molecular mechanisms underlying RNAP accumulation at the other 7 promoters are unclear at present.
Notably, 8 genes (thdF, yerA, yusL, yktD, ylxS, ymcB, yfjO, and mutS) in Table 1 showed no or weak distribution of NusA binding signals, while significant transcription signals were observed for 6 genes but not for yktD and yusL (Fig. 4B, c). These profiles might indicate nonuniform population of RNAP trafficking. In the majority fraction, RNAPs might be trapped at the promoter or promoter-proximal region without forming elongation complexes with factors, including NusA, but might initiate elongation with RNA synthesis in a minor fraction. Two genes, yktD and yusL, were exceptions, since the promoter-proximal peaks of σA and β′ were not associated with significant NusA binding and transcription signals (Fig. 4B, d). These genes are candidates for regulation by poised RNAP in B. subtilis.
DISCUSSION
We report here that the B. subtilis RNAP is evenly distributed from the promoter region to the coding sequences in the majority of TUs and that the promoter-proximal peaks mainly result from attenuation mechanisms. Most of the known attenuator sequences analyzed disclose accumulation of RNAP with NusA at the promoter-proximal regions, regardless of leader transcript detection. Interestingly, the RNAP signals distributed from promoter to attenuation sequences are significant and constant. This profile is indicative of the high density of the elongation complexes in the leader region.
It was observed that, in contrast to the distribution of the B. subtilis RNAP, that of the E. coli RNAP is generally higher at the promoter region than within the coding sequence (21, 26). Three mechanisms of RNAP accumulation at the promoter or promoter-proximal regions occur in E. coli, specifically, trapping at the promoter, promoter-proximal pausing, and attenuation of transcription (21). Reppas et al. (26) suggest that E. coli RNAP is often trapped or “poised” at the promoter region, based on the observation that 23% of Eσ70s at promoters are not associated with transcripts. In addition, RNAP trapping at the promoter and pausing at promoter-proximal regions are reported for a number of E. coli genes (3, 11, 18). However, Mooney and colleagues (21) propose that the majority of the promoter-proximal RNAP peaks reflect an elongating complex (transcription attenuation) rather than an initiating complex (RNAP poised at the promoter), based on the observation that the RNAP peaks overlap with those of NusA. While the average RNAP peak is located 130 bp downstream of σ70, the NusA peak is further downstream (190 bp from the σ70 peak) in 29 high-quality TUs analyzed by Mooney et al. (21). These differences may indicate that promoter-proximal peaks of E. coli RNAP contain multiple types of RNAP, including some fraction of RNAP-σ70 trapped at promoters or promoter-proximal sites and RNAP-NusA paused and terminated at further downstream promoter-proximal or attenuated sites.
In contrast, significant accumulation of RNAP was not observed for the majority of genes in B. subtilis, indicating that RNAP recruited to the promoter promptly leaves to form an elongation complex without trapping or pausing at the promoter-proximal site. This observation is consistent with the different in vitro properties of RNAPs of the two bacteria. E. coli RNAP forms a stable open complex, which is not observed for B. subtilis RNAP (1, 6, 23, 27, 30). Additionally, constant distribution of RNAP on TUs might be related to low activity of Rho-dependent termination in B. subtilis. In E. coli, the Rho factor is an essential abundant protein and responsible for 20 to 50% of termination of mRNA synthesis. Additionally, it has been proposed that Rho factor is involved in intragenic transcriptional termination, which occurs when translation efficiency of the mRNA is reduced (12, 24). In contrast, Rho is a weakly expressed dispensable protein in B. subtilis, and only the trp operon and the rho gene are its known targets (14). Furthermore, it has been demonstrated that two additional factors, NusG and NusA, participate in Rho-dependent termination by coupling Rho to the elongation complex (4). Interestingly, NusA is essential in B. subtilis but not in E. coli (although only in rho mutants with reduced termination efficiency), and NusG is essential in E. coli but not in B. subtilis (4). These differences might be also related to different distributions of RNAP on TUs in both bacteria.
Different biochemical characteristics of RNAP and its interactions with transcription factors in B. subtilis and E. coli may result in distinct strategies for postinitiation control of transcription. Specifically, E. coli often employs promoter trapping or promoter-proximal pausing of RNAP in addition to transcription attenuation in leader sequences, whereas B. subtilis mainly employs transcription attenuation. Further examination of the molecular basis for promoter-proximal RNAP peaks and their involvement in regulation of gene expression in both bacterial types is necessary to prove our hypothesis. In addition, studies on the dynamics of RNAP trafficking in other bacteria are essential to elucidate the relationships between the biochemical characteristics of RNAP and evolution of regulatory strategies of gene expression during the postinitiation steps of transcription.
Supplementary Material
Acknowledgments
We are grateful to Jon Hobman for the critical reading of the manuscript and Hiroki Takahashi and Hiroshi Mori for the critical discussion about the statistical analysis.
This work was supported by a KAKENHI grant-in-aid for scientific research in the Priority Area “Systems Genomics” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print on 3 September 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Artsimovitch, I., V. Svetlov, L. Anthony, R. R. Burgess, and R. Landick. 2000. RNA polymerases from Bacillus subtilis and Escherichia coli differ in recognition of regulatory signals in vitro. J. Bacteriol. 182:6027-6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57:289-300. [Google Scholar]
- 3.Choy, H. E., R. R. Hanger, T. Aki, M. Mahoney, K. Murakami, A. Ishihama, and S. Adhya. 1997. Repression and activation of promoter-bound RNA polymerase activity by Gal repressor. J. Mol. Biol. 272:293-300. [DOI] [PubMed] [Google Scholar]
- 4.Ciampi, M. S. 2006. Rho-dependent terminators and transcription termination. Microbiology 152:2515-2528. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland, W. S. 1979. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74:829-836. [Google Scholar]
- 6.Dobinson, K. F., and G. B. Spiegelman. 1987. Effect of the delta subunit of Bacillus subtilis RNA polymerase on initiation of RNA synthesis at two bacteriophage phi 29 promoters. Biochemistry 26:8206-8213. [DOI] [PubMed] [Google Scholar]
- 7.Gill, S. C., S. E. Weitzel, and P. H. von Hippel. 1991. Escherichia coli sigma 70 and NusA proteins. I. Binding interactions with core RNA polymerase in solution and within the transcription complex. J. Mol. Biol. 220:307-324. [DOI] [PubMed] [Google Scholar]
- 8.Greenblatt, J., and J. Li. 1981. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell 24:421-428. [DOI] [PubMed] [Google Scholar]
- 9.Grundy, F. J., and T. M. Henkin. 2004. Kinetic analysis of tRNA-directed transcription antitermination of the Bacillus subtilis glyQS gene in vitro. J. Bacteriol. 186:5392-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenther, M. G., S. S. Levine, L. A. Boyer, R. Jaenisch, and R. A. Young. 2007. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130:77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatoum, A., and J. Roberts. 2008. Prevalence of RNA polymerase stalling at Escherichia coli promoters after open complex formation. Mol. Microbiol. 68:17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henkin, T. M., and C. Yanofsky. 2002. Regulation by transcription attenuation in bacteria: how RNA provides instructions for transcription termination/antitermination decisions. Bioessays 24:700-707. [DOI] [PubMed] [Google Scholar]
- 13.Huber, W., J. Toedling, and L. M. Steinmetz. 2006. Transcript mapping with high-density oligonucleotide tiling arrays. Bioinformatics 22:1963-1970. [DOI] [PubMed] [Google Scholar]
- 14.Ingham, C. J., J. Dennis, and P. A. Furneaux. 1999. Autogenous regulation of transcription termination factor Rho and the requirement for Nus factors in Bacillus subtilis. Mol. Microbiol. 31:651-663. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa, S., Y. Kawai, K. Hiramatsu, M. Kuwano, and N. Ogasawara. 2006. A new FtsZ-interacting protein, YlmF, complements the activity of FtsA during progression of cell division in Bacillus subtilis. Mol. Microbiol. 60:1364-1380. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, S., Y. Ogura, M. Yoshimura, H. Okumura, E. Cho, Y. Kawai, K. Kurokawa, T. Oshima, and N. Ogasawara. 2007. Distribution of stable DnaA-binding sites on the Bacillus subtilis genome detected using a modified ChIP-chip method. DNA Res. 14:155-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 18.Laishram, R. S., and J. Gowrishankar. 2007. Environmental regulation operating at the promoter clearance step of bacterial transcription. Genes Dev. 21:1258-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal, M., B. Boese, J. E. Barrick, W. C. Winkler, and R. R. Breaker. 2003. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113:577-586. [DOI] [PubMed] [Google Scholar]
- 20.McClure, W. R. 1985. Mechanism and control of transcription initiation in prokaryotes. Annu. Rev. Biochem. 54:171-204. [DOI] [PubMed] [Google Scholar]
- 21.Mooney, R. A., S. E. Davis, J. M. Peters, J. L. Rowland, A. Z. Ansari, and R. Landick. 2009. Regulator trafficking on bacterial transcription units in vivo. Mol. Cell 33:97-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muse, G. W., D. A. Gilchrist, S. Nechaev, R. Shah, J. S. Parker, S. F. Grissom, J. Zeitlinger, and K. Adelman. 2007. RNA polymerase is poised for activation across the genome. Nat. Genet. 39:1507-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nechaev, S., M. Chlenov, and K. Severinov. 2000. Dissection of two hallmarks of the open promoter complex by mutation in an RNA polymerase core subunit. J. Biol. Chem. 275:25516-25522. [DOI] [PubMed] [Google Scholar]
- 24.Peters, J. M., R. A. Mooney, P. F. Kuan, J. L. Rowland, S. Keles, and R. Landick. 2009. Rho directs widespread termination of intragenic and stable RNA transcription. Proc. Natl. Acad. Sci. U. S. A. 106:15406-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radonjic, M., J. C. Andrau, P. Lijnzaad, P. Kemmeren, T. T. Kockelkorn, D. van Leenen, N. L. van Berkum, and F. C. Holstege. 2005. Genome-wide analyses reveal RNA polymerase II located upstream of genes poised for rapid response upon S. cerevisiae stationary phase exit. Mol. Cell 18:171-183. [DOI] [PubMed] [Google Scholar]
- 26.Reppas, N. B., J. T. Wade, G. M. Church, and K. Struhl. 2006. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Mol. Cell 24:747-757. [DOI] [PubMed] [Google Scholar]
- 27.Rojo, F., B. Nuez, M. Mencia, and M. Salas. 1993. The main early and late promoters of Bacillus subtilis phage phi 29 form unstable open complexes with sigma A-RNA polymerase that are stabilized by DNA supercoiling. Nucleic Acids Res. 21:935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahbabian, K., A. Jamalli, L. Zig, and H. Putzer. 2009. RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. EMBO J. 28:3523-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wade, J. T., and K. Struhl. 2008. The transition from transcriptional initiation to elongation. Curr. Opin. Genet. Dev. 18:130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whipple, F. W., and A. L. Sonenshein. 1992. Mechanism of initiation of transcription by Bacillus subtilis RNA polymerase at several promoters. J. Mol. Biol. 223:399-414. [DOI] [PubMed] [Google Scholar]
- 31.Wickiser, J. K., W. C. Winkler, R. R. Breaker, and D. M. Crothers. 2005. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol. Cell 18:49-60. [DOI] [PubMed] [Google Scholar]
- 32.Wu, L. J., S. Ishikawa, Y. Kawai, T. Oshima, N. Ogasawara, and J. Errington. 2009. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 28:1940-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yakhnin, A. V., and P. Babitzke. 2002. NusA-stimulated RNA polymerase pausing and termination participates in the Bacillus subtilis trp operon attenuation mechanism in vitro. Proc. Natl. Acad. Sci. U. S. A. 99:11067-11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeitlinger, J., A. Stark, M. Kellis, J. W. Hong, S. Nechaev, K. Adelman, M. Levine, and R. A. Young. 2007. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat. Genet. 39:1512-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.