Abstract
Depression and anxiety are prevalent non-motor symptoms that worsen quality of life for Parkinson’s disease (PD) patients. While dopamine (DA) cell loss is a commonly proposed mechanism, the reported efficacy of DA replacement therapy with L-DOPA on affective symptoms is inconsistent. In order to delineate the effects of DA denervation and chronic L-DOPA treatment on affective behaviors, male Sprague-Dawley rats received unilateral 6-OHDA or sham lesions and were treated daily with L-DOPA (12 mg/kg + benserazide, 15 mg/kg, sc) or vehicle (0.9% NaCl, 0.1% ascorbic acid) for 28 days before commencing investigations into anxiety (locomotor chambers, social interaction) and depression-like behaviors (forced swim test) during the OFF phase of L-DOPA. One h after final treatments, rats were killed and striatum, prefrontal cortex, hippocampus, and amygdala were analyzed via high performance liquid chromatography for monoamine levels. In locomotor chambers and social interaction, DA lesions exerted mild anxiogenic effects. Surprisingly, chronic L-DOPA treatment did not improve these effects. While DA lesion reduced climbing behaviors on day 2 of exposure to the forced swim test, chronic L-DOPA treatment did not reverse these effects. Neurochemically, L-DOPA treatment in hemiparkinsonian rats reduced NE levels in the prefrontal cortex, striatum, and hippocampus. Collectively, the present data suggest that chronic L-DOPA therapy in severely DA-lesioned rats does not improve non-motor symptoms and may impair non-dopaminergic processes, indicating that long-term L-DOPA therapy does not exert necessary cause neuroplastic changes for improving affect.
Keywords: 6-hydroxydopamine, affect, anxiety, depression, dopamine, L-DOPA, norepinephrine, Parkinson’s disease, rat, serotonin
Introduction
Parkinson’s disease (PD) is a progressive, neurodegenerative disorder characterized by the loss of dopamine (DA) neurons in the nigrostriatal pathway, resulting in motor symptoms, such as tremor, rigidity and bradykinesia. Though less acknowledged, PD patients also suffer from a variety of non-motor symptoms, including significant changes in affect that deleteriously impact quality of life (Schrag, 2006; Carod-Artal et al., 2008; McKinlay et al., 2008). For example, anxiety and/or depression are reported by more than 50% of PD patients, far exceeding rates in normal and chronic disease-affected populations (Wragg & Jeste, 1989; Richard et al., 1996; Beekman et al., 1999; Yamamoto, 2001; Barone et al., 2009). Therefore, understanding the neurobiological underpinnings of psychiatric disturbances in PD is a crucial but unmet goal.
While the pathophysiological mechanism(s) underlying increased prevalence of anxiety and depression in PD are unclear, it is likely that both dopaminergic and non-dopaminergic systems contribute. For example, Remy and colleagues (2005) found a negative correlation between decreased binding to DA/norepinephrine (NE) transporters in the ventral striatum and depressive and anxious symptoms (see also Weintraub et al., 2004) in PD patients. Moreover, animals with 6-hydroxydopamine (6-OHDA) lesions of the ventral tegmental area and substantia nigra pars compacta show increased learned helplessness behaviors (Winter et al., 2007), suggesting affective changes may be precipitated by reduced DA levels. However, early cell loss in the serotonergic raphe nuclei and noradrenergic locus coeruleus (D’Amato et al., 1987; Halliday et al., 1990; Braak et al., 2003) may also portend non-motor symptoms, an assertion supported by findings that mood changes can antedate the onset of motor symptoms in a subgroup of patients (Nilsson et al., 2001; Leentjens et al., 2003).
While L-DOPA remains the most common and effective treatment for PD motor symptoms, its purported efficacy for the reduction of mood-related symptoms is inconsistent. For example, some researchers have reported L-DOPA-mediated improvements in anxiety and depression (Maricle et al., 1995; Fetoni et al., 1999), whereas others have not (Choi et al., 2000; Kim et al., 2009). In fact there is evidence that, coincident with motor fluctuations, chronic L-DOPA may promote the development of mood fluctuations, including alterations in euphoria, anxiety and depression (Maricle et al., 1995; Kulisevsky et al., 2007). Unfortunately, there is a paucity of basic research regarding the impact of chronic L-DOPA on mood-related behaviors and concomitant neurochemical changes within the DA-depleted brain.
As such, the goals of the current study were to determine the effects of DA denervation and chronic L-DOPA treatment on rodent measures of anxiety and depression, as well as monoamine levels via high-performance liquid chromatography with electrochemical detection (HPLC-ED) within affect-related brain areas. Based on previous research (Maricle et al., 1995; Branchi et al., 2008), it was predicted that DA lesion would promote anxiety and depression-like symptoms while L-DOPA would improve aspects of these behaviors. As DA, NE, and serotonin (5-HT) are known to be involved in affect (Ressler & Nemeroff, 2000), neurochemical changes were predicted to parallel chronic behavioral effects with reductions in DA, NE, and 5-HT function associated with increased anxiety and depression-like behaviors.
Methods
Subjects
Adult male Sprague-Dawley rats (225–250 g upon arrival; Taconic Farms, Hudson, NY, USA) were housed in plastic cages (22 cm high, 45 cm deep and 23 cm wide) with free access to standard lab chow (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony was maintained on a 12/12 h light/dark cycle (lights on 0700 h) at a temperature of 22–23°C. Animals were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academic Press 1996; NIH publication number 85-23, revised 1996).
6-OHDA lesion surgeries
One week after arrival, rats received unilateral 6-OHDA or sham lesions of the left medial forebrain bundle (AP: −1.8 mm, ML: +2.0 mm, DV: −8.6 mm relative to bregma with the incisor bar positioned 5 mm below the interaural line) to destroy DA neurons. Following pretreatment with desipramine HCl (25 mg/kg, ip; Sigma, St. Louis, MO, USA) to protect NE neurons, 6-OHDA (12 μg; Sigma) .dissolved in 0.9% NaCl + 0.1% ascorbic acid. was infused at a rate of 2 μl/min for a total volume of 4 μl. The needle was withdrawn 5 min later and rats were placed in clean cages on warming pads to recover from surgery, after which they were returned to group-housing (2 rats/cage).
Pharmacological treatments
In order to test the behavioral effects of DA lesions and/or chronic L-DOPA treatment, rats received either vehicle (0.9% NaCl containing 0.1% ascorbic acid) or L-DOPA methyl ester (L-DOPA; 12 mg/kg, sc; Sigma) + DL-Serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride (benserazide; 15 mg/kg, sc; Sigma) once daily for 28 (n=28) or 75 (n=20) days, after 3 weeks of recovery from 6-OHDA or sham lesions. L-DOPA and benserazide were dissolved in Vehicle (0.9% NaCl containing 0.1% ascorbic acid) and administered at a volume of 1.0 ml/kg. Thus, four groups were formed: 1) sham-lesioned, vehicle-treated (Sham-VEH), 2) sham-lesioned, L-DOPA-treated (Sham-LD), 3) 6-OHDA-lesioned, vehicle-treated (Lesion-VEH), and 4) 6-OHDA-lesioned, L-DOPA-treated (Lesion-LD). In order to ensure that L-DOPA-induced dyskinesia did not interfere with the behavioral analyses, animals were tested 16 h after the previous treatment on day 24 (OFF phase). A subset of animals was then killed on day 28 of treatment for neurochemical tissue analyses via HPLC-ED, 60 min after treatment with vehicle or L-DOPA. The remaining animals continued their respective daily treatments during exposure to the modified forced swim test, and social interaction with a novel conspecific, each separated by at least 1 week. After behavioral testing was completed (day 75), all remaining animals were killed, 60 min after treatment with vehicle or L-DOPA for neurochemical tissue analyses via HPLC-ED.
Behavioral testing
Locomotor activity
Locomotor activity testing was conducted in 6 identical acrylic chambers measuring 40 cm long, 40 cm wide and 30 cm high (Accuscan Instruments, Columbus, OH, USA). Each chamber was surrounded by a 15 × 15 infrared photocell array interfaced with a computer that ran the Versamax and Versadat programs, which tabulated and processed behaviors in the test field. For the present experiment, the variables examined included locomotor measures such as total distance traveled (cm) and anxiety measures including center time spent (s), whole body entries (frequency), and vertical movements (frequency), for twenty 6 min periods (total of 2 h). Rats had no previous experience in the test field prior to the first test day.
Modified forced swim test
The modified forced swim test was used as a well-established measure of depressive behaviors in rats that is sensitive to both prodepressant and antidepressant medications and manipulations (Detke et al., 1995, Lucki, 1997; Deak et al., 2005). In the current study, test-naïve rats were placed into a Plexiglas cylinder (45 cm × 20 cm) filled with 30 cm of warm water (25 °C) and their responses were recorded via video camera onto recordable DVD for 15 min. Twenty-four h later and 16–20 h after their last daily treatment, rats were placed into the cylinder and their responses were recorded for an additional 5 min. Recordings were later analyzed by a blinded, trained observer every 5 s for the prevailing behavior: climbing, swimming, or immobility. Climbing was defined as attempts to escape the chamber by struggling up the sides of the cylinder. Swimming was defined as mild paddling of the limbs around the cylinder and immobility as lack of limb movements (floating), except for those necessary to stay afloat.
Social interaction test
The social interaction test was used as a measure of anxiety-like behaviors in rats. The frequency of approaches and sniffing are sensitive to both anxiolytic and anxiogenic manipulations (File & Seth, 2003). Test and non-manipulated, novel conspecifics (stimulus rats) were habituated to a 25 cm × 40 cm chamber for 15 min in a darkened room. The next day, and 16–20 h after their last daily treatment, test rats were introduced to the chamber again with a stimulus rat and their interactions were recorded for 15 min via video camera onto recordable DVD. Recordings were later tallied by a blinded, trained observer for frequency of approaches, flights, following, and anogenital or other sniffing by the test rat towards the stimulus rat. Approaches were defined as purposeful movement of the test animal towards the stimulus animal, with flights being the opposite (purposeful movement of the test animal away from the stimulus animal). Any movements in which the test animal was following the stimulus animal as it moved away from the test animal were defined as follow: sniffing was defined as visible sniffing motions of the test animal, either in contact with or close to the stimulus rat, with anogenital sniffing considered separate from other sniffing behaviors.
High-performance liquid chromatography with electrochemical detection
After 28 or 75 days of daily treatment, rats were killed by decapitation, 60 min after injection with either Vehicle or L-DOPA (12 mg/kg + benserazide, 15 mg/kg, sc). The striatum, hippocampus, amygdala and prefrontal cortex ipsilateral to sham or DA lesion were collected, flash-frozen and stored at −80°C. Reverse-phase HPLC-EC was performed on tissue samples, obtained from all rats, according to the protocol of Kilpatrick et al. (1986), a method for semiautomated catecholamine and indoleamine analysis with coulometric detection. The system included an ESA autoinjector (Model 542), an ESA solvent delivery system (1582), an external pulse dampener (ESA), an ESA column and a C-18 (100 × 4.6 mm, 5 μm packing) column (ESA). Samples were homogenized in ice-cold perchloric acid (0.1 M) with 1% ethanol and 0.02% ethylenediamine tetraacetic acid (EDTA). The homogenates were spun for 45 min at 14,400 g with the temperature maintained at 4 °C. Aliquots of supernatant were then analyzed for abundance of DA, 5-HT, NE, 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindole-3-acetic acid (5-HIAA). Samples were separated using a mobile phase composed of sodium phosphate (monobasic, anhydrous), 100 mM; EDTA, 0.05 mM; octane sulphonic acid, 1.4 mM; and acetonitrile, 9% adjusted to pH 3.0 with o-phosphoric acid. A coulometric detector configured with three electrodes (Coulochem III; ESA) measured the content of monoamines and metabolites. An ESA model 5020 guard cell (+300 mV) was positioned prior to the autoinjector. The analytical cell (ESA model 501LA; first electrode at −100 mV, second electrode at +250 mV) was located immediately after the column. The second analytical electrode emitted signals that were recorded and analyzed by EZChrom Elite software via Scientific Software Inc. module (SS420x). The final oxidation current values were plotted on a standard curve of known concentrations from 10−5 to 10−9 M, adjusted to tissue weights and expressed as nanograms (ng) of monoamine or metabolite per milligram (mg) tissue (mean +SE).
Data analyses
Two-way ANOVAs and a limited number of planned comparisons were employed for analyses of open field, social interaction, and neurochemical data.
Post-hoc comparisons were completed via least significant differences comparisons. Forced swim test data were analyzed by dependent samples t-tests to examine within-subjects variables. Alpha was set at p<0.05. Statistical analyses were conducted with Statistica Software ‘98 (Statsoft, Inc., Tulsa, OK, USA).
Results
Effects of DA lesion and chronic L-DOPA treatment on motor and anxiety-like behaviors in locomotor chambers
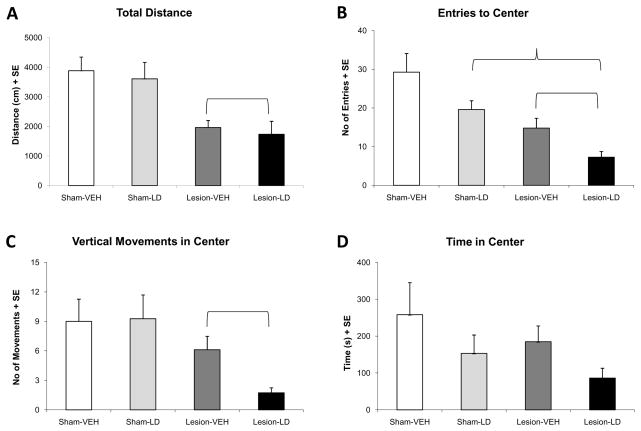
In the current study, locomotor chambers were used to examine the effect of lesion and treatment on overall movement and anxiety-like symptoms. Reduced total distance travelled is indicative of a parkinsoni an effect on motor activity (Srinivasan & Schmidt, 2004) and as expected, a main effect of lesion on total distance was observed (Figure 1A; F1,46=9.43, p<0.05). Unilateral 6-OHDA lesion significantly reduced total distance travelled compared to sham lesions by approximately 50%. Moreover, as rats were tested 16–20 h after their last L-DOPA treatment (OFF L-DOPA), there was no difference on this measure between Lesion-VEH and Lesion-LD groups.
Figure 1.
Behavioral effects of unilateral 6-OHDA lesion and chronic L-DOPA treatment on motor activity and anxiety-like behaviors measured in the locomotor chambers (n=11–17/group). Bars denote the effects of each group on (A) total distance, (B) entries to center, (C) vertical movements in center, and (D) time in center of the test field for 2 h. Main effects of lesion ( p<0.05) and treatment (
p<0.05) and treatment ( p<0.05) were determined via two-way ANOVA.
p<0.05) were determined via two-way ANOVA.
Reduced activities within the center of the test field (reduced entries, vertical movements, and time spent in the center) are traditionally considered evidence of enhanced anxiety-like behavior and are sensitive to treatment with anxiolytics (Prut & Belzung, 2003). There was a significant main effect of lesion on entries to center (Figure 1B; F1,46=23.88, p<0.05), and vertical movements in center (Figure 1C; F1,46=22.27, p<0.05), but not time in center (Figure 1D). Both DA-lesioned groups showed reduced center entries and center vertical movements versus sham-lesioned rats, likely associated with a reduction in total distance travelled. No significant main effect of treatment or interaction between lesion and treatment was observed on any measure for locomotor chamber behavior (p>0.05), suggesting that chronic L-DOPA does not improve these measures of anxiety-like behavior. In fact, the anxiogenic effects of 6-OHDA lesion appeared to be stronger in rats that received chronic L-DOPA treatment, especially for center vertical movements (>50% reduction compared to vehicle-treated rats).
Effects of DA lesion and chronic L-DOPA treatment on anxiety-like behaviors in social interaction test
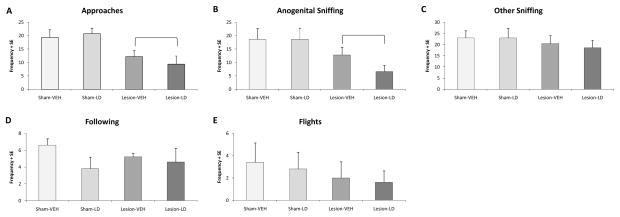
Reduced interaction with a novel conspecific has been interpreted to indicate an anxiogenic state (File & Seth, 2003). For approaches, a significant main effect of lesion was observed (Figure 2A; F1,16=16.01, p<0.05), with reductions in frequency to approach the stimulus animal in DA-lesioned rats compared to both sham-lesioned groups, regardless of treatment. A significant main effect of lesion was also observed for anogenital sniffing (Figure 2B; F1,26=8.07, p<0.05), but not other sniffing, following, or flights (Figure 2C–E). No significant main effect of treatment or interaction between lesion and treatment was found in any measure of the social interaction test, though there was a further reduction in anogenital sniffing among hemiparkinsonian rats that received chronic L-DOPA treatment (~50%) compared to those that received chronic vehicle treatment. Collectively, these data suggest that chronic L-DOPA treatment also does not improve, and may mildly exacerbate, anxiety-like behavior on these measures.
Figure 2.
Behavioral effects of unilateral 6-OHDA lesion and chronic L-DOPA treatment on anxiety-like behaviors in the social interaction test. Bars denote the effects of each group (n=5/group) on (A) approaches, (B) anogenital sniffing, (C) other sniffing, and (D) following of the stimulus animal and (E) flights away from the stimulus animal. Main effects of lesion ( p<0.05) were determined via two-way ANOVA.
p<0.05) were determined via two-way ANOVA.
Effects of DA lesion and chronic L-DOPA treatment on depression-like behaviors in the forced swim test
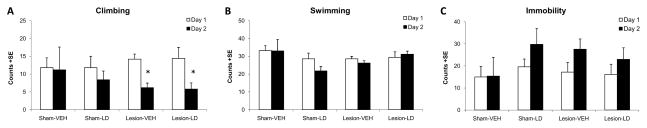
The forced swim test was employed in the current study as a behavioral measure sensitive to both antidepressant and depression-inducing treatments (Lucki, 1997; Deak et al., 2005). Depressive behaviors are inferred by increases in immobility with concomitant decreases in climbing behavior on day 2 of exposure to the test conditions (Detke et al., 1995). Such effects were observed in the current study in DA-lesioned rats, regardless of treatment. A significant main effect of day, but not lesion or treatment, on climbing behaviors was revealed via 2-way ANOVA (F1,16=21.17, p<0.05). An interaction between lesion and day (F1,16=21.17, p<0.05) showed a significant reduction in climbing on day 2 of exposure in DA-lesioned rats compared to sham-lesioned rats (Figure 3A; both p<0.05). No significant effects were observed for swimming behavior, suggesting that motor performance on this task was equivalent across groups (Figure 3B). A significant main effect of day on immobility behaviors was observed (Figure 3C; F1,16=17.14, p<0.05), but neither main effects for lesion or treatment nor any interaction were significant.
Figure 3.
Behavioral effects of unilateral 6-OHDA lesion and chronic L-DOPA treatment on depression-like behaviors in the modified forced swim test. (A) Climbing, (B) swimming, and (C) immobility behaviors for the first 5 min of day 1 of exposure (white bars) and first 5 min of day 2 of exposure (black bars) are shown for Sham-VEH, Sham-LD, Lesion-VEH, and Lesion-LD groups (n=5/group). Two-way ANOVAs revealed significant main effects of day on climbing and immobility. A significant lesion by day interaction was also found for climbing (*p<0.05).
Effects of DA lesion and chronic L-DOPA treatment on monoamine levels in affect-related brain areas
Tissue taken from the striatum, prefrontal cortex, hippocampus, and amygdala ipsilateral to lesion 1 h following either Vehicle or L-DOPA (12 mg/kg + benserazide, 15 mg/kg, sc) treatment was analyzed via HPLC-ED for monoamine content (Figure 4). Since there were no differences between rats killed on day 28 versus day 75 of L-DOPA or vehicle treatment, results include animals from both sets.
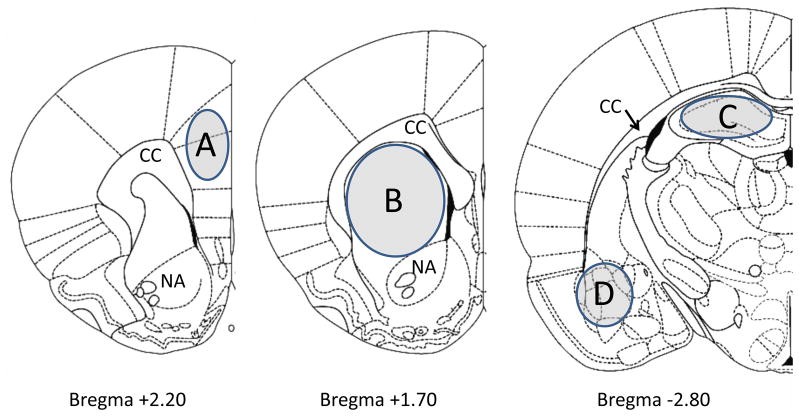
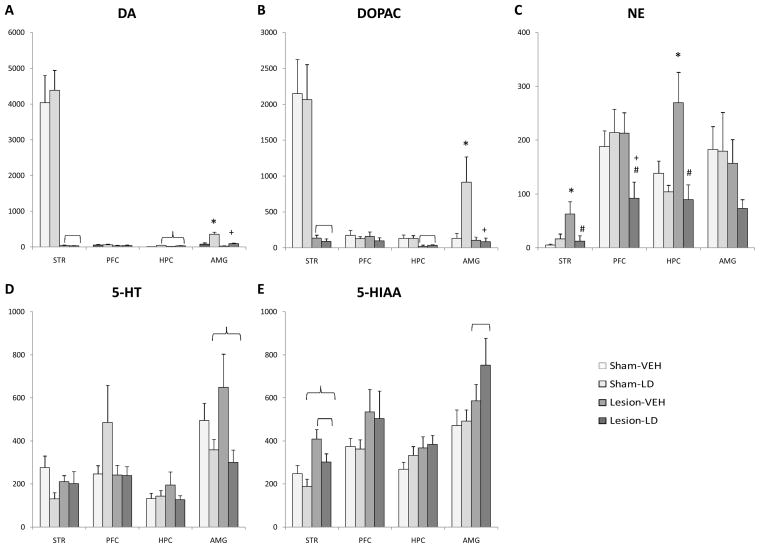
Figure 4.
Schematic representation of coronal sections of the rat brain depicting the regions that were microdissected for analysis via HPLC-ED. The sections were taken from Paxinos and Watson (1998). Shaded portions denote microdissected areas of the (A) prefrontal cortex, (B) striatum, (C) hippocampus, and (D) amygdala. Relevant anatomical structures are: CC, corpus callosum; NA, nucleus accumbens.
As expected, 6-OHDA lesions effectively reduced striatal DA and DOPAC by >98% (DA: F1,26=105.48; DOPAC: F1,26=45.97, both p<0.05; Figure 5A and B), but no significant main effect of treatment or interaction were found. While no significant effect of treatment or lesion was observed for DA or DOPAC within the prefrontal cortex, a significant main effect of treatment was observed for hippocampal DA (F1,23=15.88, p<0.05), with L-DOPA treatment increasing DA levels. Hippocampal DOPAC was reduced following 6-OHDA lesion (F1,28=11.92, p<0.05), regardless of treatment. For amygdala DA and DOPAC, there were significant main effects of lesion (DA: F1,28=20.42; DOPAC: F1,28=6.18, both p<0.05)and treatment (DA: F1,28=25.72; DOPAC: F1,28=4.92, both p<0.05), as well as their interaction (DA: F1,28=8.61; DOPAC: F1,28=5.44, both p<0.05). Upon chronic L-DOPA treatment, DA levels were increased in both sham and DA-lesioned rats compared to vehicle-treated rats (both p<0.05). L-DOPA treatment in sham-lesioned, but not DA-lesioned rats, significantly increased amygdalar DOPAC levels (both p<0.05).
Figure 5.
Neurochemical effects of unilateral 6-OHDA lesion and chronic L-DOPA treatment as determined by HPLC-ED. Monoamine levels within the striatum (STR), prefrontal cortex (PFC), hippocampus (HPC), and amygdala (AMG) are shown for (A) DA, (B) DOPAC, (C) NE, (D) 5-HT, and (E) 5-HIAA. Interactions and main effects of lesion ( p<0.05) and treatment (
p<0.05) and treatment ( p<0.05) were determined via two-way ANOVA. LSD post-hoc comparisons demonstrated significant differences between Sham-VEH (* p<0.05), Sham-LD (+ p<0.05), and Lesion-VEH (# p<0.05).
p<0.05) were determined via two-way ANOVA. LSD post-hoc comparisons demonstrated significant differences between Sham-VEH (* p<0.05), Sham-LD (+ p<0.05), and Lesion-VEH (# p<0.05).
While no significant main effects were observed on striatal NE, a significant interaction between lesion and treatment was observed (F1,27=4.82, p<0.05), with DA lesions potently enhancing striatal NE. However, chronic L-DOPA squelched this increase (both p<0.05). In the prefrontal cortex, a significant interaction was also revealed by two-way ANOVA on NE levels (F1,28=4.61, p<0.05). NE was significantly reduced in Lesion-LD rats compared to both sham-lesioned and Lesion-VEH rats (both p<0.05). Hippocampal NE was also altered by treatment (F1,30=11.88, p<0.05) and a significant interaction between treatment and lesion was revealed (F1,30=5.53, p<0.05). Similar to striatal effects, DA lesions increased and L-DOPA treatment reduced NE levels. No significant main effects or interaction were observed within the amygdala, though there appeared to be a reduction in NE levels in Lesion-LD rats (~50%).
As shown in Figure 6D and E, no significant interactions between lesion and treatment were observed in any structure for 5-HT or its metabolite, 5-HIAA. However, a significant main effect of treatment was observed for 5-HT levels in the amygdala (F1.29=7.78, p<0.05), which was likely driven by a reduction in 5-HT following L-DOPA treatment in both sham and DA-lesioned rats. In contrast, DA lesion increased 5-HIAA within the striatum (F1,29=13.41, p<0.05) and amygdala (F1,29=4.99, p<0.05). Striatal 5-HIAA levels were also affected by L-DOPA treatment (F1,29=4.95, p<0.05), as 5-HIAA was decreased in both sham and DA-lesioned rats following L-DOPA treatment.
Discussion
The results of the current study delineate the respective contributions of both unilateral DA-depletion and L-DOPA treatment on affect-related behavioral changes and neurochemical alterations in a rat model of PD. As expected, unilateral DA lesions exerted anxiogenic effects on locomotor activity and social interaction, but did not alter depression-like behaviors in DA-lesioned rats as measured by the forced swim test. Contrary to our a priori prediction, chronic L-DOPA treatment did not appear to produce the necessary plasticity to alleviate these non-motor symptoms. Furthermore, chronic L-DOPA treatment modified 5-HT and NE levels in several key structures involved in the regulation of affect. Collectively, these results suggest a role for NE and 5-HT dysfunction induced by DA cell loss and subsequent chronic L-DOPA treatment in the expression of anxiety and depression in an animal model of PD.
Effects of DA lesion on affective behaviors
Previous investigations into anxiety and depression-like behaviors in animal models of PD have been limited and contradictory. For example, Tadaiesky and colleagues (2008) observed that partial (59% striatal DA loss), bilateral 6-OHDA lesions of the striatum reduced sucrose consumption, increased immobility in the modified forced swim test, and decreased time spent in the open arms of an elevated plus maze. In contrast, a mild bilateral striatal DA lesion (36% striatal DA loss) exerted an anxiolytic effect in the elevated plus maze and during social interaction, while increasing immobility in the forced swim test without an effect of lesion on sucrose consumption (Branchi et al, 2008).
Thus, an important contribution of the present work was to clarify the contribution of DA lesions on changes in affect by using a severe 6-OHDA lesion (>95% DA loss in striatal tissue). In order to ensure the survival of rats with such lesions, a unilateral medial forebrain bundle lesion model was employed. Results from the present study support a role for DA loss in the onset of affective disorders in PD. Compared to similarly-treated sham-lesioned rats, hemiparkinsonian rats made few enteries into the center of the locomotor chamber (Figure 1C) and approached a novel conspecific less in the social interaction test (Figure 2A), suggesting an anxiogenic effect of unilateral DA lesions. Evidence of a depression-like behavioral profile in the modified forced swim test (Figure 3A) was also observed. The current findings corroborate previous investigations (Tadaiesky et al., 2008), including the depressogenic effect of substantia nigra pars compacta lesions observed within a learned helplessness paradigm (Winter et al., 2008).
Effects of L-DOPA on affective behaviors
While Winter and colleagues (2008) successfully reversed their depression-like effects in DA-lesioned rats with DA replacement therapy, no benefit of chronic L-DOPA on anxiety or depression-like behaviors was established in the current study. While the current data provide no evidence of a depressogenic effect, behavioral tests of anxiety insinuate a mild anxiogenic effect of chronic L-DOPA treatment. Despite no differences in total distance travelled in the locomotor chambers (Figure 1A), a reduction in exploratory activity in the center was observed for chronic L-DOPA-treated rats, beyond the effect of DA-lesion alone (Figure 1C, ~50% reduction). This result was corroborated by another modest anxiogenic effect observed in the social interaction test (Figure 2B, ~50% reduction in anogenital sniffing). Given that DA depletion was similar between lesion groups, these results suggest that chronic L-DOPA in the hemiparkinsonian rat does not improve non-motor symptoms and may even mildly exacerbate anxiety-like behaviors.
It is noteworthy that all DA-lesioned animals receiving L-DOPA in the present study displayed moderate to severe unilateral motor dyskinesias following daily treatment, lasting approximately 3–4 h. In fact, we found that these dyskinetic movements made behavioral testing during the ON phase impossible. However, it is worth noting that such motor fluctuations may also coincide with fluctuations in mood in PD patients. For example, Maricle and colleagues (1995) found that L-DOPA dose-dependently resulted in the development of mood fluctuations, where euphoria was associated with the ON phase while anxiety and depressed mood were associated with the OFF phase. Such effects appeared to depend upon disease progression, as L-DOPA dose only correlated with mood fluctuations in late PD but not early PD (Maricle et al., 1998), when dyskinesias would be more common and severe (Ahlskog & Muenter, 2001). In fact, mood fluctuations and affective disorders among PD patients have been specifically correlated with increased severity of dyskinesia (Menza et al., 1990; Nègre-Pagès et al., 2009). However, alterations in anxiety and depression are not solely due to a psychological effect of changes in the motor symptoms of PD, as indicated by the lack of association between improved motor symptoms and affective states (Richard et al., 2001, 2004; Kulisevsky et al., 2007).
Effects of L-DOPA on neurochemistry
One explanation for the lack of improvement following chronic L-DOPA treatment may involve alterations in non-dopaminergic monoamines implicated in affective disorders, such as NE and 5-HT. Recent research has suggested that affective disorders are associated with a hyposensitive serotonergic system and a hypersensitive noradrenergic system (Ressler & Nemeroff, 2000). In both animal models and PD patients, preclinical and clinical reports have suggested that L-DOPA treatment hinders 5-HT and NE function (Everett & Borcherding, 1970; Hashiguti et al., 1993; Borbely et al., 1999; Borah & Mohanakumar, 2007; Naivalles et al., 2010a,b). This hypothesis is substantiated by the results of the current study, as region-dependent alterations in monoamines were observed upon both DA lesion and L-DOPA treatment (Figure 5). As expected, DA lesion of the nigrostriatal pathway profoundly reduced DA and DOPAC levels in the striatum, but had little effect in limbic areas which are largely innervated by dopaminergic neurons from the VTA, including the prefrontal cortex, hippocampus, and amygdala. Unilateral DA lesion alone did not reduce 5-HT levels in any structure, while L-DOPA treatment reduced 5-HT levels within the amygdala in both sham and DA-lesioned rats, consistent with previous studies (Borah & Mohanakumar, 2007). These results corroborate recent research by Naivalles and colleagues (2010a,b) where L-DOPA treatment dose-dependently impaired 5-HT release within the prefrontal cortex and hippocampus of anesthetized, hemiparkinsonian rats. Lesion and treatment effects on striatal 5-HIAA also indicated changes in 5-HT metabolism, as both DA lesion and chronic L-DOPA treatment reduced 5-HIAA levels. On the other hand, amygdalar 5-HIAA was increased in DA-lesioned rats, likely signifying an increase in 5-HT turnover. There was no lesion-induced reduction in NE levels, likely due to pretreatment with the selective norepinephrine reuptake inhibitor, desipramine prior to 6-OHDA infusion (Fulceri et al., 2006). However, lower NE levels within the striatum, prefrontal cortex, and hippocampus were observed following chronic L-DOPA treatment in DA-lesioned rats. Reduced NE activity is traditionally associated with anxiolytic effects. However, chronic L-DOPA treatment in the current study produced only mild anxiogenic effects, which could be explained by the development of supersensitized NE receptors implicated in affective disorders (ie. α2, β receptors; Biegon & Israeli, 1988; Arango et al., 1990, 1993; Garcia-Sevilla et al., 1999).
Several potential mechanisms could explain the alterations in non-dopaminergic function upon L-DOPA treatment in hemiparkinsonian rats. First, 6-OHDA itself may have affected the integrity of other monoaminergic systems. However, this is unlikely given the use of desipramine prior to 6-OHDA lesion to block uptake into NE neurons, and its lack of specificity for 5-HT neurons opposes this suggestion (Figures 5C and 5D). In addition, several studies have reported an increase in both serotonergic dorsal raphe nuclei firing (Zhang et al., 2007; Kaya et al., 2008; Wang et al., 2009a) and locus coeruleus firing following 6-OHDA lesions in rat models of PD (Guiard et al., 2008; Wang et al., 2009b). Such an effect may explain the increase in striatal and hippocampal NE observed in Lesion-VEH rats (Figure 5C), but does not explain the dampening effect of chronic L-DOPA treatment. Second, L-DOPA itself may alter the synthesis of NE and/or 5-HT. This could account for the effects of L-DOPA on 5-HT as it is formed from tryptophan via tryptophan hydroxylase and aromatic amino acid decarboxylase (AADC), the same enzyme that transforms L-DOPA into DA. Exogenous L-DOPA administration may impair 5-HT function at two levels: 1) by inhibiting tryptophan hydroxylase (Hashiguti et al., 1993; Kuhn & Arthur, 1999) or 2) by competing for conversion via AADC. However, L-DOPA would be predicted to increase NE, as NE is formed directly from L-DOPA and would presumably be enhanced following exogenous L-DOPA treatment. Finally and perhaps more parsimonious with the current findings, NE and 5-HT neurons act as surrogates for the DA system following severe DA loss, compromising normal monoaminergic function. For example, NE and 5-HT fibers form functional synapses that have been shown to take up exogenously administered L-DOPA and convert and release L-DOPA-derived DA into the striatum as a “false neurotransmitter” (Ng et al., 1970; Kannari et al., 2001; Carta et al., 2007; Arai et al., 2008; Eskow et al., 2009). This phenotypic alteration has been suggested to result in reductions in 5-HT following L-DOPA treatment in hemiparkinsonian rats (Carta et al., 2008; Naivalles et al., 2010a,b). However, no previous studies have reported effects on NE or directly tested the mechanism underlying these findings.
One limitation of the current study was the methodological dissociation between the neurochemical and behavioral data. Tissue for neurochemical analysis was obtained 60 min after respective treatments during the ON phase of L-DOPA, since it was expected that neurotransmitter changes would be strongest at the point of highest L-DOPA plasma levels (Sato et al., 1994). Therefore, behavioral analyses are likely indicative of durable alterations in brain function in response to chronic DA replacement therapy, while acute changes in neurotransmitter levels were measured via HPLC in the current study. Furthermore, whole tissue neurochemistry was obtained in the current study, including both extracellular and intracellular stores of monoamines. Due to the nature of the lesion and the dyskinesias induced by L-DOPA treatment in all DA-lesioned rats, only OFF phase behavioral data could be assessed. However, this may be considered a strength of the current research, as anxiety and depressed mood are more severe during this phase of L-DOPA treatment (Maricle et al., 1996). Therefore, future studies could allow for both OFF phase neurochemical analyses and for measures of functional extracellular efflux of neurotransmitter, such as microdialysis or in vivo voltammetry (Young, 1993; Robinson et al., 2003).
The nature of the unilateral model should also be considered. Though issues with L-DOPA-related side effects have already been discussed, the unilateral 6-OHDA lesion may allow for contralateral compensation, there by subverting the observed behavioral effects (Pierucci et al., 2009). In addition, the severity of the lesion resulted in the development of significant motor disability (Figure 1A). As such, it is difficult to make extrapolations to affective processes due to the involvement of motor ability in many of the tasks used in the current study. However, the addition of both sham-lesioned and vehicle-treated controls clarifies this issue within the multiple testing procedures employed. A bilateral lesion may be another informative alternative. However, a more moderate DA lesion would be required to ensure survival and could not address the involvement of L-DOPA-induced motor/mood fluctuations that is only evidenced after substantial DA denervation.
Conclusions
Monoaminergic impairment has been implicated in the development and expression of affective disorders (Ressler & Nemeroff, 2000). Though depression and anxiety in PD have traditionally been attributed to DA cell loss and may reportedly be alleviated by chronic L-DOPA treatment, our novel findings suggest that such treatment does not improve non-motor symptoms and insinuates a liability toward affective problems among PD patients via impairment of the function of other monoaminergic systems. Further knowledge of the mechanism underlying these effects is certainly essential to potentially improve the treatment of affective disorders, and ultimately quality of life, for the PD patient.
Acknowledgments
The authors would like to acknowledge Dr. Hiroyuki Arakawa for his guidance regarding the social interaction testing. Supported by NINDS grant NIH NS059600 (CB) and the Center for Development and Behavioral Neuroscience at Binghamton University.
Supported by NINDS grant NIH NS059600 (CB) and the Center for Development and Behavioral Neuroscience at Binghamton University.
References
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–58. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Arai A, Tomiyama M, Kannari K, Kimura T, Suzuki C, Watanabe M, et al. Reuptake of L-DOPA-derived extracellular DA in the striatum of a rodent model of Parkinson’s disease via norepinephrine transporter. Synapse. 2008;62:632–5. doi: 10.1002/syn.20535. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–47. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Sved AF, Mann JJ. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res. 1993;630:271–82. doi: 10.1016/0006-8993(93)90666-b. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–11. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- Biegon A, Israeli M. Regionally selective increases in beta-adrenergic receptor density in the brains of suicide victims. Brain Res. 1998;442:199–203. doi: 10.1016/0006-8993(88)91453-9. [DOI] [PubMed] [Google Scholar]
- Borah A, Mohanakumar KP. Long-term L-DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol. 2007;27:985–96. doi: 10.1007/s10571-007-9213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely K, Brooks RA, Wong DF, Burns RS, Cumming P, Gjedde A, et al. NMSP binding to dopamine and serotonin receptors in MPTP-induced parkinsonism: relation to dopa therapy. Acta Neurol Scand. 1999;100:42–52. doi: 10.1111/j.1600-0404.1999.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Armida M, Cassano T, Pèzzola A, Potenza RL, et al. Nonmotor symptoms in Parkinson’s disease: investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J Neurosci Res. 2008;86:2050–61. doi: 10.1002/jnr.21642. [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ, Ziomkowski S, Mourão Mesquita H, Martínez-Martin P. Anxiety and depression: main determinants of health-related quality of life in Brazilian patients with Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:102–8. doi: 10.1016/j.parkreldis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–33. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A. Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog Brain Res. 2008;172:465–78. doi: 10.1016/S0079-6123(08)00922-9. [DOI] [PubMed] [Google Scholar]
- Choi C, Sohn YH, Lee JH, Kim J. The effect of long-term levodopa therapy on depression level in de novo patients with Parkinson’s disease. J Neurol Sci. 2000;172:12–6. doi: 10.1016/s0022-510x(99)00198-7. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Zweig RM, Whitehouse PJ, Wenk GL, Singer HS, Mayeux R, et al. Aminergic systems in Alzheimer’s disease and Parkinson’s disease. Ann Neurol. 1987;22:229–36. doi: 10.1002/ana.410220207. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–34. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. The role of the dorsal raphe nucleus in the development, expression, and treatment of L-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse. 2009;63:610–20. doi: 10.1002/syn.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett GM, Borcherding JW. L-DOPA: effect on concentrations of dopamine, norepinephrine, and serotonin in brains of mice. Science. 1970;168:847–50. [PubMed] [Google Scholar]
- Fetoni V, Soliveri P, Monza D, Testa D, Girotti F. Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry. 1999;66:541–4. doi: 10.1136/jnnp.66.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, et al. Nigrostriatal damage with 6-OHDA: validation of routinely applied procedures. Ann NY Acad Sci. 2006;1074:344–8. doi: 10.1196/annals.1369.032. [DOI] [PubMed] [Google Scholar]
- García-Sevilla JA, Escribá PV, Ozaita A, La Harpe R, Walzer C, Eytan A, et al. Up-regulation of immunolabeled alpha2A-adrenoceptors, Gi coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. J Neurochem. 1999;72:282–91. doi: 10.1046/j.1471-4159.1999.0720282.x. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11:625–39. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–85. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Hashiguti H, Nakahara D, Maruyama W, Naoi M, Ikeda T. Simultaneous determination of in vivo hydroxylation of tyrosine and tryptophan in rat striatum by microdialysis-HPLC: relationship between dopamine and serotonin biosynthesis. J Neural Transm Gen Sect. 1993;93:213–23. doi: 10.1007/BF01244998. [DOI] [PubMed] [Google Scholar]
- Kannari K, Yamato H, Shen H, Tomiyama M, Suda T, Matsunaga M. Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J Neurochem. 2001;76:1346–53. doi: 10.1046/j.1471-4159.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- Kaya AH, Vlamings R, Tan S, Lim LW, Magill PJ, Steinbusch HW, et al. Increased electrical and metabolic activity in the dorsal raphe nucleus of Parkinsonian rats. Brain Res. 2008;1221:93–7. doi: 10.1016/j.brainres.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–76. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park SY, Cho YJ, Hong KS, Cho JY, Seo SY, et al. Nonmotor symptoms in de novo Parkinson disease before and after dopaminergic treatment. J Neurol Sci. 2009;287:200–4. doi: 10.1016/j.jns.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr L-DOPA-quinone inactivates tryptophan hydroxylase and converts the enzyme to a redox-cycling quinoprotein. Brain Res Mol Brain Res. 1999;73:78–84. doi: 10.1016/s0169-328x(99)00238-7. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, Pascual-Sedano B, Barbanoj M, Gironell A, Pagonabarraga J, García-Sánchez C. Acute effects of immediate and controlled-release levodopa on mood in Parkinson’s disease: A double-blind study. Mov Disord. 2007;22:62–7. doi: 10.1002/mds.21205. [DOI] [PubMed] [Google Scholar]
- Lancia AJ, Williams EA, McKnight LV, Zahm DS. Vulnerabilities of ventral mesencephalic neurons projecting to the nucleus accumbens following infusions of 6-hydroxydopamine into the medial forebrain bundle in the rat. Brain Res. 2004;997:119–27. doi: 10.1016/j.brainres.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Van den Akker M, Metsemakers JF, Lousberg R, Verhey FR. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Mov Disord. 2003;18:414–8. doi: 10.1002/mds.10387. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–32. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maricle RA, Nutt JG, Valentine RJ, Carter JH. Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson’s disease: a double-blind, placebo-controlled study. Neurology. 1995;45:1757–60. doi: 10.1212/wnl.45.9.1757. [DOI] [PubMed] [Google Scholar]
- Maricle RA, Valentine RJ, Carter J, Nutt JG. Mood response to levodopa infusion in early Parkinson’s disease. Neurology. 1998;50:1890–2. doi: 10.1212/wnl.50.6.1890. [DOI] [PubMed] [Google Scholar]
- Menza MA, Sage J, Marshall E, Cody R, Duvoisin R. Mood changes and “on-off” phenomena in Parkinson’s disease. Mov Disord. 1990;5:148–51. doi: 10.1002/mds.870050210. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Grace RC, Dalrymple-Alford JC, Anderson T, Fink J, Roger D. A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson’s disease patients without dementia. Parkinsonism Relat Disord. 2008;14:37–42. doi: 10.1016/j.parkreldis.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Naivalles S, Bioulac B, Gross C, De Deurwaedere P. Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis. 2010a doi: 10.1016/j.nbd.2010.01.012. in press. [DOI] [PubMed] [Google Scholar]
- Naivalles S, Benazzouz A, Bioulac B, Gross C, De Deurwaedere P. High-frequency stimulation of the subthalamic nucleus and L-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson’s disease. J Neurosci. 2010b;30:2356–2364. doi: 10.1523/JNEUROSCI.5031-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nègre-Pagès L, Grandjean H, Lapeyre-Mestre M, Montastruc JL, Fourrier A, Lépine JP, et al. Anxious and depressive symptoms in Parkinson’s disease: The French cross-sectionnal DoPaMiP study. Mov Disord. 2010 doi: 10.1002/mds.22760. in press. [DOI] [PubMed] [Google Scholar]
- Ng KY, Chase TN, Colburn RW, Kopin IJ. L-Dopa-induced release of cerebral monoamines. Science. 1970;170:76–7. doi: 10.1126/science.170.3953.76. [DOI] [PubMed] [Google Scholar]
- Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson’s disease for patients with major affective disorder: a register study. Acta Psychiatr Scand. 2001;104:380–6. doi: 10.1034/j.1600-0447.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pierucci M, Di Matteo V, Benigno A, Crescimanno G, Esposito E, Di Giovanni G. The unilateral nigral lesion induces dramatic bilateral modification on rat brain monoamine neurochemistry. Ann NY Acad Sci. 2009;1155:316–23. doi: 10.1111/j.1749-6632.2008.03679.x. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–22. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Richard IH, Schiffer RB, Kurlan R. Anxiety and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1996;8:383–92. doi: 10.1176/jnp.8.4.383. [DOI] [PubMed] [Google Scholar]
- Richard IH, Justus AW, Kurlan R. Relationship between mood and motor fluctuations in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:35–41. doi: 10.1176/jnp.13.1.35. [DOI] [PubMed] [Google Scholar]
- Richard IH, Frank S, McDermott MP, Wang H, Justus AW, Ladonna KA, et al. The ups and downs of Parkinson disease: a prospective study of mood and anxiety fluctuations. Cogn Behav Neurol. 2004;17:201–7. [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–73. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Sato S, Koitabashi T, Koshiro A. Pharmacokinetic and pharmacodynamic studies of L-dopa in rats. I. Pharmacokinetic analysis of L-dopa in rat plasma and striatum. Biol Pharm Bull. 1994;17:1616–21. doi: 10.1248/bpb.17.1616. [DOI] [PubMed] [Google Scholar]
- Schrag A. Quality of life and depression in Parkinson’s disease. J Neurol Sci. 2006;248:151–7. doi: 10.1016/j.jns.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. Behavioral and neurochemical effects of noradrenergic depletions with N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine in 6-hydroxydopamine-induced rat model of Parkinson’s disease. Behav Brain Res. 2004;151:191–9. doi: 10.1016/j.bbr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da Cunha C, Takahashi RN. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience. 2008;156:830–40. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang QJ, Liu J, Wu ZH, Wang T, Gui ZH, et al. Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal firing of the midbrain raphe nuclei 5-HT neurons and a decrease of their response to 5-HT (1A) receptor stimulation in the rat. Neuroscience. 2009a;159:850–61. doi: 10.1016/j.neuroscience.2008.12.051. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang QJ, Liu J, Wu ZH, Wang S. Firing activity of locus coeruleus noradrenergic neurons increases in a rodent model of Parkinsonism. Neurosci Bull. 2009b;5:15–20. doi: 10.1007/s12264-009-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52:784–8. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- Winter C, von Rumohr A, Mundt A, Petrus D, Klein J, Lee T, et al. Lesions of dopaminergic neurons in the substantia nigra pars compacta and in the ventral tegmental area enhance depressive-like behavior in rats. Behav Brain Res. 2007;184:133–41. doi: 10.1016/j.bbr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Wragg RE, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry. 1989;146:577–87. doi: 10.1176/ajp.146.5.577. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Depression in Parkinson’s disease: its prevalence, diagnosis, and neurochemical background. J Neurol. 2001;248:III5–11. doi: 10.1007/pl00022917. [DOI] [PubMed] [Google Scholar]
- Young AM. Intracerebral microdialysis in the study of physiology and behavior. Rev Neurosci. 1993;4:373–95. doi: 10.1515/revneuro.1993.4.4.373. [DOI] [PubMed] [Google Scholar]
- Zhang QJ, Gao R, Liu J, Liu YP, Wang S. Changes in the firing activity of serotonergic neurons in the dorsal raphe nucleus in a rat model of Parkinson’s disease. Sheng Li Xue Bao. 2007;59:183–9. [PubMed] [Google Scholar]