Abstract
Compartmentalization of the activities of RNA polymerase sigma factors is a hallmark of formation of spores by Bacillus subtilis. It is initiated soon after the asymmetrically located sporulation division takes place with the activation of σF in the smaller cell, the prespore. σF then directs a signal via the membrane protease SpoIIGA to activate σE in the larger mother cell by processing of pro-σE. Here, we show that σE can be activated in the prespore with little effect on sporulation efficiency, implying that complete compartmentalization of σE activity is not essential for spore formation. σE activity in the prespore can be obtained by inducing transcription in the prespore of spoIIGA or of sigE*, which encodes a constitutively active form of σE, but not of spoIIGB, which encodes pro-σE. We infer that σE compartmentalization is partially attributed to a competition between the compartments for the activation signaling protein SpoIIR. Normally, SpoIIGA is predominantly located in the mother cell and as a consequence confines σE activation to it. In addition, we find that CsfB, previously shown to inhibit σG, is independently inhibiting σE activity in the prespore. CsfB thus appears to serve a gatekeeper function in blocking the action of two sigma factors in the prespore: it prevents σG from becoming active before completion of engulfment and helps prevent σE from becoming active at all.
Formation of spores by Bacillus subtilis results from a primitive cell differentiation involving two distinct cell types, the smaller prespore (also called the forespore) and the larger mother cell. These cells are formed following an asymmetrically located division that takes place about 1 h after the start of spore formation. The prespore is subsequently engulfed by the mother cell and develops into a mature spore. The mother cell is necessary for spore formation but ultimately lyses. Formation of a heat-resistant, dormant spore takes about 7 h at 37°C. A fundamental trait of cell differentiation is the existence of distinct programs of gene expression in the different cell types. This is exemplified in the sporulation of B. subtilis by the action of distinct RNA polymerase sigma factors (reviewed in reference 18). The expression of different genes in the two cells is governed to a considerable extent by the activation of four RNA polymerase σ factors. The first sigma factor to become active in the prespore is σF, and it does so soon after the formation of the division septum (17). σF directs transcription of about 50 genes (42, 46), including one critical for intercellular signaling, spoIIR. Expression of spoIIR leads rapidly to the activation of σE in the mother cell (27, 33); σE directs transcription of about 250 genes (10, 42). Upon completion of engulfment, σF and σE are replaced by σG and σK, respectively. σG directs transcription of about 110 genes in the prespore (42, 46), and σK directs transcription of about 140 genes in the mother cell (10, 42). In this paper, we focus on the compartmentalized activation of σE.
σE is synthesized as pro-σE, an inactive precursor (45). It is activated by cleavage of the 27-residue pro sequence (31, 43). Pro-σE is encoded by spoIIGB, which is cotranscribed with spoIIGA (34, 43). Transcription of the spoIIG operon is directed by the housekeeping σ factor, σA (29). Transcription is activated by the master regulator Spo0A (41) and commences soon after the start of spore formation, before the spore septum is formed (16, 28). Ordinarily, pro-σE is processed to active σE only after septation because it also requires the action of SpoIIR, which is synthesized after septum formation (27). SpoIIR is synthesized in the prespore but is exported to the intermembrane septal space, where it is thought to activate SpoIIGA. σE becomes active exclusively in the mother cell (8). It is activated within 4 min of spoIIR transcription in the prespore (11). The activity of σE is strictly confined to the mother cell, and it is thought that specific regulatory controls exist to prevent it from becoming active in the prespore (14, 25, 37). In addition, after septum formation there is a large increase in Spo0A-directed transcription of the spoIIG operon in the mother cell but not in the prespore (14, 15).
There have been several reports of strains engineered to activate σE before formation of the spore septum. Generally in these cases, the σE activity prevented septum formation so that the prespore was not formed (14, 37, 48). Fujita and Losick (14) did report rare organisms displaying σE activity in both the prespore and the mother cell of a strain in which the spoIIG operon was expressed constitutively; however, they did not comment further about those organisms. Attempts to obtain substantial σE activity in the prespore of B. subtilis by placing its structural gene under the control of a σF-directed promoter have been largely unsuccessful, even when sigE*, which encodes a form of σE (σE*) that is active without the need for processing (25), was used. It is thought that degradation of σE (or σE*) in the prespore is a critical factor in restricting σE activity to the mother cell (14, 25, 37).
In this paper, we reexamine the question of activating σE in the prespore. We reasoned that the prespore-specific degradation of σE might be under developmental control and tested the possibility that such a control might be directed from the mother cell. We find evidence of a σE-directed signal from the mother cell that blocks σE from becoming active in the prespore. We also find evidence that the product of a σF-directed gene, CsfB, inhibits σE activity in the prespore. We find that under some conditions, it is possible to obtain σE activity exclusively in the prespore or in both the prespore and the mother cell. We show that organisms displaying σE activity in both the prespore and the mother cell are capable of forming heat-resistant spores so that complete compartmentalization of σE activity is not essential for spore formation.
MATERIALS AND METHODS
Media.
B. subtilis was grown in modified Schaeffer's sporulation medium (MSSM) or on Schaeffer's sporulation agar as described previously (5, 36). When required, the medium was supplemented with chloramphenicol at 5 μg/ml, erythromycin at 1.5 μg/ml, neomycin at 3.5 μg/ml, spectinomycin at 100 μg/ml, or tetracycline at 10 μg/ml. Escherichia coli was grown on Luria-Bertani lysogeny broth agar containing 100 μg of ampicillin/ml when required.
Strains.
The B. subtilis strains used are listed in Table 1. B. subtilis 168 strain BR151 (trpC2 metB10 lys-3) was used as the parent strain. Plasmids were constructed in E. coli DH5α, and their structures were confirmed by restriction enzyme digestions and also by PCR.
TABLE 1.
B. subtilis strains used
| Straina | Relevant genotype |
|---|---|
| SL14574 | spoIIG::PspoIIQ-spoIIG thrC::PspoIID-gfp |
| SL14578 | spoIIG::PspoIIQ-spoIIG thrC::PspoIID-gfp ΔcsfB::cat |
| SL14613 | spoIIG::PspoIIQ-spoIIG thrC::PspoIID-gfp ΔcsfB::cat amyE::spoIIG |
| SL14631 | spoIIG::PspoIIQ-spoIIG thrC::PspoIID-gfp amyE::spoIIG |
| SL14656 | amyE::PspoIIQ-sigE* thrC::PspoIID-gfp ΔcsfB::cat ΔspoIIGB::spc |
| SL14657 | amyE::PspoIIQ-sigE* thrC::PspoIID-gfp ΔspoIIGB::spc |
| SL14679 | amyE::PspoIIQ-spoIIGB thrC::PspoIID-gfp ΔcsfB::cat |
| SL14711 | amyE::PspoIIQ-spoIIG PsspA-gfp@sspAb |
| SL14712 | amyE::PspoIIQ-spoIIG thrC::PspoIID-gfp |
| SL14715 | amyE::PspoIIQ-spoIIG thrC::PspoIID-gfp ΔcsfB::spc |
| SL14816 | amyE::PspoIIQ-spoIIG thrC::PspoIIQ-gfp |
| SL14868 | amyE::PspoIIQ-spoIIG thrC::PcotEp1-gfp |
| SL15041 | amyE::PspoIIQ-spoIIG ΔcsfB::spc ΔspoIIIG::cat |
| SL15062 | amyE::PspoIIQ-spoIIGA thrC::PspoIID-gfp ΔcsfB::spc |
| SL15139 | spoIIR::PspoIIQ-spoIIR thrC::PspoIID-gfp ΔcsfB::cat |
| SL15143 | amyE::PspoIIQ-spoIIG thrC::PspoIIID-gfp |
| SL15145 | amyE::PspoIIQ-spoIIG ΔcsfB::cat thrC::PspoIID-gfp |
| SL15175 | amyE::PspoIIQ-spoIIG ΔcsfB::cat |
| SL15365 | spoIIG::PspoIIQ-spoIIG amyE::PspoIIG-spoIIG ΔcsfB::cat thrC::PspoIID-gfp ΔspoIIIG::spc |
| SL15366 | spoIIG::PspoIIQ-spoIIG amyE::spoIIG thrC::PspoIID-gfp ΔspoIIIG::spc |
All strains are in the genetic background of B. subtilis 168 strain BR151 (trpC2 lys-3 metB10). They have all its auxotrophic markers. All strains were developed in the course of the present study.
@, the fusion has been introduced by single-crossover (Campbell-like) recombination.
The sigE* gene, encoding σE*, which is active without processing, was cloned under the control of the σF-directed PspoIIQ. The pro-less form of the open reading frame (ORF), starting with the 28th codon of pro-σE and including the rest of the ORF, was amplified by PCR. The PCR product was cloned downstream of PspoIIQ in pEIA99 (4) to give pVK333, which is designed to integrate the PspoIIQ-sigE* construct at the amyE locus of B. subtilis by double crossover. As a result of the cloning, the codon for Tyr, which is the 28th codon of pro-σE, is preceded by just two codons, for Met and His, and a strong ribosome binding site ([RBS] GGAGG).
The spoIIG operon, retaining its RBS but not its promoter, was amplified by PCR and cloned downstream of PspoIIQ in pEIA99 to give pVK339, containing amyE::PspoIIQ-spoIIG. The promoterless spoIIGA gene, retaining its RBS, was amplified by PCR and cloned downstream of PspoIIQ in pEIA99 to give pVK239, containing amyE::PspoIIQ-spoIIGA. The constructs from pVK333, pVK339, and pVK239 were introduced by double crossover into the amyE locus of B. subtilis BR151 by transformation, selecting for the appropriate resistance marker. The constructs were subsequently moved into other strains by transformation.
PspoIID-gfp fusions in two locations were used as indicators of σE activity, located at ppsD (169°) and thrC (284°); the origin of replication of the circular chromosome is at 0°. Construction of thrC::PspoIID-gfp was described previously (3). The ppsD::PspoIID-gfp fusion was constructed in two stages. First, a 450-bp portion of ppsD was PCR amplified and inserted upstream of the spoIID promoter (−291 to +22) in pMLK5169 (a gift from Margaret Karow) to form pVK177. Next, a promoterless gfp was isolated from a derivative of pGreenTIR (35) described previously (2) and inserted downstream of the spoIID promoter in pVK177, replacing lacI, to form pVK196. B. subtilis BR151 was transformed with pVK196, selecting for Cmr and screening for single-crossover (Campbell-like) integration of the plasmid at ppsD. The site of integration of pVK196 was confirmed by linkage analysis. DNA was prepared from one such transformant and used to transform appropriate strains. To construct PcotEp1-gfp, the same promoterless gfp fragment was inserted downstream of PcotEp1 in pDF1264, which was a gift from Patrick Stragier. As a result of this manipulation, the spoIIQ of pDF1264 was replaced with gfp. The construct was introduced by double crossover into the thrC locus of B. subtilis BR151. Vectors for the expression of PspoIIID-gfp and PgerE-gfp were also designed for insertion into thrC by double crossover. For PspoIIID-gfp, a 400-bp DNA region that contains the promoter of spoIIID was PCR amplified from B. subtilis BR151 chromosomal DNA and cloned upstream of gfp in pVK370, which is a laboratory construct. For PgerE-gfp, the lacZ gene of thrC::PgerE-lacZ in B. subtilis (6) was replaced with gfp using the lacZ-to-gfp replacement vector pVK209 (2). Details of plasmid and strain construction are available on request.
Fluorescence microscopy.
The conditions for growth and imaging of the samples were essentially as described previously (5). Images were captured using a Leica DM IRE2 microscope with a TCS SL confocal system.
Other methods.
The methods for transformation of B. subtilis and for sporulation by exhaustion in MSSM and other methods were essentially those described previously (4). The initiation of spore formation in MSSM is taken to occur at the end of exponential growth; time after the initiation of spore formation is indicated as T2 for 2 h, T3 for 3 h, etc.
RESULTS AND DISCUSSION
σE activity in the prespore.
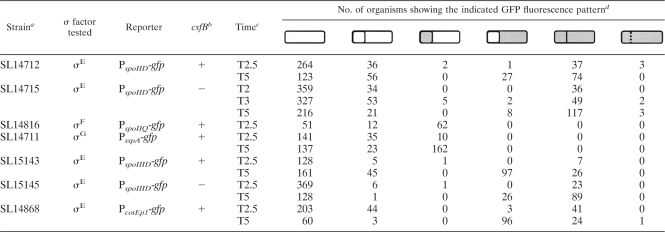
In order to obtain σE activity in the prespore, we performed three genetic manipulations. First, we utilized sigE*, which encodes a form of σE (σE*) that is active without the need for processing (25). Second, we placed sigE* under the control of the strong σF-directed PspoIIQ promoter so that it would be expressed only in the prespore and located the construct at the origin-proximal amyE locus to ensure optimal transcription. Third, we inactivated the parental spoIIGB locus, which encodes pro-σE, so as to block any σE-directed signal from the mother cell that might prevent σE from becoming active in the prespore. The resulting strain, SL14657, displayed σE activity in the prespore (Fig. 1 A). By 2.5 h after the end of exponential growth (T2.5), 20% of organisms had undergone the asymmetrically located sporulation division. Of those, 21% displayed σE activity, and in all cases it was localized to the prespore (Table 2). At T5, 70% of organisms had formed the sporulation septum, and 37% of those displayed a signal in the prespore; again, none displayed any σE activity in the mother cell. The strains were blocked in spore formation and had the abortively disporic phenotype typical of spoIIGB mutants. Interestingly, the σE activity was often confined to just one of the two prespores in the abortively disporic organisms (Fig. 1A; Table 2). Time-lapse studies (data not shown) indicated that it was the first of the two prespores to be formed that displayed σE activity. We obtained a similar result with spoIIGB intact but spoIIR disrupted so that pro-σE was made under its normal control but could not be processed to σE (data not shown).
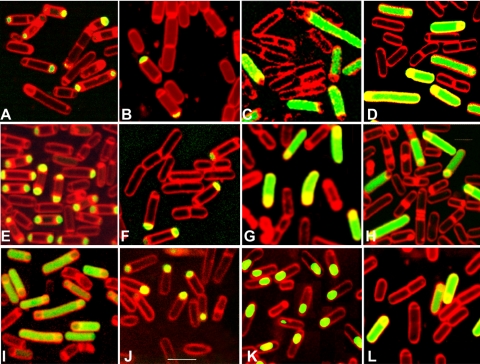
FIG. 1.
Localization of activity of σE and other sporulation-associated sigma factors in strains with various modifications to expression of the spoIIG operon. Examples of bacteria stained with FM4-64 (red) and expressing GFP (green) from gfp transcriptional fusions to promoters directed by different sigma factors are shown. Promoters are as follows: σE-directed PspoIID (A to H and L), σE-directed PcotEp1 (I), σF-directed PspoIIQ (J), and σG-directed PsspA (K). Strains are either csfB+ (A to D and I to K) or csfB null (E to H and L). (A) Strain SL14657 at T5 (amyE::PspoIIQ-sigE* ΔspoIIGB::spc). (B) SL14574 at T2.5 (spoIIG::PspoIIQ-spoIIG). (C) SL14631 at T2.5 (spoIIG::PspoIIQ-spoIIG amyE::spoIIG). (D) SL14712 at T2.5 (amyE::PspoIIQ-spoIIG). (E) SL14656 at T5 (amyE::PspoIIQ-sigE* ΔspoIIGB::spc ΔcsfB::cat). (F) SL14578 at T2.5 (spoIIG::PspoIIQ-spoIIG ΔcsfB::cat). (G) SL14613 at T2.5 (spoIIG::PspoIIQ-spoIIG amyE::spoIIG ΔcsfB::cat). (H) SL14715 at T2.5 (amyE::PspoIIQ-spoIIG ΔcsfB::spc). (I) SL14868 at T2.5 (amyE::PspoIIQ-spoIIG). (J) SL14816 at T2.5 (amyE::PspoIIQ-spoIIG). (K) SL14711 at T5 (amyE::PspoIIQ-spoIIG). (L) SL15062 at T2.5 (amyE::PspoIIQ-spoIIGA ΔcsfB::spc). The scale bar shown in panel J is 3 μm and is applicable to all images.
TABLE 2.
Expression of σE activity in the prespore
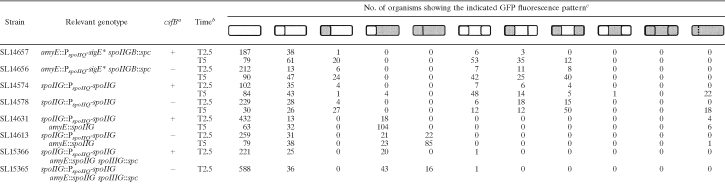 |
a Strains were either csfB+ or csfB null (−).
b Samples were analyzed at the indicated times given in h after the start of spore formation.
c σE activity was visualized using a PspoIID-gfp fusion. Cultures were induced to form spores in MSSM. Schematic representations are given of the different types of organisms present in the culture; shaded areas indicate GFP fluorescence. Membranes were visualized by staining with FM4-64. Some organisms of strains SL14575 and SL14578 appeared to have defective septa, indicated by a dotted line; the defective septa would disrupt compartmentalized GFP fluorescence.
Processing of pro-σE in the prespore.
We next tested to see if the wild-type form of pro-σE could be processed to active σE in the prespore. Ordinarily, expression of the spoIIG operon, which encodes pro-σE and SpoIIGA, is induced soon after the start of spore formation and before the sporulation division (16, 28). However, processing of the inactive pro-σE to active σE by SpoIIGA is confined to the mother cell following septum formation (8) and requires the action of SpoIIR, which is formed in the prespore from the σF-controlled spoIIR locus (27, 33). Thus, σE activation in the mother cell is dependent on an intercompartmental signal across the septum.
To test for processing of pro-σE in the prespore, we replaced the promoter of the spoIIG operon with the σF-directed spoIIQ promoter so as to confine its transcription to the prespore. This substitution indeed resulted in σE activity being largely confined to the prespore (Fig. 1B; Table 2, strain SL14574) although at T5, for reasons that are not clear, some organisms showed activity in the mother cell. The activation of σE was blocked by inactivation of spoIIR (data not shown) so that in this genetic background SpoIIR acts as an intracompartmental signal to activate processing of pro-σE. The strain did not form spores and was blocked at stage II of sporulation. The septum, as indicated by FM4-64 staining, appeared to be disrupted in a number of organisms in the T5 sample, making quantitation for that sample difficult.
Activation of σE in the mother cell can prevent it from becoming active in the prespore.
The strains described in the experiments in the previous sections had a genetic background in which normal spoIIG expression was disrupted. This was done to block a suspected σE-directed signal from the mother cell that would prevent σE from becoming active in the prespore. To test for such a signal, the strain SL14631, which is isogenic with SL14574 (spoIIG::PspoIIQ-spoIIG) except for the insertion of an intact copy of the spoIIG operon under its own promoter at the ectopic amyE locus, was tested. The change restored σE activity to the mother cell. All organisms displaying σE activity now had activity in the mother cell (Fig. 1C and Table 2). Importantly, few organisms showed any σE activity in the prespore, and that activity was weak. The strain formed spores efficiently, and no abortively disporic organisms were detected.
These results are consistent with a σE-directed signal from the mother cell largely preventing σE from becoming active in the prespore. However, they also fit an alternative explanation, namely, that the critical factor is the activation of σE, not its activity. In particular, enhanced expression of the protease SpoIIGA in the mother cell outcompetes any SpoIIGA in the prespore for activation by SpoIIR, thus ensuring that pro-σE is processed by activated SpoIIGA only in the mother cell. This possibility is considered in detail below, in the section on compartmentalization of σE activity.
σE activity in the prespore is regulated by CsfB.
The csfB locus is transcribed in the prespore from a σF-directed promoter (7). The CsfB protein has been shown to inhibit premature activation of σG in the prespore (1, 6, 26). CsfB can also impair σE activity (6). We wished to test the possibility that CsfB might be a developmentally regulated check on σE becoming active in the prespore. Consistent with this possibility, inactivation of csfB increased σE* activity in the prespores of a spoIIGB mutant that was expressing sigE* from a σF-directed promoter. Most notably, a higher proportion of abortively disporic organisms displayed σE* activity in both prespores in the csfB mutant SL14656 than in the csfB+ strain SL14657 (37% and 12%, respectively, at T5) (Table 2; compare Fig. 1A and E). The effect is unlikely to be an indirect consequence of CsfB acting on σG because a strain isogenic with SL14656 but containing a reporter for σG displayed no σG activity (data not shown). Inactivation of csfB also resulted in a substantial increase in the proportion of prespores expressing σE activity in a strain expressing the spoIIG locus under the σF-directed PspoIIQ promoter (Table 2, compare strains SL14578 and SL14574).
The effect of csfB inactivation was dramatic in a strain that had a second copy of spoIIG expressed from the σF-directed PspoIIQ promoter, in addition to spoIIG expressed from its natural promoter. Expression was largely confined to the mother cell of the csfB+ strain (SL14631) (Fig. 1C and Table 2). However, with csfB inactivated, the location of σE activity changed: the majority of green fluorescent protein (GFP)-expressing organisms now displayed activity in both the mother cell and the prespore (Fig. 1G and Table 2, strain SL14613). This distinction was retained when the structural gene for σG was inactivated: in the csfB+ strain σE activity was confined to the mother cell, whereas in the csfB mutant, there was substantial σE activity in the prespore (Table 2, strains SL15366 and SL15365, respectively).
Together, the results indicate that CsfB helps curtail σE activity in the prespore. CsfB does so independently of its role in inhibiting σG. CsfB also acts to curtail the activity of σE*, which is active without processing. We think it plausible that, by analogy with its action on σG (1, 6, 26), CsfB directly inhibits σE activity. That is to say, CsfB inhibits the activity of two sigma factors. It serves a gatekeeper function in the prespore, helping to prevent σE from becoming active at all in the prespore and to prevent σG from becoming active prematurely before completion of engulfment. In an otherwise wild-type background, this gatekeeper role of CsfB for σE is redundant as inactivation of csfB does not result in σE activity in the prespore (data not shown), but the role is revealed in the genetic backgrounds used here. The results show that it is possible to have σE active in both the prespore and the mother cell. In the following section we explore how σE activity in both compartments can also be achieved by enhancing spoIIG transcription even if csfB is intact.
Enhanced spoIIG transcription in the prespore can result in σE being active in both the prespore and the mother cell.
Results described above suggested that activation of σE in, or a σE-directed signal from, the mother cell helped prevent σE from becoming active in the prespore (Table 2, compare strains SL14574 and SL14631). Implicit in the conclusion is the importance of timing: if σE was activated sooner in the prespore, then its activity in the prespore might not be blocked by activation and/or activity in the mother cell. Additionally, earlier expression of σE in the prespore might help it escape the inhibitory effects of CsfB. A key factor in the timing of gene expression directed by σF in the prespore is the location of the gene on the chromosome (30, 49); origin-distal genes enter the prespore later than origin-proximal genes so that their expression is delayed (47). In strain SL14631 the σF-directed spoIIG locus is at an origin-distal position, 135°, so that its transcription in the prespore is delayed relative to origin-proximal genes. We tested the effect of earlier transcription of spoIIG in the prespore by introducing the PspoIIQ-spoIIG construct at the origin-proximal amyE locus, at 25°, in a strain that has spoIIG expressed from its own promoter at its natural locus. The change in location to 25° indeed altered the location of σE activity. In a sample taken 2.5 h after the start of spore formation, over 90% of organisms with active σE displayed activity in both the prespore and the mother cell (Fig. 1D and Table 3, strain SL14712,). For SL14715, at T5, the figure was about 78%, with 20% having activity predominantly or exclusively in the mother cell and about 2% displaying activity predominantly in the prespore. In the same genetic background, σF and σG activities were completely confined to the prespore (strains SL14816 and SL14711, respectively) (Fig. 1J and K and Table 3) so that the presence of σE activity in both compartments was not a consequence of loss of septum integrity. A likely cause of the heterogeneity of location for σE activity with SL14712 is variability (noise) in the expression of the genes encoding σE, or its activators, combined with some type of switch that results in a bimodal distribution in σE expression (9, 12).
TABLE 3.
Expression pattern of different sigma factors in strains with enhanced spoIIG transcription in the prespore
a Cultures were induced to form spores in MSSM.
b Strains were either csfB+ or csfB null (−).
c Samples were analyzed at the indicated times given in hours after the start of spore formation.
d All strains were spoIIG+ and also contained amyE::PspoIIQ-spoIIG to enhance early transcription of spoIIG in the prespore. Schematic representations are given of the different types of organism present in the cultures; shaded areas indicate GFP fluorescence. Membranes were visualized by staining with FM4-64.
Expression in both the mother cell and the prespore was also observed with two other σE-directed promoters, cotEp1 (strain SL14868) (Fig. 1I and Table 3) and spoIIID (strain SL15143) (Table 3). These promoters were weaker than the promoter for spoIID. In samples taken later in sporulation (T5), but not in earlier samples (T2.5), fluorescence in the prespore was often very faint for cotEp1 and spoIIID, making scoring difficult (only prespores displaying substantial fluorescence are scored as positive in Table 3). We suspect that this behavior indicates relatively short-lived expression of σE in the prespore, combined with its turnover. Inactivation of csfB increased the proportion of organisms at T5 clearly displaying σE activity in both the prespore and the mother cell for the weakly expressed spoIIID promoter (Table 3, strain SL15145 compared to SL15143); it had much less effect on the stronger spoIID promoter (Table 3, compare strains SL14715 and SL14712).
σE activity in the prespore does not block spore formation.
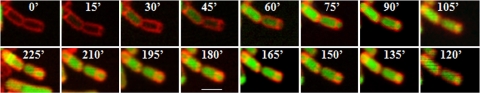
Surprisingly, strains displaying σE activity in both the prespore and the mother cell did not appear compromised in their abilities to form heat-resistant spores. Results of typical experiments are shown in Table 4. Much of the sporulating population of strain SL14712 showed σE activity in both the prespore and the mother cell (Table 3). To analyze this behavior further, individual sporulating organisms of strain SL14712 were tracked by time-lapse microscopy. Activity was detected in both compartments at various stages of prespore engulfment (Fig. 2). The development of phase-bright spores in organisms that had σE activity in both the prespore and the mother cell is illustrated in a movie (see Fig. S1 in the supplemental material). Because of GFP stability, it was not possible to say for how long σE remained active during the maturation of the prespore. However, the results do indicate that complete compartmentalization of σE activity is not essential for spore formation even though σE activity is ordinarily confined to the mother cell.
TABLE 4.
Effect on spore formation of σE being active in both the prespore and the mother cell
| Relevant genotype (strain) | Location of σE activitya | No. of heat-resistant spores/ml (×108)b |
|---|---|---|
| spo+ (SL4) | MC | 5.0, 3.2 |
| amyE::PspoIIQ-spoIIG (SL14712) | PS + MC | 5.0, 4.0, 4.0, 4.6 |
| amyE::PspoIIQ-spoIIG csfB::cat (SL14715) | PS + MC | 3.0, 2.0 |
| amyE::PspoIIQ-spoIIG csfB::cat (SL15175) | PS + MC | 4.0, 3.5 |
MC, mother cell; PS, prespore.
Sporulation was assessed 18 to 20 h after the end of exponential growth. Heat treatment was for 25 min at 80°C. Each value represents a different culture.
FIG. 2.
Engulfment of a prespore expressing σE activity. Successive images from time-lapse microscopy of strain SL14712, amyE::PspoIIQ-spoIIG thrC::PspoIID-gfp. Membranes were stained with FM4-64 (red); GFP (green) indicates the location of σE activity. Time in each image is the time in minutes (′) from the first frame. Scale bar, 2 μm. Bacteria were placed on agarose pads containing MSSM and incubated at 33°C.
The skin element is not excised in the prespores of strains that display σE activity in the prespore.
The σE regulon contains more than 200 genes (10, 42). They direct a variety of functions that are required for spore formation. A striking example of a late-expressed function is the excision of the 48-kb skin element from the genome of the mother cell (44). Transcription of the gene encoding the recombinase for skin excision, spoIVCA, is directed by σE. We tested for spoIVCA promoter activity but found the signal too weak to reach any conclusion about the location of its expression. Excision of skin is required to join together the 5′ and 3′ ends of sigK, the structural gene for σK, whose activity in the mother cell is required for spore formation. The mother cell ultimately dies, and skin is not excised in the developing spore (the germ line); many strains of B. subtilis retain skin in their genome. We detected σK activity only in the mother cell of strains with the amyE::PspoIIQ-spoIIG construct that gave σE activity in both the prespore and the mother cell (data not shown). Consequently, we suspected that the skin element had not been excised from the prespore. To test directly for skin excision, we utilized a skin element containing a cat gene (23) which conferred chloramphenicol resistance. This construct was present in a strain expressing σE activity in both the prespore and the mother cell. The strain was maintained and induced to form spores in the absence of chloramphenicol. We tested 25 colonies derived from heat-resistant spores formed in each of 12 separate sporulation experiments (i.e., a total of 300 colonies). We detected no loss of the cat gene. This result indicated that the skin element was not excised in the prespore. Similar results were obtained with csfB+ and csfB strains. Three different promoters, PspoIID, PcotEp1 and PspoIIID, were used to demonstrate σE activity in the prespore. We infer that B. subtilis is able to tolerate a considerable amount of σE activity in the wrong cell compartment without compromising its ability to form spores. However, skin was not excised in the prespore, indicating that the σE regulon was not fully functional in the prespore. The intensity of GFP expression from the different promoters suggests that σE activity in the prespore is lower than its normal activity in the mother cell although we do not have firm quantitative data. We had previously shown that σF and σG do not impair each other's activity when they are coexpressed in the prespore, indicating that their activity is not limited by competition for core polymerase (5); thus, we think it unlikely that σE activity is limited by competition with σF although we cannot exclude that possibility. Structural factors might also limit the functioning of various members of the regulon in the prespore. Suggestive of such an explanation, Ramamurthi and Losick (38) have shown that a product of the σE regulon, SpoVM, homes only to convex membranes so that even if it were expressed in the prespore, it would not localize properly.
Compartmentalization of σE activity.
The ability to change the location of σE activity helped us to explore the mechanism of compartmentalization of σE activity. Pro-σE and SpoIIGA are synthesized before the spore septum is formed. Ordinarily, pro-σE is processed to active σE only after septation because it requires the action of SpoIIR, which is expressed only after septation (27). σE becomes active in the mother cell within 4 min of spoIIR transcription in the prespore (11) but does not become active in the prespore (8). The spoIIR locus is weakly transcribed (27), but once it is expressed, SpoIIR or pro-σE or SpoIIGA could be the limiting factor that helps restrict σE activation to the mother cell. These possibilities were tested by increasing expression of each of the corresponding structural genes in the prespore from the strong σF-directed spoIIQ promoter. The strains contained the intact spoIIG operon at its natural location. They also contained a csfB mutation in order to prevent inhibition of σE activity in the prespore by CsfB.
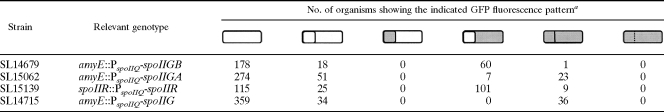
Enhanced transcription of spoIIGB (encoding pro-σE) in the prespore did not change the location of σE activity, which remained in the mother cell (Table 5, strain SL14679). However, enhanced transcription of spoIIGA in the prespore resulted in a substantial number of organisms expressing σE activity in the prespore as well as the mother cell (23 organisms out of 30 displaying σE activity) (Table 5, strain SL15062; Fig. 1L). When expression of spoIIR was increased by replacing its natural promoter with the stronger spoIIQ promoter, σE activity remained predominantly in the mother cell (Table 5, strain SL15139). Some organisms also displayed σE activity in the prespore (9 out of 110), but the effect was less dramatic than with enhanced spoIIGA expression. As discussed above, enhanced expression of the spoIIG operon also resulted in σE activity in both the prespore and the mother cell. Together, the results suggest that limitation of the processing machinery, SpoIIGA and SpoIIR, normally helps prevent σE from becoming active in the prespore.
TABLE 5.
Enhanced expression of spoIIGA in the prespore results in σE activity in the prespore
a σE activity was visualized using a PspoIID-gfp fusion. Strains contained the csfB::cat mutation. Cultures were induced to form spores in MSSM. Samples were analyzed 2.5h after the start of spore formation. Schematic representations are given of the different types of organism present in the cultures; shaded areas indicate GFP fluorescence. Membranes were visualized by staining with FM4-64. For clarity, data for SL14715 (2h) have been recapitulated from Table 3.
The SpoIIR protein is exported from the prespore into the intercellular space (19) and is thought to interact with membrane-located SpoIIGA, activating it to cause the conversion of pro-σE into active σE (22). The spoIIR locus is poorly transcribed (27), and once it is expressed, we suggest that SpoIIGA proteins in the membranes of the prespore and the mother cell compete for the limited amount of SpoIIR that has been made. Ordinarily, SpoIIGA in the mother cell predominates, and so σE activation is confined to the mother cell. However, increased SpoIIGA expression in the prespore can result in σE also becoming active in the prespore. This interpretation also fits with the results of Ju et al. (24, 25), who expressed a fusion of the pro sequence to gfp in the prespore and detected no processing unless spoIIGA was also expressed in the prespore. Less effectively, increased expression of SpoIIR improves the chances of SpoIIGA being activated in the prespore.
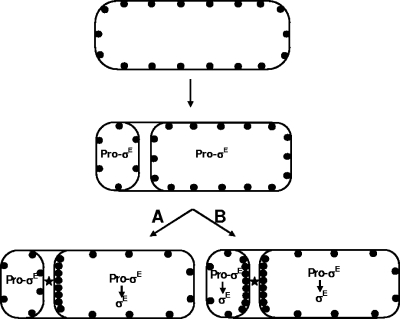
The question then becomes, Why is SpoIIGA normally predominantly in the mother cell? We think that a diffusion and capture model (13, 40) may account, at least in part, for the initial compartmentalization of σE activation. This model is illustrated in Fig. 3. SpoIIGA is a membrane protein that likely inserts nonspecifically into the cell membrane before septation (13, 32, 43). As a consequence, we suggest that upon septation, the majority of SpoIIGA that has been synthesized before septation will be in the membrane surrounding the mother cell (perhaps a 5-fold excess over the amount in the membrane around the prespore). SpoIIGA is freely diffusible within its membrane and can diffuse into the face of the septal membrane. SpoIIR is exported from the prespore and localizes to the sporulation septum (39), where presumably it interacts with SpoIIGA from either the prespore or the mother cell membrane. Because there is excess SpoIIGA in the mother cell membrane, the mother cell wins out in the competition for the limited SpoIIR available, and σE becomes active only in the mother cell. SpoIIGA functions as dimer or higher multimer (22), which may enhance this selectivity. Additionally, some component of the septum (not necessarily SpoIIR) may capture and hence help concentrate SpoIIGA in the septum. Once σE is active in the mother cell, it may direct a signal to the prespore to block activation in the prespore. The studies of Fawcett et al. (13) on SpoIIGA-GFP localization in B. subtilis and Bacillus megaterium are consistent with the model in Fig. 3. Fawcett et al. found that SpoIIGA is initially located in the peripheral membrane and then becomes concentrated at the septum; indeed, they proposed that SpoIIGA is sequestered (captured) by some unknown component of the septum (13).
FIG. 3.
Schematic representation of a model for the compartmentalization of σE activation. The membrane protein SpoIIGA is indicated by filled circles, and SpoIIR is indicated by a star. SpoIIGA is first synthesized before the spore septum is formed and is distributed randomly throughout the cell membrane. As a consequence, upon septation the large majority of SpoIIGA is located in the mother cell. Some component of the recently completed septum captures SpoIIGA that has diffused from the peripheral membrane. When SpoIIR is synthesized, it is secreted into the space between the septal membranes. There, it can interact with SpoIIGA from either the mother cell or the prespore. (SpoIIR may or may not be the component that captures SpoIIGA in the septum.) Because the preponderance of SpoIIGA is in the mother cell, mother cell SpoIIGA wins out in the competition for the very limited amount of SpoIIR. As a consequence, only SpoIIGA in the mother cell is activated, and so conversion of pro-σE to active σE occurs only in the mother cell (shown in path A). However, if expression of SpoIIGA in the prespore is artificially enhanced, then activation of σE can also occur in the prespore (path B).
A second factor in the normal compartmentalization of σE activity is enhanced transcription in the mother cell of the spoIIG operon (14). It remains unclear if the increase in spoIIG transcription occurs before or after σE first becomes active in the mother cell. The increase occurs soon after septation (14). However, σE becomes active in the mother cell within 4 min of σF-directed spoIIR transcription in the prespore (11), and σF activation is thought to follow very rapidly after septum formation (17, 20, 21). If increased spoIIG transcription occurs before σE activation, then it could be important for both establishing and maintaining compartmentalization; if spoIIG transcription increases after σE activation, then its role is in maintaining compartmentalization. This Spo0A-mediated increase in spoIIG transcription (14, 15) would “lock in” σE activity in the mother cell.
Prespore-specific proteolysis of σE is also important for enforcing compartmentalization (14, 25, 37), and we think that this proteolysis may be activated by a σE-directed signal from the mother cell. CsfB provides an additional barrier to inappropriate σE activation in the prespore. The existence of such multiple controls suggests that compartmentalizing σE activity to the mother cell is important for spore formation. It is therefore surprising to find that B. subtilis can still form spores efficiently despite a substantial loss of σE compartmentalization. However, only a small selective advantage, below differences detectable in our assay, would be sufficient to ensure complete compartmentalization of σE activity in the mother cell in a wild-type strain.
It is interesting to compare the proposed model for compartmentalizing σE in the mother cell (Fig. 3) with the models for compartmentalization of σF in the prespore (20, 21). Models for both sigma factors invoke cell size asymmetry together with sequestration in the septum of a membrane protein critical to activation, SpoIIGA for σE and SpoIIE for σF. Yet opposite results are achieved with respect to cell compartment. The actual activation mechanisms are very different: release from the anti-sigma factor SpoIIAB for σF because of activation of the anti-anti-sigma factor SpoIIAA by SpoIIE-mediated dephosphorylation (20, 21) and cleavage of the inactive precursor pro-σE for σE (31, 43). All the components for activation of σF are already present before septation. Indeed, SpoIIE is involved in formation of the septum and initially is probably present in comparable levels on both faces of the septum (18). It is thought to be the sudden change in ratio of soluble components to septum-bound components, which accompanies formation of the prespore but not the mother cell, that shifts the equilibrium, triggering σF activation in the prespore; this switch is then locked in by hysteresis (20, 21).
For σE, the critical component SpoIIR is synthesized only after the septum has formed (48). In addition, we suggest that SpoIIGA comes from peripheral membrane to the septum after completion of septation so that it is predominantly on the mother cell face (Fig. 3) (13). Consequently, the excess of SpoIIGA already present in the mother cell face of the septum wins out in the competition to be activated by the small amount of SpoIIR that has been made so that σE activation is confined to the mother cell (Fig. 3).
Supplementary Material
Acknowledgments
We thank Margaret Karow, Marta Perego, and Patrick Stragier for kindly providing strains. We thank Richard Losick for critical comments on the manuscript. We thank Michael Elowitz for helpful discussions.
This work was supported by Public Health Services grant GM43577 from the National Institutes of Health.
Footnotes
Published ahead of print on 27 August 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Camp, A. H., and R. Losick. 2008. A novel pathway of intercellular signalling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol. Microbiol. 69:402-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chary, V. K., M. Busuioc, J. A. Renye, Jr., and P. J. Piggot. 2005. Vectors that facilitate the replacement of transcriptional lacZ fusions in Streptococcus mutans and Bacillus subtilis with fusions to gfp or gusA. FEMS Microbiol. Lett. 247:171-176. [DOI] [PubMed] [Google Scholar]
- 3.Chary, V. K., M. Meloni, D. W. Hilbert, and P. J. Piggot. 2005. Control of the expression and compartmentalization of σG activity during sporulation of Bacillus subtilis by regulators of σF and σE. J. Bacteriol. 187:6832-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chary, V. K., and P. J. Piggot. 2003. Postdivisional synthesis of the Sporosarcina ureae DNA translocase SpoIIIE either in the mother cell or in the prespore enables Bacillus subtilis to translocate DNA from the mother cell to the prespore. J. Bacteriol. 185:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chary, V. K., P. Xenopoulos, and P. J. Piggot. 2006. Blocking chromosome translocation during sporulation of Bacillus subtilis can result in prespore-specific activation of σG that is independent of σE and of engulfment. J. Bacteriol. 188:7267-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chary, V. K., P. Xenopoulos, and P. J. Piggot. 2007. Expression of the σF-directed csfB locus prevents premature appearance of σG activity during sporulation of Bacillus subtilis. J. Bacteriol. 189:8754-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decatur, A., and R. Losick. 1996. Identification of additional genes under the control of the transcription factor σF of Bacillus subtilis. J. Bacteriol. 178:5039-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driks, A., and R. Losick. 1991. Compartmentalized expression of a gene under the control of sporulation transcription factor σE in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 88:9934-9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubnau, D., and R. Losick. 2006. Bistability in bacteria. Mol. Microbiol. 61:564-572. [DOI] [PubMed] [Google Scholar]
- 10.Eichenberger, P., M. Fujita, S. T. Jensen, E. M. Conlon, D. Z. Rudner, S. T. Wang, C. Ferguson, K. Haga, T. Sato, J. S. Liu, and R. Losick. 2004. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2:e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldar, A., V. K. Chary, P. Xenopoulos, M. E. Fontes, O. C. Loson, J. Dworkin, P. J. Piggot, and M. B. Elowitz. 2009. Partial penetrance facilitates developmental evolution in bacteria. Nature 460:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elowitz, M. B., A. J. Levine, E. D. Siggia, and P. S. Swain. 2002. Stochastic gene expression in a single cell. Science 297:1183-1186. [DOI] [PubMed] [Google Scholar]
- 13.Fawcett, P., A. Melnikov, and P. Youngman. 1998. The Bacillus SpoIIGA protein is targeted to sites of spore septum formation in a SpoIIE-independent manner. Mol. Microbiol. 28:931-943. [DOI] [PubMed] [Google Scholar]
- 14.Fujita, M., and R. Losick. 2002. An investigation into the compartmentalization of the sporulation transcription factor σE in Bacillus subtilis. Mol. Microbiol. 43:27-38. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, M., and R. Losick. 2003. The master regulator for entry into sporulation in Bacillus subtilis becomes a cell-specific transcription factor after asymmetric division. Genes Dev. 17:1166-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gholamhoseinian, A., and P. J. Piggot. 1989. Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J. Bacteriol. 171:5747-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harry, E. J., K. Pogliano, and R. Losick. 1995. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 177:3386-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68:234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmeister, A. E., A. Londono-Vallejo, E. Harry, P. Stragier, and R. Losick. 1995. Extracellular signal protein triggering the proteolytic activation of a developmental transcription factor in B. subtilis. Cell 83:219-226. [DOI] [PubMed] [Google Scholar]
- 20.Iber, D., J. Clarkson, M. D. Yudkin, and I. D. Campbell. 2006. The mechanism of cell differentiation in Bacillus subtilis. Nature 441:371-374. [DOI] [PubMed] [Google Scholar]
- 21.Igoshin, O. A., C. W. Price, and M. A. Savageau. 2006. Signalling network with a bistable hysteretic switch controls developmental activation of the sigma transcription factor in Bacillus subtilis. Mol. Microbiol. 61:165-184. [DOI] [PubMed] [Google Scholar]
- 22.Imamura, D., R. Zhou, M. Feig, and L. Kroos. 2008. Evidence that the Bacillus subtilis SpoIIGA protein is a novel type of signal-transducing aspartic protease. J. Biol. Chem. 283:15287-15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, M., R. Grau, and M. Perego. 2000. Differential processing of propeptide inhibitors of Rap phosphatases in Bacillus subtilis. J. Bacteriol. 182:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ju, J., T. Luo, and W. G. Haldenwang. 1997. Bacillus subtilis Pro-sigmaE fusion protein localizes to the forespore septum and fails to be processed when synthesized in the forespore. J. Bacteriol. 179:4888-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju, J., T. Luo, and W. G. Haldenwang. 1998. Forespore expression and processing of the SigE transcription factor in wild-type and mutant Bacillus subtilis. J. Bacteriol. 180:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmazyn-Campelli, C., L. Rhayat, R. Carballido-Lopez, S. Duperrier, N. Frandsen, and P. Stragier. 2008. How the early sporulation sigma factor σF delays the switch to late development in Bacillus subtilis. Mol. Microbiol. 67:1169-1180. [DOI] [PubMed] [Google Scholar]
- 27.Karow, M. L., P. Glaser, and P. J. Piggot. 1995. Identification of a gene, spoIIR, that links the activation of σE to the transcriptional activity of σF during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 92:2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney, T. J., and C. P. Moran, Jr. 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J. Bacteriol. 169:3329-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenney, T. J., K. York, P. Youngman, and C. P. Moran, Jr. 1989. Genetic evidence that RNA polymerase associated with σA factor uses a sporulation-specific promoter in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 86:9109-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khvorova, A., V. K. Chary, D. W. Hilbert, and P. J. Piggot. 2000. The chromosomal location of the Bacillus subtilis sporulation gene spoIIR is important for its function. J. Bacteriol. 182:4425-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaBell, T. L., J. E. Trempy, and W. G. Haldenwang. 1987. Sporulation-specific sigma factor sigma 29 of Bacillus subtilis is synthesized from a precursor protein, P31. Proc. Natl. Acad. Sci. U. S. A. 84:1784-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Londono-Vallejo, J. A. 1997. Mutational analysis of the early forespore/mother-cell signalling pathway in Bacillus subtilis. Microbiology 143:2753-2761. [DOI] [PubMed] [Google Scholar]
- 33.Londono-Vallejo, J. A., and P. Stragier. 1995. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 9:503-508. [DOI] [PubMed] [Google Scholar]
- 34.Masuda, E. S., H. Anaguchi, T. Sato, M. Takeuchi, and Y. Kobayashi. 1990. Nucleotide sequence of the sporulation gene spoIIGA from Bacillus subtilis. Nucleic Acids Res. 18:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, W. G., and S. E. Lindow. 1997. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191:149-153. [DOI] [PubMed] [Google Scholar]
- 36.Piggot, P. J., and C. A. Curtis. 1987. Analysis of the regulation of gene expression during Bacillus subtilis sporulation by manipulation of the copy number of spo-lacZ fusions. J. Bacteriol. 169:1260-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogliano, K., A. E. Hofmeister, and R. Losick. 1997. Disappearance of the σE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 179:3331-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramamurthi, K. S., and R. Losick. 2009. Negative membrane curvature as a cue for subcellular localization of a bacterial protein. Proc. Natl. Acad. Sci. U. S. A. 106:13541-13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubio, A., and K. Pogliano. 2004. Septal localization of forespore membrane proteins during engulfment in Bacillus subtilis. EMBO J. 23:1636-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudner, D. Z., Q. Pan, and R. M. Losick. 2002. Evidence that subcellular localization of a bacterial membrane protein is achieved by diffusion and capture. Proc. Natl. Acad. Sci. U. S. A. 99:8701-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satola, S., P. A. Kirchman, and C. P. Moran, Jr. 1991. Spo0A binds to a promoter used by σA RNA polymerase during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 88:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steil, L., M. Serrano, A. O. Henriques, and U. Volker. 2005. Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology 151:399-420. [DOI] [PubMed] [Google Scholar]
- 43.Stragier, P., C. Bonamy, and C. Karmazyn-Campelli. 1988. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell 52:697-704. [DOI] [PubMed] [Google Scholar]
- 44.Stragier, P., B. Kunkel, L. Kroos, and R. Losick. 1989. Chromosomal rearrangement generating a composite gene for a developmental transcription factor. Science 243:507-512. [DOI] [PubMed] [Google Scholar]
- 45.Trempy, J. E., J. Morrison-Plummer, and W. G. Haldenwang. 1985. Synthesis of sigma 29, an RNA polymerase specificity determinant, is a developmentally regulated event in Bacillus subtilis. J. Bacteriol. 161:340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, S. T., B. Setlow, E. M. Conlon, J. L. Lyon, D. Imamura, T. Sato, P. Setlow, R. Losick, and P. Eichenberger. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 358:16-37. [DOI] [PubMed] [Google Scholar]
- 47.Wu, L. J., and J. Errington. 1998. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol. Microbiol. 27:777-786. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, L., M. L. Higgins, P. J. Piggot, and M. L. Karow. 1996. Analysis of the role of prespore gene expression in the compartmentalization of mother cell-specific gene expression during sporulation of Bacillus subtilis. J. Bacteriol. 178:2813-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zupancic, M. L., H. Tran, and A. E. Hofmeister. 2001. Chromosomal organization governs the timing of cell type-specific gene expression required for spore formation in Bacillus subtilis. Mol. Microbiol. 39:1471-1481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.