Abstract
LolA accommodates the acyl chains of lipoproteins in its hydrophobic cavity and shuttles between the inner and outer membranes through the hydrophilic periplasm to place lipoproteins in the outer membrane. The LolA(I93C/F140C) derivative, in which Cys replaces Ile at position 93 and Phe at position 140, strongly inhibited growth in the absence of a reducing agent because of the lethal intramolecular disulfide bond between the two Cys residues. Expression of I93C/F140C was found to activate the Cpx two-component system, which responds to cell envelope stress. The inhibition of growth by I93C/F140C was partly suppressed by overproduction of LolCDE, which is an ATP-binding cassette transporter and mediates the transfer of lipoproteins from the inner membrane to LolA. A substantial portion of the oxidized form, but not the reduced one, of I93C/F140C expressed on LolCDE overproduction was recovered in the membrane fraction, whereas wild-type LolA was localized in the periplasm even when LolCDE was overproduced. Moreover, LolCDE overproduction stabilized I93C/F140C and therefore caused an increase in its level. Taken together, these results indicate that oxidized I93C/F140C stably binds to LolCDE, which causes strong envelope stress.
There are more than 90 different species of lipoproteins in the Escherichia coli envelope, most of which are localized on the periplasmic side of the outer membrane (29, 31) They each have an N-terminal cysteine covalently modified with three acyl chains, and are anchored to membranes via these lipid tails (25). Although some of these proteins have been shown to be involved in important cellular processes, such as biogenesis of the outer membrane (1, 14, 24, 35), drug transport (11), and signal transduction (7), the functions of the majority of them remain unknown. The Lol system, composed of five Lol proteins, is required for the sorting and targeting of outer membrane-specific lipoproteins (30).
Lipoprotein precursors are sequentially processed to their mature forms on the periplasmic side of the inner membrane after their translocation across the inner membrane by Sec translocon (21). Those destined for the outer membrane then each form a complex with LolCDE (36), a member of ATP-binding cassette (ABC) transporter family, in the inner membrane. LolA, a periplasmic lipoprotein-specific carrier, receives lipoproteins from LolCDE in an ATP hydrolysis-dependent manner and forms a water-soluble complex with a lipoprotein (13). The complex then traverses the periplasmic space from the inner to the outer membrane, where lipoproteins are transferred from LolA to a lipoprotein receptor, LolB (14), in a mouth-to-mouth manner (20). Finally, lipoproteins are anchored to the outer membrane through the action of LolB (32).
LolA is composed of 11 antiparallel β-sheets and 3 α-helices, which form an incomplete β-barrel structure with a lid covering the barrel (28). The cavity formed inside the barrel is hydrophobic and is considered to be the binding site for the acyl chains of lipoproteins. To elucidate the role of the opening and closing of the hydrophobic cavity in lipoprotein transfer reactions, the LolA(I93C/F140C) mutant, in which cysteine replaces Ile93 in the α2 helix and Phe140 in the β10 strand, was previously constructed (34). In I93C/F140C expressed in the periplasm, an intramolecular disulfide bond was formed between the two cysteine residues. This oxidized form of I93C/F140C was fixed in the closed conformation and was unable to release lipoproteins from the inner membrane, suggesting that opening of the cavity is crucial for the LolA function (34). Biochemical studies subsequently showed that the LolA cavity indeed undergoes opening and closing upon the binding and release of lipoproteins, respectively (19). Moreover, it was found that only the closed form of LolA is active in the lipoprotein release reaction.
I93C/F140C exhibited the strongest growth inhibition among the LolA mutants so far isolated, although it was fully active in the presence of a reducing agent (34). We show here that oxidized I93C/F140C strongly activates the Cpx two-component system (23) that responds to cell envelope stress, whereas overproduction of LolCDE partly suppresses the toxic effect of I93C/F140C.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
An ara+ revertant of MC4100 [Δ(argF-lac)U169 araD139 rpsL150 thiA1 relA1 flbB5301 deoC1 ptsF25 rbsR] (2) selected on a MacConkey arabinose plate was used as the wild-type strain, MY201. The cpx::spc allele was transferred by P1 transduction from PAD299 [MC4100 λRS88 (degP′-lacZ+) cpxR::spc] (17) to MY201, resulting in MY202. C43(DE3) (16) was used for experiments involving overexpression of LolCDE under the control of the T7 promoter. Plasmid pDegP-LacZ, carrying the degP-lacZ fusion gene, was described previously (17). Plasmids pSW77 and pSWIF, which encode the wild-type LolA tagged with FLAG at the C terminus and the I93C/F140C mutant, respectively, were described previously (34). Plasmid pCOLA-CDE, which carries a DNA fragment encoding LolC with a His tag at the N terminus and LolDE under the control of the T7 promoter, was constructed as follows. The DNA fragment encoding the N-terminal half of LolC was amplified by PCR with a pair of oligonucleotides as primers (C3Sal [GAGTCGACTTTCAGCGGCTCATCCAGCCAC] and C5Bam [CAGGATCCGATGTACCAACCTGTCGCTCTA] and pKM401 (12) as a template. The amplified fragment was digested with BamHI and SalI and then cloned into the same site of pCOLA Duet-1 (Novagen). The resulting plasmid was digested with SalI and PstI and then ligated with the SalI-PstI fragment of pKM401, encoding the C-terminal half of LolC and the entire LolD and LolE. This plasmid encodes LolCDE under the control of PT7 lac.
Cells were grown in LB medium at 37°C with aeration. When required, chloramphenicol and kanamycin were added to a final concentration of 25 μg/ml each. Ampicillin was added to 100 μg/ml.
Western blotting.
SDS-PAGE was carried out as described by Laemmli (10), and proteins were transferred to polyvinylidene difluoride membranes for immunodetection. Anti-FLAG antibodies were obtained from Sigma. Anti-LolC (37), -LolD (18), and -LolE (37) antisera were described previously. Anti-DegP antiserum was kindly provided by S. Matsuyama (Rikkyo University, Japan). Western blots were visualized with LAS4000, and bands were quantitated using Multi Gauge software.
Other methods.
Total membranes were prepared as described previously (9). β-Galactosidase activity was measured as described by Miller (15). The growth of cells was monitored by measuring the optical density at 660 nm (OD660) of the culture.
RESULTS
LolA(I93C/F140C) inhibits growth and activates the Cpx envelope stress response system.
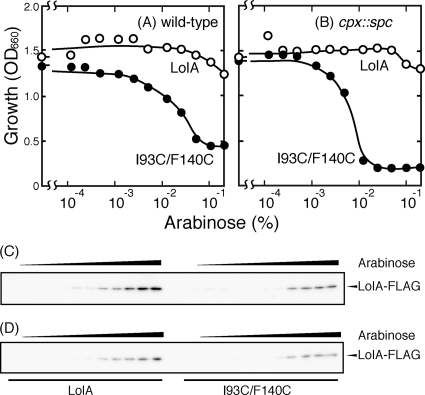
E. coli cells were transformed with a plasmid encoding the wild-type LolA or I93C/F140C. The cells were grown to the early logarithmic phase of growth, and then various concentrations of arabinose were added to induce the expression of LolA and I93C/F140C controlled by the ara promoter. As observed previously (34), expression of I93C/F140C inhibited the growth of MY201 cells, depending on the arabinose concentration, even though chromosomally encoded wild-type LolA was always expressed (Fig. 1 A). Arabinose caused growth inhibition at a concentration of as low as 0.005%, and maximal growth inhibition was observed with 0.1% arabinose. The growth inhibition nearly paralleled the amount of I93C/F140C determined by Western blotting with an anti-FLAG antibody (Fig. 1C).
FIG. 1.
Growth inhibition by LolA(I93C/F140C). (A and B) E. coli MY201 (wild type) (A) and MY202 (cpx::spc) (B) cells harboring either pSW77, which encodes wild-type LolA (open circles), or pSWIF, which encodes LolA(I93C/F140C) (closed circles), were grown to the early logarithmic phase of growth (OD660 = 0.2), and then the expression of FLAG-tagged LolA proteins was induced for 3 h by the addition of various concentrations of arabinose. Cellular growth was then measured as OD660. (C and D) Cell lysates were prepared from MY201 (C) and MY202 (D) cells and examined by SDS-PAGE and Western blotting with anti-FLAG peptide antibodies. The amounts of cell lysates examined for LolA(I93C/F140C) were five times higher than those examined for wild-type LolA because the mutant protein was unstable and expressed at a low level.
The envelope stress response is one of the global stress responses induced under various conditions that disturb the biogenesis and maintenance of this compartment (23). When cells lacked the Cpx two-component stress response system, growth inhibition by I93C/F140C was more significant (Fig. 1B), while overproduction of wild-type LolA had little inhibitory effect, suggesting that I93C/F140C induces the stress response and that Cpx-regulated factors alleviate this toxic effect of I93C/F140C.
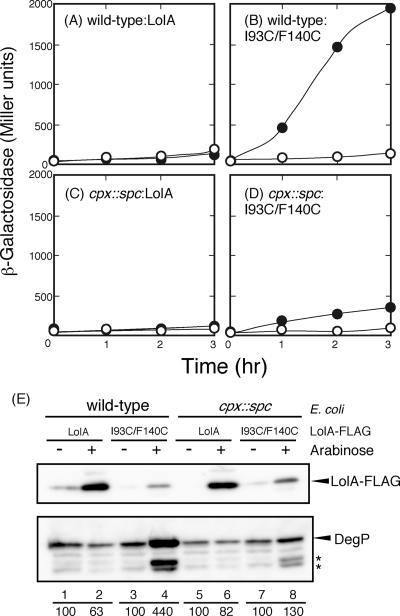
The degP gene is under control of the two major regulatory systems for the stress response, the CpxAR two-component system and σE (5). Expression of DegP, which is a periplasmic chaperone/protease, is induced by various forms of envelope stress and is essential for growth at high temperature, indicating that DegP is important for counteracting the stress conditions in the periplasm. We therefore examined whether or not I93C/F140C expression induces degP expression by measuring the activity of β-galactosidase encoded by the degP promoter-lacZ fusion gene (17).
The wild-type strain exhibited an increase in β-galactosidase activity upon I93C/F140C expression (Fig. 2 B). In contrast, expression of I93C/F140C only marginally increased the β-galactosidase activity in the cpxR::spc strain (Fig. 2D). Induction of the wild-type LolA did not increase the β-galactosidase activity whether the Cpx two-component system was present or not (Fig. 2A and C).
FIG. 2.
Induction of degP expression by LolA(I93C/F140C). (A to D) MY201 (wild type) (A and B) and MY202 (cpx::spc) (C and D) cells harboring either pSW77 (A and C) or pSWIF (B and D) were transformed with pDegP-LacZ carrying the degP-lacZ fusion gene. The cells were grown to the early logarithmic phase of growth (OD660 = 0.2), and then the expression of LolA proteins was induced with 0.2% arabinose at zero time (closed circles) or was not induced (open circles). Samples were taken periodically to measure β-galactosidase activity as described previously (15). (E) Cell lysates were prepared at 2 h after the addition of arabinose, and equivalent amounts were analyzed by Western blotting with anti-FLAG peptide and anti-DegP antisera. Bands (asterisks) migrating faster than that of DegP most likely represent its self-cleaved forms (8). The amounts of intact DegP were densitometrically determined and expressed as percentages, taking the amount of DegP in the absence of arabinose as 100.
The levels of the DegP and LolA proteins were determined by Western blotting with anti-DegP and anti-FLAG antisera, respectively (Fig. 2E). Expression of I93C/F140C in wild-type cells increased the level of DegP (lane 4), although the amount of I93C/F140C was smaller than that of wild-type LolA (lane 2). Disruption of the Cpx two-component system significantly reduced the level of DegP induced by I93C/F140C (lane 8). Taken together, these results indicate that the oxidized form of I93C/F140C strongly induces the Cpx two-component system.
Overproduction of LolCDE partly suppresses the toxic effect of LolA(I93C/F140C).
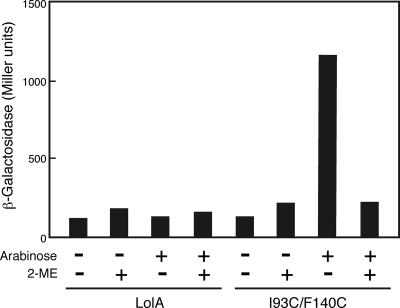
We previously showed that the growth inhibition by I93C/F140C was abolished when the disulfide bond between Cys93 and Cys140 was reduced with 2-mercaptoethanol (34), indicating that only the oxidized form of I93C/F140C is toxic. β-Galactosidase activity was determined after expression of I93C/F140C in the presence and absence of 2-mercaptoethanol (Fig. 3). Consistent with the previous observation, expression of β-galactosidase was induced only by the oxidized form of I93C/F140C.
FIG. 3.
Effect of 2-mercapthoethanol on the induction of degP. Wild-type cells harboring a plasmid carrying the degP-lacZ fusion gene and either pSW77 or pSWIF were grown to the early logarithmic phase of growth (OD660 = 0.2), and then the expression of plasmid-encoded LolA proteins was induced with 0.2% arabinose in the presence and absence of 20 mM 2-mercapthoethanol (2-ME) for 2 h. β-Galactosidase activity was then measured as described for Fig. 2A.
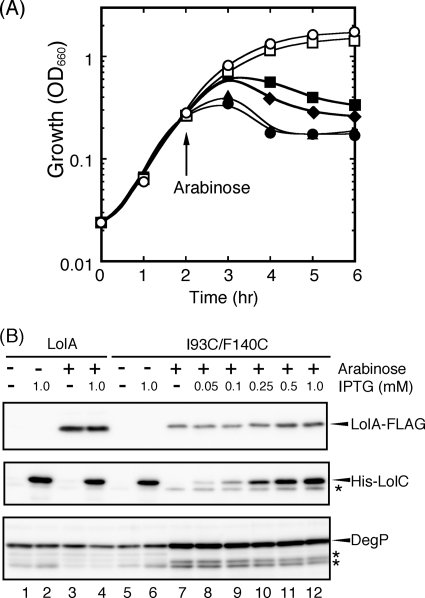
I93C/F140C exhibited a dominant-negative phenotype (34), suggesting that it is partly functional, i.e., that it can interact with LolCDE but cannot bind lipoproteins. If this is the case, overproduction of LolCDE might neutralize the toxicity of I93C/F140C. To express LolCDE together with I93C/F140C encoded by pSWIF, the lolCDE genes were subcloned into pCOLA Duet-1, followed by transformation into C43(DE3) cells carrying pSWIF. The genes encoding I93C/F140C and LolCDE are under the control of the ara and T7 lac promoters, respectively, in this strain. Expression of I93C/F140C alone inhibited the growth of this strain, as observed with other strains. When LolCDE was expressed prior to the induction of I93C/F140C, the growth inhibition was partially alleviated, depending on the IPTG (isopropyl-β-d-thiogalactopyranoside) concentration (Fig. 4 A). The oxidized form of I93C/F140C is unstable and is expressed at only a low level (34). On the other hand, the amount of I93C/F140C was significantly increased with the cooverexpression of LolCDE (Fig. 4B). When the amount of LolCDE in the inner membrane reached the maximum at 1 mM IPTG, the amount of I93C/F140C was also maximum. Although overexpression of LolCDE partly suppressed the toxic effect of I93C/F140C, the level of DegP remained high (Fig. 4B), presumably because the suppression of toxicity was incomplete.
FIG. 4.
LolCDE overexpression suppresses the growth inhibition by LolA(I93C/F140C). (A) An overnight culture of C43(DE3) cells harboring both pSWIF (I93C/F140C) and pCOLA-CDE (LolCDE) was diluted 200-fold with fresh medium containing 0 (closed circles), 0.05 (closed triangles), 0.25 (closed diamonds), or 1 (closed squares) mM IPTG, followed by cultivation for 2 h. Arabinose (0.2%) was then added to induce I93C/F140C. As controls, growth in the absence of arabinose with (open squares) or without (open circles) 1 mM IPTG was also examined. (B) Total cell lysates were prepared from equivalent amounts of cells at the end of the experiments shown in panel A and then subjected to SDS-PAGE and Western blotting with anti-FLAG peptide and anti-LolC and anti-DegP antisera. The band indicated by an asterisk below the His-LolC band represents chromosomally encoded wild-type LolC, and those indicated by asterisks below DegP represent those mentioned in the legend to Fig. 2E. As a control, C43(DE3) cells harboring pSW77 (wild-type LolA) and pCOLA-CDE (LolCDE) were also examined as indicated.
Membrane localization of LolA(I93C/F140C).
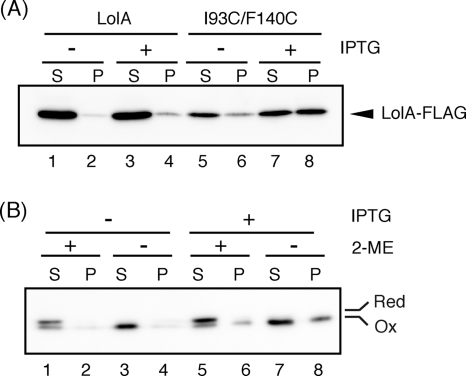
It was shown recently by means of in vivo photo-cross-linking that the region around the entrance of the hydrophobic cavity of LolA physically interacts with LolC (20). It therefore seemed likely that the toxic effect of I93C/F140C is suppressed by the formation of the I93C/F140C-LolCDE complex. If this is the case, I93C/F140F might be cofractionated with the inner membrane containing LolCDE, whereas wild-type LolA is always recovered in the periplasmic fraction. Localization of I93C/F140C was examined in the presence and absence of LolCDE overexpression (Fig. 5). A total cell lysate of C43(DE3)/pCOLA-CDE cells harboring pSW77 or pSWIF was fractionated into total membranes and soluble fractions by ultracentrifugation, which were then analyzed by SDS-PAGE and Western blotting using anti-FLAG antibodies. Most wild-type LolA molecules were recovered in the soluble fraction irrespective of LolCDE overproduction (Fig. 5A, lanes 1 to 4). In marked contrast, when LolCDE was overproduced, the total amount of I93C/F140C was increased and a substantial amount of I93C/F140C was found in the membrane fraction (lanes 7 and 8).
FIG. 5.
Localization of LolA(I93C/F140C). (A) C43(DE3)/pCOLA-CDE cells were transformed with either pSW77 or pSWIF. An overnight culture of the cells was diluted 200-fold with fresh medium containing or not containing 1 mM IPTG, followed by incubation for 2 h. Plasmid-encoded LolA was then induced with 0.2% arabinose for 3 h. Lysates were prepared by sonication from the same amounts of cells and fractionated into supernatant (S) and total membrane (P) fractions by centrifugation at 10,000 × g for 30 min. Equivalent amounts of fractions were analyzed by SDS-PAGE and immunoblotting with anti-FLAG antibodies. (B) Overnight-grown C43(DE3)/pCOLA-CDE/pSWIF cells were incubated in the presence and absence of 1 mM IPTG for 2 h as for panel A. Expression of I93C/F140C was induced with 0.2% arabinose in the presence of 10 mM 2-mercapthoethanol for 3 h. Cells were then collected by centrifugation and washed twice with phosphate-buffered saline to remove the 2-mercapthoethanol. The cells were resuspended in the original volume of fresh medium and then divided into two portions; one portion was treated with 10 mM 2-mercapthoethanol, while the other was not. After a further 30 min of incubation with aeration, cell lysates were prepared from both cultures and fractionated as for panel A. Proteins were analyzed by SDS-PAGE without a reducing agent to separate the oxidized and reduced forms of I93C/F140C, followed by analysis by immunoblotting with anti-FLAG antibodies.
The effect of the redox state on the membrane localization of I93C/F140C was further examined (Fig. 5B). Cells were grown in the presence of 2-mercapthoethanol, and then the expression of I93C/F140C was induced. When the OD660 of the culture reached 1.0, cells were collected and washed to remove the 2-mercapthoethanol. The washed cells were then incubated for 30 min with or without 2-mercapthoethanol. The localization of I93C/F140C was then examined as for Fig. 4A. SDS-PAGE was carried out without a reducing agent. The cell fractionation procedures did not affect the redox state of I93C/F140C (data not shown). When cells were incubated in the presence of 2-mercaptoethanol, most I93C/F140 molecules were present in a reduced form, which migrated more slowly than the oxidized form on SDS-PAGE and was recovered exclusively in the soluble fraction, as observed with wild-type LolA (Fig. 5B, lanes 1 and 5). On the other hand, when cells were incubated for 30 min in the absence of 2-mercaptoethanol, I93C/F140C existed only in the oxidized form (lanes 3, 7, and 8). Overproduction of LolCDE increased the total amount of I93C/F140C but did not affect the redox state of I93C/F140C. However, a significant amount of oxidized I93C/F140C was recovered in the membrane fraction when LolCDE was overproduced (lane 8). A small fraction of I93C/F140C existed in the oxidized form even in the presence of 2-mercaptoethanol and was recovered in membrane fraction (lane 6), while the reduced form of I93C/F140C was always recovered in the soluble fraction. Taken together, these results indicate that only oxidized LolA(I93C/F140C) forms a stable complex with LolCDE.
DISCUSSION
E. coli cells respond to envelope stress and show changes in the expression of various “stress genes,” including those that encode DegP, PpiA, and DsbA (23). Both the Cpx and σE regulons function to maintain cell envelope integrity (23). It is known that activation of the σE regulon is initiated by the binding of the C-terminal peptide derived from β-barrel outer membrane proteins to the PDZ domain of DegS, causing the cleavage of anti-σE factor RseA (33). Although a number of inducing cues have been identified, the precise molecular signals that activate the Cpx regulon are not well understood. It has been reported that overproduction of pilus components such as PapE, PapG, and BfpA activates the Cpx system, suggesting that misfolded pilus component proteins are direct signals for Cpx activation (23). It was thought that the σE pathway monitors the biogenesis of β-barrel outer membrane proteins, while the Cpx system monitors the biogenesis of cell surface structures (23). However, it was recently reported that the Cpx regulon also plays an important role in the response to misassembly of β-barrel outer membrane proteins (6). The detailed molecular events in the network of envelope stress response systems, including σE, Cpx, and Bae (22) regulons, remain to be elucidated.
Stress caused by disulfide bond formation has been reported. CastilloKeller and Misra reported that the introduction of a disulfide bond into OmpC was lethal in cells lacking DegP (3). This lethal effect was abolished by the expression of DegP even if it lacked proteolytic activity, because the misfolded OmpC derivative was captured by the DegP mutant. Slamti and Waldor found that the formation of an aberrant disulfide bond in the periplasm induced the Cpx pathway of Vibrio cholerae (26). These observations indicate that an aberrant structure formed in the periplasm generally induces the Cpx pathway. Based on these data, it might be possible that the oxidized structure of I93C/F140C itself induces the Cpx pathway. On the other hand, the LolA function is essential for the outer membrane sorting of lipoproteins, including essential LolB (14), BamD (formerly YfiO) (1), and LptE (35), which are required for the outer membrane sorting of lipoproteins, β-barrel proteins, and lipopolysaccharide, respectively. Therefore, defects in the sorting of outer membrane components might function as signals for Cpx activation. We previously reported that overproduction of inner membrane lipoprotein YafY (17) activates the Cpx system, as that of outer membrane lipoprotein NlpE (4, 27) does. Accumulation of mislocalized lipoproteins in the inner membrane could therefore be the reason for the Cpx activation. It should be noted that the toxic effect of oxidized I93C/F140C was partially suppressed by overproduction of LolCDE (Fig. 4A) even though the total amount of I93C/F140C significantly increased with LolCDE overexpression (Fig. 4B). These results suggest that the formation of a nonfunctional LolCDE-I93C/F140C complex is lethal to E. coli and causes envelope stress. We have not previously observed the activation of stress response systems by defects in the lipoprotein-sorting pathway. It is important to reveal what is critical for activation of the stress response system.
It seems likely that the toxic I93C/F140C is neutralized by LolCDE, as in the case of the lethal OmpC derivative captured by DegP. Indeed, the proportion of LolA(I93C/F40C) molecules recovered in the membrane fraction remarkably increased with LolCDE overproduction, whereas that of wild-type LolA was little affected (Fig. 5A). Taken together, these results indicate that the oxidized form of I93C/F140C more strongly binds to LolCDE. It was recently found by means of an in vivo photo-cross-linking technique that LolA specifically interacts with LolC but not LolE (20), despite their similar structures (37). It therefore seems likely that I93C/F140C binds to the LolC subunit of LolCDE. However, suppression of the toxic effect of oxidized I93C/F140C by overproduction of LolC alone could not be examined because it is highly toxic (data not shown). Moreover, a possible LolCDE-I93C/F140C complex was not detected after solubilization of membranes with a detergent (data not shown), suggesting that the interaction between I93C/F140C and LolCDE is not strong enough to maintain the complex in a detergent solution.
The crystal structures of LolA and LolB are very similar to each other (28). Both proteins have a hydrophobic cavity, which is formed by an incomplete β-barrel and covering loops. The hydrophobic cavity undergoes opening and closing upon the binding and release of lipoproteins, respectively (19). However, the functions of the two proteins are clearly different. LolA interacts with LolCDE (20) but cannot target the lipid phase, whereas lipid targeting is a critical function of LolB (32). Binding of I93C/F140C to LolCDE most likely represents the physiological function, but the LolA derivative remains bound to LolCDE because of the fixed closed structure. I93C/F140C is an interesting LolA derivative for clarification of the detailed molecular events at the step of lipoprotein release from the inner membrane.
Acknowledgments
This work was supported by grants to H.T. from the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
Published ahead of print on 27 August 2010.
REFERENCES
- 1.Bos, M. P., V. Robert, and J. Tommassen. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61:191-214. [DOI] [PubMed] [Google Scholar]
- 2.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 3.CastilloKeller, M., and R. Misra. 2003. Protease-deficient DegP suppresses lethal effects of a mutant OmpC protein by its capture. J. Bacteriol. 185:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese, P. N., and T. J. Silhavy. 1997. The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 5.Erickson, J. W., and C. A. Gross. 1989. Identification of the σE subunit of Escherichia coli RNA polymerase: a second alternate σ factor involved in high-temperature gene expression. Genes Dev. 3:1462-1471. [DOI] [PubMed] [Google Scholar]
- 6.Gerken, H., O. P. Leiser, D. Bennion, and R. Misra. 2010. Involvement and necessity of the Cpx regulon in the event of aberrant β-barrel outer membrane protein assembly. Mol. Microbiol. 75:1033-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagiwara, D., M. Sugiura, T. Oshima, H. Mori, H. Aiba, T. Yamashino, and T. Mizuno. 2003. Genome-wide analyses revealing a signaling network of the RcsC-YojN-RcsB phosphorelay system in Escherichia coli. J. Bacteriol. 185:5735-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jomaa, A., J. Iwanczyk, J. Tran, and J. Ortega. 2009. Characterization of the autocleavage process of the Escherichia coli HtrA protein: implications for its physiological role. J. Bacteriol. 191:1924-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaback, H. R. 1971. Bacterial membranes. Methods Enzymol. 22:99-120. [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 11.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masuda, K., S. Matsuyama, and H. Tokuda. 2002. Elucidation of the function of lipoprotein-sorting signals that determine membrane localization. Proc. Natl. Acad. Sci. U. S. A. 99:7390-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuyama, S., T. Tajima, and H. Tokuda. 1995. A novel periplasmic carrier protein involved in the sorting and transport of E. coli lipoproteins destined for the outer membrane. EMBO J. 14:3365-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuyama, S., N. Yokota, and H. Tokuda. 1997. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 16:6947-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 16.Miroux, B., and J. E. Walker. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260:289-298. [DOI] [PubMed] [Google Scholar]
- 17.Miyadai, H., K. Tanaka-Masuda, S. Matsuyama, and H. Tokuda. 2004. Effects of lipoprotein overproduction on the induction of DegP (HtrA) involved in the quality control of Escherichia coli periplasm. J. Biol. Chem. 279:39807-39813. [DOI] [PubMed] [Google Scholar]
- 18.Narita, S., K. Kanamaru, S. Matsuyama, and H. Tokuda. 2003. A mutation in the membrane subunit of an ABC transporter LolCDE complex causing outer membrane localization of lipoproteins against their inner membrane-specific signals. Mol. Microbiol. 49:167-177. [DOI] [PubMed] [Google Scholar]
- 19.Oguchi, Y., K. Takeda, S. Watanabe, N. Yokota, K. Miki, and H. Tokuda. 2008. Opening and closing of the hydrophobic cavity of LolA coupled to lipoprotein binding and release. J. Biol. Chem. 283:25414-25420. [DOI] [PubMed] [Google Scholar]
- 20.Okuda, S., and H. Tokuda. 2009. Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB. Proc. Natl. Acad. Sci., U. S. A. 106:5877-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugsley, A. 1993. The complete general secretory pathway in Gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45:1599-1611. [DOI] [PubMed] [Google Scholar]
- 23.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz, N., D. Kahne, and T. J. Silhavy. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57-66. [DOI] [PubMed] [Google Scholar]
- 25.Sankaran, K., and H. C. Wu. 1994. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J. Biol. Chem. 269:19701-19706. [PubMed] [Google Scholar]
- 26.Slamti, L., and M. K. Waldor. 2009. Genetic analysis of activation of the Vibrio cholerae Cpx pathway. J. Bacteriol. 191:5044-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda, K., H. Miyatake, N. Yokota, S. Matsuyama, H. Tokuda, and K. Miki. 2003. Crystal structures of bacterial lipoprotein localization factors, LolA and LolB. EMBO J. 22:3199-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokuda, H. 2009. Biogenesis of outer membranes in Gram-negative bacteria. Biosci. Biotechnol. Biochem. 73:465-473. [DOI] [PubMed] [Google Scholar]
- 30.Tokuda, H., and S. Matsuyama. 2004. Sorting of lipoproteins to the outer membrane in E. coli. Biochim. Biophys. Acta 1693:5-13. [DOI] [PubMed] [Google Scholar]
- 31.Tokuda, H., S. Matsuyama, and K. Tanaka-Masuda. 2007. Structure, function and transport of lipoproteins in Escherichia coli, p. 67-79. In M. Ehrmann (ed.), The periplasm. ASM Press, Washington, DC.
- 32.Tsukahara, J., K. Mukaiyama, S. Okuda, S. Narita, and H. Tokuda. 2009. Dissection of the LolB function; lipoprotein binding, membrane targeting, and incorporation of lipoproteins into lipid bilayers. FEBS J. 276:4496-4504. [DOI] [PubMed] [Google Scholar]
- 33.Walsh, N. P., B. M. Alba, B. Bose, C. A. Gross, and R. T. Sauer. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61-71. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe, S., Y. Oguchi, K. Takeda, K. Miki, and H. Tokuda. 2008. Introduction of a lethal redox switch that controls the opening and closing of the hydrophobic cavity in LolA. J. Biol. Chem. 283:25421-25427. [DOI] [PubMed] [Google Scholar]
- 35.Wu, T., A. C. McCandlish, L. S. Groenberger, S. S. Chng, T. J. Silhavy, and D. Kahne. 2006. Identification of a protein complex that assembles lipopolysaccharides in the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 103:11754-11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakushi, T., K. Masuda, N. Narita, S. Matsuyama, and H. Tokuda. 2000. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat. Cell Biol. 2:212-218. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda, M., A. Iguchi-Yokoyama, S. Matsuyama, H. Tokuda, and S. Narita. 2009. Membrane topology and functional importance of the periplasmic region of ABC transporter LolCDE. Biosci. Biotechnol. Biochem. 73:2310-2316. [DOI] [PubMed] [Google Scholar]