Abstract
Lipid A deacylase PagL, which detoxifies endotoxin, is latent in Salmonella enterica. This study determined the biological significance of this latency. PagL latency was beneficial for bacteria in producing a robust permeation barrier through lipid A modifications under host-mimetic conditions that induced the modification enzymes, including PagL.
The outer layer of the outer membrane in enteric Gram-negative bacteria is exclusively occupied by lipopolysaccharide (LPS), which contains lipid A as the membrane anchor, while the inner layer contains phospholipids. This asymmetric lipid bilayer serves as a permeation barrier to a large number of noxious compounds. The strength of this barrier is due to the strong lateral interactions between LPS molecules and the low fluidity of the saturated fatty acid portion of lipid A in the outer membrane (reviewed in reference 20). Large hydrophilic compounds are excluded by narrow porin channels, and lipophilic compounds cross the asymmetric bilayer very slowly.
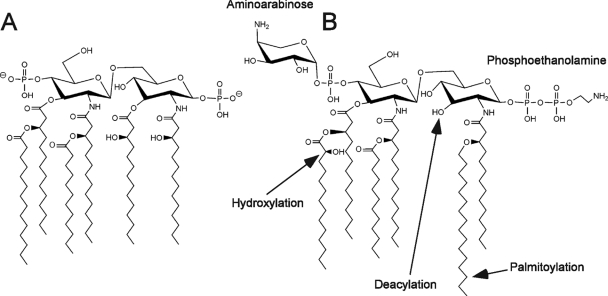
The prototype lipid A structure synthesized in Salmonella enterica serovar Typhimurium (S. Typhimurium) is shown in Fig. 1 A. In S. Typhimurium, lipid A is further modified by enzymes that are induced upon activation of the two-component regulatory system PhoP-PhoQ (Fig. 1B) (9). PhoP-PhoQ is essential for Salmonella virulence (3, 6, 18), and PhoP-PhoQ-regulated lipid A modifications are involved in many aspects of virulence. PhoQ is a sensor histidine kinase that responds to environmental conditions, including those within mammalian tissues. The host environment is experimentally mimicked by magnesium limitation and/or mild acid pH in the culture medium (3, 4, 6, 18, 21). In response to specific environmental signals, PhoQ phosphorylates PhoP, leading to the activation of pagL and pagP, which encode outer membrane lipid A 3-O-deacylase and outer membrane lipid A palmitoyltransferase, respectively (2, 22). Lipid A 3-O-deacylation by PagL and palmitoylation by PagP reduce the ability of lipid A to activate host Toll-like receptor 4, indicating that PhoP-PhoQ-dependent lipid A modifications help pathogens evade innate immune recognition (12). The regulation of lpxO, which encodes lipid A hydroxylase, is also mediated, at least in part, by PhoP-PhoQ (5, 9). Activation of PhoP-PhoQ leads to the activation of a second two-component regulatory system, PmrA-PmrB (8, 10). PmrA-PmrB promotes the attachment of aminoarabinose and phosphoethanolamine to phosphate groups on lipid A, which are involved in bacterial resistance to cationic antimicrobial peptides (7, 15). Furthermore, PhoP-PhoQ activation produces a more robust permeation barrier in the outer membrane, and lipid A modifications are involved in the generation of this enhanced barrier (19). Mg2+ ions decreased membrane permeability strongly in a phoP-null strain but only modestly in a PhoP-constitutive strain (19), implying a biological relevance of lipid A modifications by magnesium limitation.
FIG. 1.
Structures of the prototype lipid A (A) and modified lipid A (B) of S. Typhimurium.
Previous studies did not detect PagL-dependent lipid A deacylation when S. Typhimurium was grown under PhoP-PhoQ-activating conditions that induce PagL expression (11, 13, 22). In contrast, PagL-dependent lipid A deacylation was observed in pmrA-null and pmrE-null strains, both of which lacked aminoarabinose modification of lipid A (11, 13). These findings cannot be simply ascribed to the substrate specificity of PagL, since many lipid A species that are not modified with aminoarabinose exist in S. Typhimurium grown under PhoP-PhoQ-activating conditions (13). Therefore, it is thought that PagL is latent under these conditions and that aminoarabinose modification of lipid A is involved in the regulation of latency (13). PagL latency is consistent with an emerging paradigm of outer membrane enzyme regulation (1). It should be noted that PagL-dependent lipid A deacylation, which is beneficial for invading bacteria by allowing them to avoid Toll-like receptor 4 responses, would occur under some specific conditions such as those which activate PhoP-PhoQ without induction of lipid A aminoarabinose modification. Furthermore, we have identified several amino acid residues in the extracellular loops of PagL that are essential for latency but not for deacylase activity (17). The amino acid residues essential for latency were also necessary for PagL to associate with LPS (16). However, the biological significance of latency remains unknown.
The influx rate of a lipophilic agent, ethidium bromide, is increased by a pmrA-null mutation in an S. Typhimurium strain with a PhoP-constitutive phenotype (19). The rate-limiting step of this influx is crossing of the asymmetric bilayer in the outer membrane. Therefore, these observations suggest that pmrA-dependent lipid A modifications, such as aminoarabinose and phosphoethanolamine attachment, help generate a more robust permeation barrier through PhoP-PhoQ activation. On the other hand, lipid A is deacylated by PagL in a pmrA strain under PhoP-PhoQ-activating conditions (13). These observations led us to examine whether PagL-dependent lipid A deacylation increases the membrane permeability of the pmrA mutant strain.
Effects of PagL on PmrA-dependent production of robust membrane permeability.
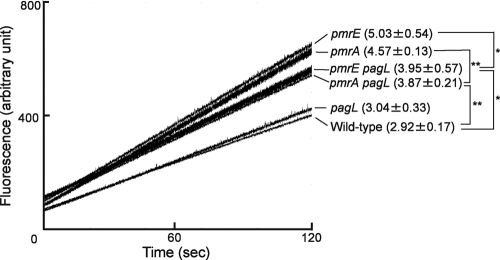
To examine outer membrane permeability, ethidium influx rates were examined as previously described (19). S. Typhimurium strains were cultivated overnight at 37°C in magnesium-limited (10 μM MgCl2) N-minimal medium (pH 5.8), which activates PhoP-PhoQ (11, 15). The precultures were diluted 1:10 with fresh medium and then grown at 37°C for 24 h. The stationary-phase cells were suspended in 50 mM phosphate buffer (pH 7.0) and then diluted to an optical density at 600 nm of 0.4 at room temperature. To inactivate the efflux pumps, the cells were treated with 100 μM carbonyl cyanide meta-chlorophenylhydrazone for 5 min prior to the addition of ethidium bromide (final concentration of 10 μg/ml). Fluorescence was monitored with a Shimadzu RF-5300 spectrofluorometer. The ethidium influx rate of pmrA mutant strain JSG421 (pmrA::Tn10d) (8) was higher than that of the parental wild-type strain (S. Typhimurium 14028s), indicating that PmrA is involved in the production of a robust membrane barrier (Fig. 2). The influx rate of pmrA pagL double-mutant strain KCS208 (pmrA::Tn10d ΔpagL) (11) was lower than that of the pmrA single-mutant strain, indicating that PagL-dependent lipid A deacylation increased the permeability of the pmrA mutant strain membrane (Fig. 2). On the other hand, the influx rate of the pmrA pagL mutant strain was higher than that of the wild-type strain, suggesting that PmrA-dependent lipid A modifications, such as aminoarabinose and phosphoethanolamine attachment, are also involved in generating a robust permeation barrier (Fig. 2). Furthermore, we examined pmrE-null strain KCS041 (pmrE1::Tn10d), in which aminoarabinose modification of lipid A is defective and PagL is released from latency (11, 13), and pmrE pagL double-mutant strain KCS209 (pmrE1::Tn10d ΔpagL) (11). The pmrE mutant strains behaved similar to pmrA mutant strains (Fig. 2). In addition, the influx rate of pagL-null strain KCS216 (ΔpagL) (11) was similar to that of the wild-type strain (Fig. 2), which was consistent with previous observations that lipid A was not deacylated by PagL when the wild-type strain was cultivated under PhoP-PhoQ-activating conditions, despite the induction of PagL (11, 13). These results, taken together, suggest that PagL latency is involved in the production of PmrA-dependent robust membrane permeability.
FIG. 2.
Ethidium influx into S. Typhimurium mutant strains. Ethidium influx into the wild-type and pmrA, pmrE, pagL, pmrA pagL, and pmrE pagL mutant strains was monitored for 120 s after the addition of ethidium to the cell suspension, and a representative set of assays is shown. The influx rates (increased fluorescence per second) from 30 to 90 s are shown in parentheses (average ± standard deviation of triplicate results). *, P < 0.05; **, P < 0.01 (Student's t test).
Nonlatent PagL increased membrane permeability.
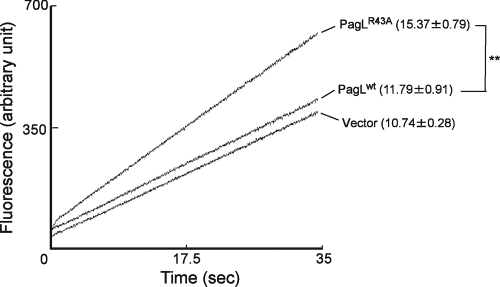
To further confirm that PagL latency is beneficial for a robust permeation barrier during PhoP-PhoQ activation, a nonlatent PagLR43A mutant protein (17), which has lost latency but retained its enzymatic activity, was used for the analysis. A low-copy-number cloning vector, pWKS30 (24), and its derivatives that express recombinant wild-type PagL (pWKS30-pagL-His6) (13) or nonlatent PagLR43A (pWKS30-pagLR43A-His6) (17) were introduced into S. Typhimurium pagL mutant strain KCS216 (11). The transformants were grown in magnesium-limited N-minimal medium (pH 7.4) containing 50 μg/ml ampicillin as previously described (17), and ethidium influx was examined. Compared with the strain expressing wild-type PagL, the strain expressing nonlatent PagLR43A had an increased influx rate (Fig. 3), suggesting that lipid A deacylation increased membrane permeability. The wild-type PagL-expressing strain had an influx rate similar to that of the strain that was transformed with the control vector (Fig. 3), which was consistent with previous observations that PagL-dependent lipid A deacylation was not induced by introducing wild-type PagL into S. Typhimurium pagL mutant strains (13, 17).
FIG. 3.
Ethidium influx into S. Typhimurium expressing the nonlatent PagLR43A mutant protein. Ethidium influx into S. Typhimurium pagL mutant strains transformed with pWKS30 (Vector), pWKS30-pagLR43A-His6 (PagLR43A), or pWKS30-pagL-His6 (PagLwt) was monitored for 35 s, and a representative set of assays is shown. The influx rates (increased fluorescence per second) from 10 to 30 s are shown in parentheses (average ± standard deviation of triplicate results). **, P < 0.01 (Student's t test).
The role of PagL latency was further examined by analyzing the resistance to lipophilic antimicrobial agents, which are expected to cross the outer membrane through the asymmetric bilayer region (19, 23). We determined the MICs of antimicrobial agents, including lipophilic agents such as rifampin, fusidic acid, novobiocin, erythromycin, and mupirocin. The MICs were determined in 96-well microtiter plates using a standard 2-fold broth microdilution method. Magnesium-limited N-minimal medium (pH 7.4) containing 50 μg/ml ampicillin was used as the assay medium. The transformants were precultured in the assay medium and then used for the analysis, with an inoculum size of approximately 3,000 cells per well. Each experiment was repeated at least twice. The strain expressing recombinant nonlatent PagLR43A was more susceptible to some lipophilic agents, such as rifampin, fusidic acid, and novobiocin, than was the strain expressing wild-type PagL (Table 1). In addition, the strain expressing PagLR43A was more susceptible to the large compound vancomycin than was the strain expressing wild-type PagL (Table 1). The susceptibility of Gram-negative bacteria to vancomycin increases when the outer membrane is perturbed with the polymyxin B nanopeptide, which binds LPS (14, 23). These results suggest that PagL latency is important for bacterial resistance to some lipophilic antimicrobial agents. To further confirm this susceptibility, cell growth in the assay medium was examined in the presence of the antimicrobial agents. In the presence of rifampin (0.25 μg/ml), fusidic acid (160 μg/ml), novobiocin (20 μg/ml), or vancomycin (2.5 μg/ml), the PagLR43A-expressing strain had a lower cell growth rate than the strain expressing wild-type PagL and the strain transformed with the control vector (data not shown). In contrast, there were no differences in cell growth in the absence of these agents (data not shown). Taken together, these results indicate that PagL latency is important for a robust permeation barrier in the outer membrane.
TABLE 1.
MICs of lipophilic antimicrobial agents and vancomycin against the S. Typhimurium pagL mutant strain transformed with recombinant PagL expression plasmids
| Agent | MIC (μg/ml) for pagL strain expressing: |
||
|---|---|---|---|
| Vector | PagLR43A | Wild-type PagL | |
| Rifampin | 0.5 | 0.13 | 0.5 |
| Fusidic acid | 320 | 160 | 320 |
| Novobiocin | 40 | 20 | 40 |
| Erythromycin | 8 | 8 | 8 |
| Mupirocin | 8 | 8 | 8 |
| Vancomycin | 10 | 5 | 10 |
Biological effects of lipid A modifications are difficult to dissociate from one another because they are more or less coregulated. PagL latency in S. Typhimurium grown under PhoP-PhoQ-activating conditions is a part of the coregulation machinery (13). Here, we demonstrated that PagL-dependent lipid A deacylation makes the outer membrane more permeable to lipophilic agents and that PagL latency is involved in generating a robust permeation barrier when S. Typhimurium is grown under PhoP-PhoQ-activating conditions. The reduced fluidity of saturated acyl chains consisting of the lipid A portion is responsible for the protective functions of the LPS leaflet in the outer membrane, and this fluidity increases as the numbers of saturated acyl chains in lipid A decreases (20). Therefore, PagL latency is suspected to be involved in maintaining the low fluidity of the outer membrane of S. Typhimurium.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 10 September 2010.
REFERENCES
- 1.Bishop, R. E. 2008. Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane. Biochim. Biophys. Acta 1778:1881-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop, R. E., H. S. Gibbons, T. Guina, M. S. Trent, S. I. Miller, and C. R. Raetz. 2000. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 19:5071-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 4.García Véscovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons, H. S., S. Lin, R. J. Cotter, and C. R. Raetz. 2000. Oxygen requirement for the biosynthesis of the S-2-hydroxymyristate moiety in Salmonella typhimurium lipid A. Function of LpxO, a new Fe2+/alpha-ketoglutarate-dependent dioxygenase homologue. J. Biol. Chem. 275:32940-32949. [DOI] [PubMed] [Google Scholar]
- 6.Groisman, E. A., E. Chiao, C. J. Lipps, and F. Heffron. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. U. S. A. 86:7077-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 8.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 10.Kato, A., and E. A. Groisman. 2004. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 18:2302-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki, K., K. China, and M. Nishijima. 2007. Release of the lipopolysaccharide deacylase PagL from latency compensates for a lack of lipopolysaccharide aminoarabinose modification-dependent resistance to the antimicrobial peptide polymyxin B in Salmonella enterica. J. Bacteriol. 189:4911-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2004. 3-O-deacylation of lipid A by PagL, a PhoP/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signaling through Toll-like receptor 4. J. Biol. Chem. 279:20044-20048. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2005. Inhibition of Salmonella enterica serovar Typhimurium lipopolysaccharide deacylation by aminoarabinose membrane modification. J. Bacteriol. 187:2448-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam, C., J. Hildebrandt, E. Schütze, and A. F. Wenzel. 1986. Membrane-disorganizing property of polymyxin B nonapeptide. J. Antimicrob. Chemother. 18:9-15. [DOI] [PubMed] [Google Scholar]
- 15.Lee, H., F. F. Hsu, J. Turk, and E. A. Groisman. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 186:4124-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manabe, T., M. Kawano, and K. Kawasaki. 2010. Mutations in the lipid A deacylase PagL which release the enzyme from its latency affect the ability of PagL to interact with lipopolysaccharide in Salmonella enterica serovar Typhimurium. Biochem. Biophys. Res. Commun. 396:812-816. [DOI] [PubMed] [Google Scholar]
- 17.Manabe, T., and K. Kawasaki. 2008. Extracellular loops of lipid A 3-O-deacylase PagL are involved in recognition of aminoarabinose-based membrane modifications in Salmonella enterica serovar Typhimurium. J. Bacteriol. 190:5597-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. U. S. A. 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata, T., W. Tseng, T. Guina, S. I. Miller, and H. Nikaido. 2007. PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:7213-7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prost, L. R., M. E. Daley, V. Le Sage, M. W. Bader, H. Le Moual, R. E. Klevit, and S. I. Miller. 2007. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 26:165-174. [DOI] [PubMed] [Google Scholar]
- 22.Trent, M. S., W. Pabich, C. R. Raetz, and S. I. Miller. 2001. A PhoP/PhoQ-induced lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083-9092. [DOI] [PubMed] [Google Scholar]
- 23.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]