Abstract
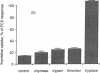
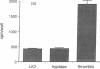
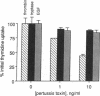
Mast cells appear to promote fibroblast proliferation, presumably through secretion of growth factors, although the molecular mechanisms underlying this mitogenic potential have not been explained fully by known mast cell-derived mediators. We report here that tryptase, a trypsin-like serine proteinase of mast cell secretory granules, is a potent mitogen for fibroblasts in vitro. Nanomolar concentrations of dog tryptase strongly stimulate thymidine incorporation in Chinese hamster lung and Rat-1 fibroblasts and increase cell density in both subconfluent and confluent cultures of these cell lines. Tryptase-induced cell proliferation appears proteinase-specific, as this response is not mimicked by pancreatic trypsin or mast cell chymase. In addition, low levels of tryptase markedly potentiate DNA synthesis stimulated by epidermal growth factor, basic fibroblast growth factor, or insulin. Inhibitors of catalytic activity decrease the mitogenic capacity of tryptase, suggesting, though not proving, the participation of the catalytic site in cell activation by tryptase. Differences in Ca++ mobilization and sensitivity to pertussis toxin suggest that tryptase and thrombin activate distinct signal transduction pathways in fibroblasts. These data implicate mast cell tryptase as a potent, previously unrecognized fibroblast growth factor, and may provide a molecular link between mast cell activation and fibrosis.
Full text
PDF

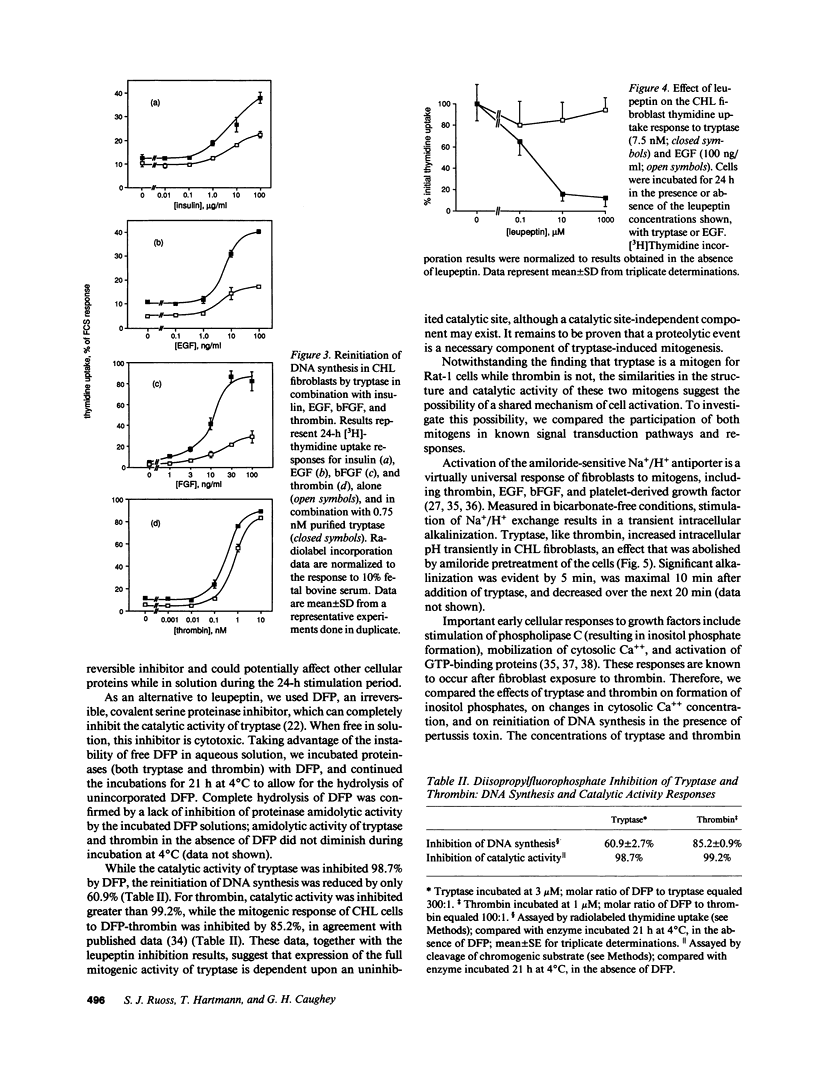
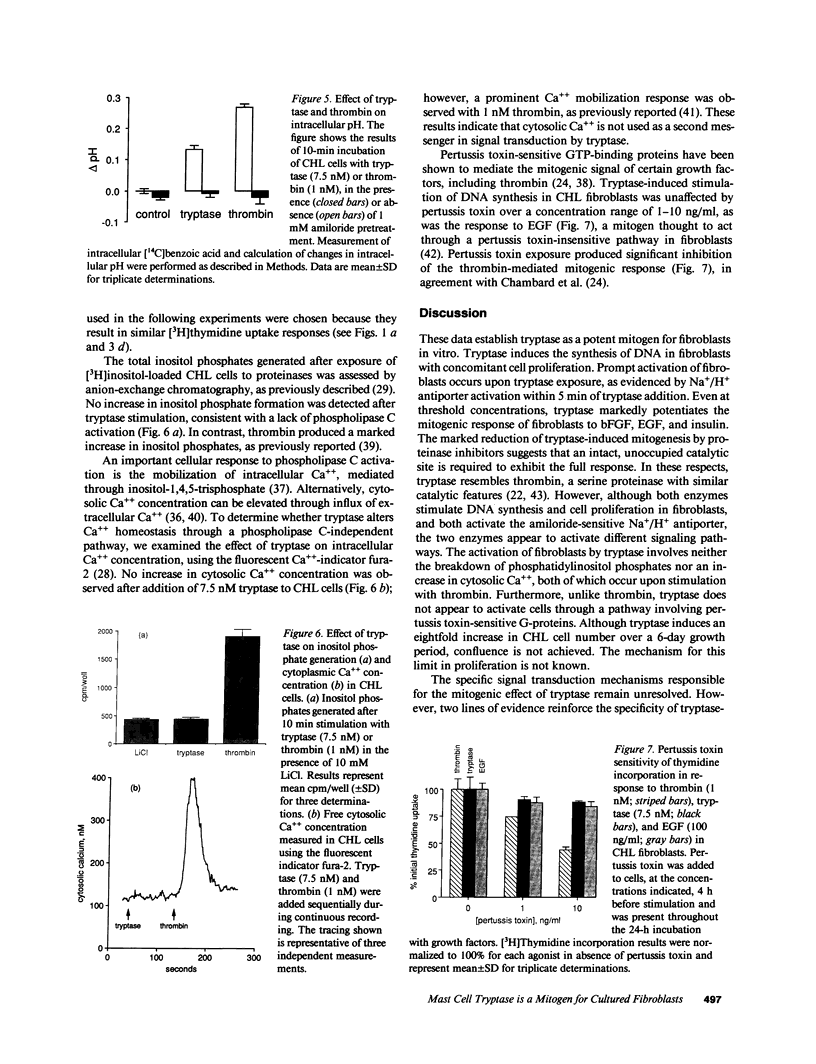


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter S. C., Kramps J. A., Janoff A., Schwartz L. B. Interactions of human mast cell tryptase with biological protease inhibitors. Arch Biochem Biophys. 1990 Jan;276(1):26–31. doi: 10.1016/0003-9861(90)90005-j. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R., Benezra M., Eldor A., Hy-Am E., Fenton J. W., 2nd, Wilner G. D., Vlodavsky I. Thrombin immobilized to extracellular matrix is a potent mitogen for vascular smooth muscle cells: nonenzymatic mode of action. Cell Regul. 1990 May;1(6):453–463. doi: 10.1091/mbc.1.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A. J., Mann K. G., Wilner G. D. Identification of a thrombin sequence with growth factor activity on macrophages. Proc Natl Acad Sci U S A. 1986 Feb;83(4):976–980. doi: 10.1073/pnas.83.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Cell surface action of thrombin is sufficient to initiate division of chick cells. Cell. 1978 Aug;14(4):811–823. doi: 10.1016/0092-8674(78)90337-9. [DOI] [PubMed] [Google Scholar]
- Caughey G. H., Lazarus S. C., Viro N. F., Gold W. M., Nadel J. A. Tryptase and chymase: comparison of extraction and release in two dog mastocytoma lines. Immunology. 1988 Feb;63(2):339–344. [PMC free article] [PubMed] [Google Scholar]
- Caughey G. H., Viro N. F., Lazarus S. C., Nadel J. A. Purification and characterization of dog mastocytoma chymase: identification of an octapeptide conserved in chymotryptic leukocyte proteinases. Biochim Biophys Acta. 1988 Jan 29;952(2):142–149. doi: 10.1016/0167-4838(88)90109-4. [DOI] [PubMed] [Google Scholar]
- Caughey G. H., Viro N. F., Ramachandran J., Lazarus S. C., Borson D. B., Nadel J. A. Dog mastocytoma tryptase: affinity purification, characterization, and amino-terminal sequence. Arch Biochem Biophys. 1987 Nov 1;258(2):555–563. doi: 10.1016/0003-9861(87)90377-8. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. On scleroderma. Mast cells, endothelial cells, and fibroblasts. JAMA. 1989 Sep 1;262(9):1206–1209. doi: 10.1001/jama.262.9.1206. [DOI] [PubMed] [Google Scholar]
- Cox S. W., Eley B. M. Tryptase-like activity in crevicular fluid from gingivitis and periodontitis patients. J Periodontal Res. 1989 Jan;24(1):41–44. doi: 10.1111/j.1600-0765.1989.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Franzén, Norrby K. Local mitogenic effect of tissue mast cell secretion. Cell Tissue Kinet. 1980 Nov;13(6):635–642. doi: 10.1111/j.1365-2184.1980.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Goto T., Befus D., Low R., Bienenstock J. Mast cell heterogeneity and hyperplasia in bleomycin-induced pulmonary fibrosis of rats. Am Rev Respir Dis. 1984 Nov;130(5):797–802. doi: 10.1164/arrd.1984.130.5.797. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hartmann T., Seuwen K., Roussel M. F., Sherr C. J., Pouysségur J. Functional expression of the human receptor for colony-stimulating factor 1 (CSF-1) in hamster fibroblasts: CSF-1 stimulates Na+/H+ exchange and DNA-synthesis in the absence of phosphoinositide breakdown. Growth Factors. 1990;2(4):289–300. doi: 10.3109/08977199009167024. [DOI] [PubMed] [Google Scholar]
- Jordana M., Befus A. D., Newhouse M. T., Bienenstock J., Gauldie J. Effect of histamine on proliferation of normal human adult lung fibroblasts. Thorax. 1988 Jul;43(7):552–558. doi: 10.1136/thx.43.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Fulmer J. D., Crystal R. G. Ultrastructure of pulmonary mast cells in patients with fibrotic lung disorders. Lab Invest. 1979 Jun;40(6):717–734. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- L'Allemain G., Seuwen K., Velu T., Pouyssegur J. Signal transduction in hamster fibroblasts overexpressing the human EGF receptor. Growth Factors. 1989;1(4):311–321. doi: 10.3109/08977198909000255. [DOI] [PubMed] [Google Scholar]
- Leroy E. C., Smith E. A., Kahaleh M. B., Trojanowska M., Silver R. M. A strategy for determining the pathogenesis of systemic sclerosis. Is transforming growth factor beta the answer? Arthritis Rheum. 1989 Jul;32(7):817–825. [PubMed] [Google Scholar]
- Magnaldo I., L'Allemain G., Chambard J. C., Moenner M., Barritault D., Pouysségur J. The mitogenic signaling pathway of fibroblast growth factor is not mediated through polyphosphoinositide hydrolysis and protein kinase C activation in hamster fibroblasts. J Biol Chem. 1986 Dec 25;261(36):16916–16922. [PubMed] [Google Scholar]
- Marks R. M., Roche W. R., Czerniecki M., Penny R., Nelson D. S. Mast cell granules cause proliferation of human microvascular endothelial cells. Lab Invest. 1986 Sep;55(3):289–294. [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Possible involvement of a GTP-binding protein, the substrate of islet-activating protein, in receptor-mediated signaling responsible for cell proliferation. J Biol Chem. 1987 Sep 15;262(26):12463–12467. [PubMed] [Google Scholar]
- Norrby K., Enerbäck L., Franzén L. Mast cell activation and tissue cell proliferation. Cell Tissue Res. 1976 Jul 30;170(3):289–303. doi: 10.1007/BF00219412. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Malgaroli A., Meldolesi J., Vicentini L. M. EGF raises cytosolic Ca2+ in A431 and Swiss 3T3 cells by a dual mechanism. Redistribution from intracellular stores and stimulated influx. Exp Cell Res. 1987 May;170(1):175–185. doi: 10.1016/0014-4827(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Raben D. M., Cunningham D. D. Effects of EGF and thrombin on inositol-containing phospholipids of cultured fibroblasts: stimulation of phosphatidylinositol synthesis by thrombin but not EGF. J Cell Physiol. 1985 Dec;125(3):582–590. doi: 10.1002/jcp.1041250330. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Schechter N. M., Choi J. K., Slavin D. A., Deresienski D. T., Sayama S., Dong G., Lavker R. M., Proud D., Lazarus G. S. Identification of a chymotrypsin-like proteinase in human mast cells. J Immunol. 1986 Aug 1;137(3):962–970. [PubMed] [Google Scholar]
- Scher W. The role of extracellular proteases in cell proliferation and differentiation. Lab Invest. 1987 Dec;57(6):607–633. [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986 Jun 5;261(16):7372–7379. [PubMed] [Google Scholar]
- Schwartz L. B., Irani A. M., Roller K., Castells M. C., Schechter N. M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987 Apr 15;138(8):2611–2615. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Seldin D., Austen K. F. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981 Apr;126(4):1290–1294. [PubMed] [Google Scholar]
- Schwartz L. B. Mediators of human mast cells and human mast cell subsets. Ann Allergy. 1987 Apr;58(4):226–235. [PubMed] [Google Scholar]
- Sekizawa K., Caughey G. H., Lazarus S. C., Gold W. M., Nadel J. A. Mast cell tryptase causes airway smooth muscle hyperresponsiveness in dogs. J Clin Invest. 1989 Jan;83(1):175–179. doi: 10.1172/JCI113855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Hougland M. W., Johnson D. A. Human lung tryptase. Purification and characterization. J Biol Chem. 1984 Sep 10;259(17):11046–11051. [PubMed] [Google Scholar]
- Takizawa H., Ohta K., Hirai K., Misaki Y., Horiuchi T., Kobayashi N., Shiga J., Miyamoto T. Mast cells are important in the development of hypersensitivity pneumonitis. A study with mast-cell-deficient mice. J Immunol. 1989 Sep 15;143(6):1982–1988. [PubMed] [Google Scholar]
- Trabucchi E., Radaelli E., Marazzi M., Foschi D., Musazzi M., Veronesi A. M., Montorsi W. The role of mast cells in wound healing. Int J Tissue React. 1988;10(6):367–372. [PubMed] [Google Scholar]
- Wagner M. M., Edwards R. E., Moncrieff C. B., Wagner J. C. Mast cells and inhalation of asbestos in rats. Thorax. 1984 Jul;39(7):539–544. doi: 10.1136/thx.39.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Watanabe K., Oishi T., Aiba M., Kageyama K. Mast cells in the rat alveolar septa undergoing fibrosis after ionizing irradiation. Ultrastructural and histochemical studies. Lab Invest. 1974 Nov;31(5):555–567. [PubMed] [Google Scholar]
- Witting J. I., Pouliott C., Catalfamo J. L., Fareed J., Fenton J. W., 2nd Thrombin inhibition with dipeptidyl argininals. Thromb Res. 1988 May 15;50(4):461–467. doi: 10.1016/0049-3848(88)90195-8. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Chen L. B., Buchanan J. M. Effects of protease treatment on growth, morphology, adhesion, and cell surface proteins of secondary chick embryo fibroblasts. Cell. 1976 Mar;7(3):407–412. doi: 10.1016/0092-8674(76)90170-7. [DOI] [PubMed] [Google Scholar]