Abstract
Background and aims
Feeding a western-style diet (WD) enriched in saturated fat protects against chronic alcoholic hepatitis. However, saturated fat induces lipotoxicity in cultured hepatocytes. The purpose of the present study was to elucidate the influence of WD on acute hepatic injury and healing.
Methods
Male C57BL/6 mice were fed a purified control diet (CD), or WD enriched in palmitate and cholesterol. After 3 weeks, carbon tetrachloride (CCl4) was administered (0.1 μl/g, ip). Hepatic inflammation and proliferation were assessed by immunostaining for neutrophils and ICAM-1, and Ki67, respectively. Cytokine expression was analyzed by real-time PCR. Protein levels of peroxisome proliferator-activated receptor-γ (PPAR-γ) were assessed by western blotting.
Results
Feeding WD resulted in markedly greater histological evidence of necrosis and enhanced ALT activity (188 ± 6.2U/l) compared to CD-fed mice (99.1 ± 6.3U/l) by day 2 post CCl4. In contrast, WD blunted leukocyte accumulation in necrotic areas and expression of cytokines (TNF-α and IL-6) involved in tissue regeneration. Diminished repair was further indexed by lower collagen αI and Ki67 expression in mice fed WD. Finally, feeding WD as well as treatment of cultured hepatocytes with palmitic acid up-regulated expression of PPAR-γ, shown previously to prevent hepatic repair following CCl4 exposure.
Conclusions
These findings suggest that impaired healing in WD-fed mice blunted recovery from acute injury and underscore the complex relationship between diet and hepatic injury.
Keywords: steatohepatitis, inflammation, saturated fat, peroxisome proliferator-activated receptor-gamma
Introduction
The typical “western” diet (WD) includes high amounts of saturated fat. Several previous studies have addressed the effects of saturated fatty acids on injury mechanisms; however, results remain equivocal. In rats, hepatic injury and inflammation were prevented or reversed when saturated fat was added to an ethanol-containing diet 1–3. Protection from early alcoholic hepatitis was attributed to reductions in lipid peroxidation and endotoxemia. More recently, You et al. demonstrated that feeding a western-style diet high in palmitic acid blunted alcoholic steatohepatitis in mice 4; the observed protection was thought to involve enhanced expression of adipose tissue-derived adiponectin. On the other hand, cholesterol and saturated fatty acids have been linked to endothelial dysfunction in baboons fed a high cholesterol/high saturated fat diet5. Thus, it appears that the beneficial effect of feeding WD is a phenomenon uniquely associated with chronic alcohol exposure.
The present study examined the influence of feeding WD on hepatic injury and subsequent healing mechanisms. For this purpose we used a well-characterized model of acute carbon tetrachloride (CCl4) exposure to induce injury in mice that were fed WD for 3 weeks. Contrary to findings in the chronic ethanol exposure model, the present study provides molecular and biochemical evidence that feeding WD markedly enhanced CCl4-induced injury, and blunted markers of healing and regeneration. Taken together, these findings demonstrate that the saturated fat component of WD is not directly hepato-protective as suggested by results obtained from rodents chronically exposed to ethanol.
Materials and Methods
Animal Treatment
Male C57BL/6 mice (4–6 wk) were fed control diet (CD) or western diet (WD) that was enriched in saturated fat (36% calories), cholesterol (1.5% w/w) and sucrose (46% of calories). After 3 weeks of feeding, 0.1 μl/g body weight of CCl4 (Sigma-Aldrich, St. Louis, MO) was administered intraperitoneally as a 2% solution in olive oil; control mice received only olive oil. Blood and tissue samples were collected on days 1, 2, 3, 4, or 7 after CCl4. The protocols used for handling mice were approved by the Louisiana State University Health Sciences Center Animal Care Committee and were in accordance with the guidelines set by the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Histology and Immunohistochemistry
Sections of liver preserved in formalin-free zinc fixative (BD Pharmingen) were embedded in paraffin and stained with hematoxylin and eosin. Hepatic staining for neutrophils, ICAM-1 and Ki67 (a marker of regeneration) was examined using a standard immunohistochemical procedure. Briefly, sections were deparaffinized and treated with 3% hydrogen peroxide to inactivate endogenous peroxidases. Epitope unmasking was performed by immersing sections in antigen retrieval solution A (BD Pharmingen) and heating to 120°C for 20–30 min. After cooling, blocking buffer (10% FBS) was applied at room temperature for 30 min followed by sequential application of rat anti-mouse neutrophil (Serotec, Raleigh, NC; diluted 1:1000) or rat anti-mouse CD54 antibody (Serotec, Raleigh, NC; diluted 1:100), biotin-conjugated goat anti-rat IgG (1:1000; 30 min), and streptavidin-conjugated horseradish peroxidase 30 min). The procedure for immunostaining for expression of Ki67 (1:100; Vector Laboratories, Burlingame, CA) was similar except that the secondary antibody was HRP-conjugated bovine anti-rabbit (1:1000). Finally, sections were incubated in diaminobenzidine according to the manufacturer instructions (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin. The number of Ki67-positive hepatocytes was counted in 3 randomly selected 100 mm2 areas on each section.
Serum Transaminase Activity
Serum was prepared from blood samples collected from the vena cava. Alanine aminotransferase (ALT) activity was determined using a kinetic spectrophotometric assay (Thermo Electron, Louisville, CO).
Cell Culture and Palmitate Treatment
Human C3A hepatocytes (ATCC, Manassas, VA) were grown to confluence on 48-well plates using Eagle’s Minimum Essential Media (EMEM) supplemented with 10% Fetal Bovine Serum (FBS) as the culture medium. For treatment of C3A cells with palmitate (Sigma-Aldrich, St. Louis, MO), a 5 mM stock solution was prepared in methanol. The stock was diluted to the desired concentrations using EMEM that contained 1% BSA as a carrier.
RNA Isolation and Reverse Transcription
Total RNA was extracted from samples of liver tissue or C3A hepatocytes using the QiagenRNeasy mini kit (Qiagen, Chatsworth, CA) according to the manufacturer’s instructions. From each sample, 250 ng of total RNA was used as a template for cDNA synthesis. The reverse transcription reaction was performed in a 25 μl mixture, which contained 10X RT Buffer, 25 mM MgCl2, 10 mM dNTP mix, 50 μM random hexamers, 20U/μl RNase inhibitor and 50U/μl multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA). Incubations were performed in a Mastercycler-personal (Eppendorf, Westbury, NY) for 10 min at room temperature followed by 60 min at 42°C and 5 min at 95°C.
Quantitative Real-Time Polymerase Chain Reaction
For real-time PCR analysis, cDNA was amplified using primers listed in Table 1 for CYP2E1, collagen αI, PPARγ, TNFα, and IL-6 (Taqman Gene expression Assays-on-demand; Applied Biosystems, Foster City, CA). Reactions were assembled according to the manufacturer’s instructions with individual 20 μl reactions consisting of 10 μl of Taqman master mix, 1 μl of primer (Applied Biosystems, Foster City, CA), 8 μl of Nuclease-free water and 1 μl of cDNA. All reactions were performed in duplicate using an ABI Prism 7700 Sequence Detection System. Raw data were analyzed using the RQ manager 1.2 software. The amount of mRNA relative to the 18s ribosomal subunit was calculated using the comparative Ct (ΔCt) method as described previously 6.
Table 1.
Expression Assays used for Real-time PCR assessment of mRNA levels
| Gene Symbol | Gene Name | Accession # | Assay ID | Context Sequence |
|---|---|---|---|---|
| TNFα | Tumor necrosis factor-α | X02611.1 | Mm00443258_m1 | CCCAAAGGGATGAGAAGTTCCCAAA |
| TGFβ1 | Transforming growth factor-β1 | NM_011577.1 | Mm00441724_m1 | GGACCGCAACAACGCCATCTATGAG |
| IL-6 | Interleukin 6 | NM_031168.1 | Mm00446190_m1 | AATGAGAAAAGAGTTGTGCAATGGC |
| PPAR gamma | Peroxisome proliferator activated receptor-γ | NM_011146.1 | Mm0044095_m1 | TCAGTGGAGACCGCCCAGGCTTGCT |
| Col1α1 | Procollagen α1I | NM_007742.3 | Mm00801666_g1 | ATGACCGATGGATTCCCGTTCGAGT |
| CCL2 | Macrophage Chemoatractant protein | NM_011333.1 | Mm00441242_m1 | GCTCAGCCAGATGCAGTTAACGCCC |
Western Blot Analysis
Nuclear protein was extracted using a protein extraction kit (Chemicon International, Temecula, CA). Approximately 50 μg of total protein diluted in Laemmli sample buffer (Bio-Rad, Hercules, CA) was separated on a 4–15% gel and transferred to nitrocellulose membrane. Nonspecific binding to the membrane was minimized by incubation of the membrane in blocking buffer (20 mmol/L Tris-HCl (pH 7.6), 8.5% NaCl, 0.1% Tween 20 containing 5% nonfat dry milk) at room temperature for 1 h. Primary antibody directed against PPAR-γ (1:100 dilution; Cell Signaling Technology, Inc., Danver, MA) was applied overnight at 4°C. Horseradish peroxidase-conjugated goat anti-rat IgG (1:2000 in blocking buffer) was applied for 1 h at room temperature. Visualization was achieved using ECL-Plus detection reagents (Amersham Biosciences, Piscataway, NJ).
Statistical Analysis
Data are presented as mean ± SEM of 4 (control) or 5 (CCl4) observations per dietary treatment. Cell culture experiments are the mean of 3 separate measurements. Statistical analysis of static adhesion results was performed using one-way ANOVA; all other data was analyzed with two-way ANOVA and Tukey’s multiple comparisons test; p< 0.05 was selected as the level of significance.
Results
Feeding a western diet enhanced CCl4-induce hepatic injury
Mice were given unrestricted access to CD or WD that was enriched in calories derived from butter fat. As expected, mice fed WD weighed significantly more (33.9 ± 0.9g) than mice fed CD (25.9 ± 1.1g). Upon histological evaluation, livers of mice from the vehicle control groups appeared normal. To determine the influence of these diets on injury, CCl4 was administered and mice were sacrificed up to 7 days post CCl4 exposure. Histological evidence of moderate pericentral necrosis was observed in both dietary groups beginning 1 day after CCl4 (Fig. 1). Feeding WD resulted in significantly greater hepatocellular necrosis in zones 2 and 3 after CCl4 exposure for 2–7 days. Serum ALT activity was measured to index hepatic injury. A time-dependent increase in ALT was observed with peak activity occurring at the 2-day time point in both dietary groups (Fig. 2). Consistent with histological findings, serum ALT activity in the WD group was elevated significantly above diet-matched controls and CD-fed mice at each subsequent time point. In fact, serum ALT activity returned to baseline levels in CD-fed mice 7 days after CCl4 exposure, yet remained elevated significantly in mice fed WD.
Figure 1. CCl4-induced hepatic injury.
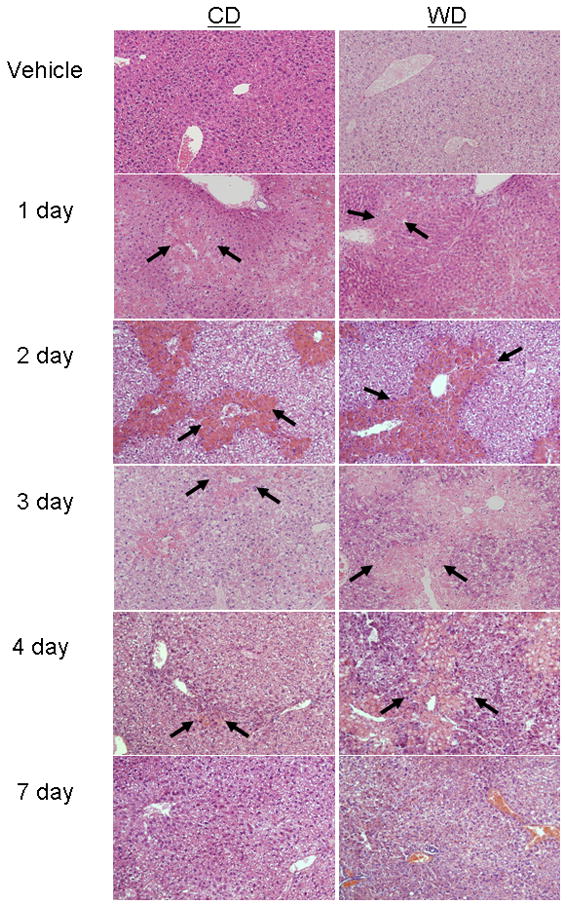
Male C57BL/6 mice were fed control (CD) or western (WD) diet for 3 weeks, treated with vehicle or CCl4 (0.1 μl/g) and sacrificed after up to 7 days. Photomicrographs are liver sections representative of at least 4 observations/group stained with hematoxylin and eosin. Original magnification = 32x. Centrilobular necroinflammation peaked at day 2 in mice fed CD and was nearly resolved by day 7. Although initial injury was similar among the dietary groups, feeding WD enhanced injury at days 2–7. Arrows denote borders of necrotic areas.
Figure 2. Serum ALT activity.
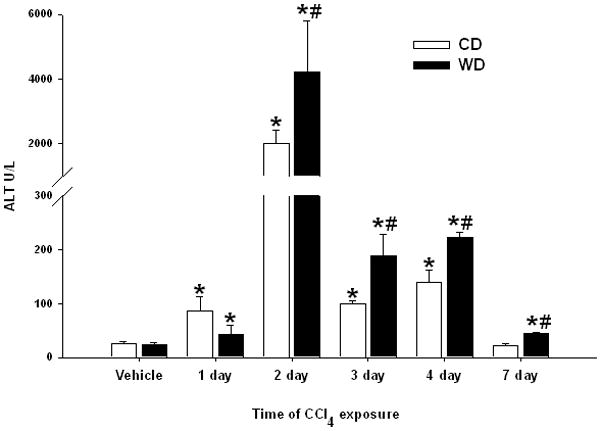
Male C57BL/6 mice were fed control (CD) or western (WD) diet for 3 weeks and treated with vehicle or CCl4 (0.1 μl/g). Serum alanine aminotransferase activity (ALT) was assessed in vehicle controls, and 1, 2, 4, or 7 days after administration of CCl4. Data (mean ± SEM) were analyzed using two-way ANOVA and Tukey’s multiple comparisons test; *p< 0.05 versus diet-matched control rats, #p< 0.05 compared to CD-fed mice. Data without a common letter differ statistically.
Healing and regeneration are impaired in mice fed western diet
Representative photomicrographs of livers immunostained for Ki67 content are presented in Figure 3A. The expression of this regeneration marker was minimal in both dietary control groups. Treatment with CCl4 maximally enhanced panlobular immunostaining in livers of CD-fed mice on day 3 post exposure; however, this affect was significantly less pronounced in mice fed WD as summarized in Figure 3B. These findings demonstrate that healing and regeneration are impaired in mice fed WD.
Figure 3. Hepatic regeneration is diminished in mice fed western diet.

Sections of liver collected 3 days after CCl4 exposure were immuno-stained for the presence of Ki67 to index hepatic regeneration. (A) Representative photomicrographs indicate positive staining in nuclei near necrotic areas (brown nuclei). (B) The percentage of Ki67-positive hepatocytes was calculated in 3 random areas on each section of liver (n= 4 sections/treatment group). Statistical analysis was performed using one-way ANOVA and Bonnferoni’s multiple comparisons test. *p< 0.05 versus diet-matched controls; #p< 0.05 compared to corresponding CD-fed mice treated with CCl4. Data without a common letter are statistically different.
To characterize the healing capacity in livers of mice fed CD or WD, expression of pro-collagen α1 was assessed. Although a significant increase was observed in both dietary groups, the expression of this marker was approximately 4-fold lower in the WD group compared to the CD group (Fig. 4). Pro-collagen mRNA expression returned to baseline by 7 days post CCl4.
Figure 4. Hepatic collagen α1 expression is blunted in WD-fed mice.

mRNA expression collagen α1 was determined using pre-developed assays for real-time PCR according to the manufacturer’s instructions (Applied Biosystems). Values were calculated using a comparative CT method and presented as mean ± SEM of at least 4 observations/group. Statistical analysis was performed using two-way ANOVA and Tukey’s multiple comparisons test. *p< 0.05 versus diet-matched controls; #p< 0.05 compared to corresponding CD-fed mice treated with CCl4. Data without a common letter are statistically different.
Neutrophil accumulation and cytokine expression are attenuated in mice fed western diet
As shown in Fig. 5, immunohistochemical staining revealed that necrotic areas in mice fed CD were associated with marked neutrophil accumulation 1 day after CCl4. In contrast, neutrophil infiltration was notably reduced by feeding WD. A similar pattern of neutrophil accumulation was observed on days 2–4 (data not shown). To further characterize the hepatic inflammatory state, expression of ICAM-1 was assessed. CCl4 exposure significantly enhanced the extent of protein content in the liver at each time point (Fig. 5). Although feeding WD did not influence baseline neutrophil content or ICAM-1 levels, this diet blunted CCl4-induced increases in ICAM-1 expression, consistent with reduced neutrophil accumulation. Next, the influence of diet on hepatic mRNA expression of the pro-inflammatory cytokines TNF-α and IL-6 was analyzed by real-time PCR. As expected, expression of these cytokines was enhanced significantly by CCl4 exposure in mice fed CD (Fig. 6). Expression returned toward baseline by day 3 post exposure; therefore, no additional time points were evaluated. Feeding western diet attenuated the induction of both TNF-α and IL-6. Since cytokines are important stimuli for regeneration and healing 7, these findings suggest that the pattern of injury observed in mice fed WD may be associated with impaired healing.
Figure 5. Hepatic inflammation.

Immunohistochemical staining for neutrophils (left panels) and ICAM-1 (right panels) 1 day after administration of vehicle or CCl4. Minimal brown staining in mice fed western diet (WD) indicated diminished hepatic neutrophil accumulation and ICAM-1 content. Original magnification = 40x.
Figure 6. The relative mRNA expression of TNF-α and IL-6.

Expression of (A) TNF-α and (B) IL-6 were assessed using pre-developed assays for real-time PCR according to the manufacturer’s instructions (Applied Biosystems). Values were calculated using a comparative CT method and presented as mean ± SEM of at least 4 observations/group. Statistical analysis was performed using two-way ANOVA and Tukey’s multiple comparisons test. *p< 0.05 versus diet-matched controls; #p< 0.05 compared to corresponding CD-fed mice treated with CCl4. Data without a common letter are statistically different.
Western diet and palmitate alter PPAR-γ expression
We investigated expression levels of PPAR-γ because this nuclear receptor has been shown to participate in early CCl4-induced injury 8;9. In comparison to CD-fed controls, mice fed WD displayed a 5-fold induction of PPAR-γ expression (Fig. 7A). To examine the direct effect of saturated fatty acid, we performed immunoblot analysis of PPAR-γ protein levels in extracts of C3A hepatocytes treated with palmitate. Palmitate stimulated a dose-dependant increase in PPAR-γ levels (Fig 7B). Diminished expression at higher doses of palmitate was associated with a loss of cell viability (data not shown). These findings are consistent with the idea that impaired nuclear signaling mechanisms in WD-fed mice may have contributed to the observed response to CCl4.
Figure 7. Expression of PPAR-γ in response to western diet and palmitate.

(A) After 3 weeks of feeding WD, the relative mRNA expression of the nuclear receptor PPAR-γ was evaluated by real-time PCR. Values were calculated using a comparative CT method and presented as mean ± SEM of at least four observations/group. Statistical analysis was performed using Student’s t test; *p< 0.05 versus CD-fed mice treated with CCl4. Data without a common letter are statistically different. (B) Protein levels of PPAR-γ were analyzed by immunoblot of nuclear extracts from C3A cells treated with palmitate as described in Methods. CD= control diet; WD= western diet.
Discussion
The present study examined the progression of acute liver injury in mice that were fed a western-style diet. The time course of CCl4 injury has been described extensively First, CCl4 is metabolized primarily by the cytochorme P450 2E1 component of the hepatic mixed function oxidase system. The resulting trichloromethyl radical is then converted to the more electrophylic trichloromethylperoxyl radical. Both radicals initiate the peroxidation of polyunsaturated fatty acids in the endoplasmic reticulum and mitochondria within minutes of a bolus administration of CCl4. Necrosis is evident within 12 h and peaks with approximately 1–3 days, depending on the administered dose of CCl4. Dying hepatocytes trigger the influx of leukocytes into necrotic areas, which produce cytokines that cause the proliferation of existing viable hepatocytes as well as the transformation of hepatic stellate cells into myofibroblasts to stimulate the repair process. Injury diminishes within approximately 3 days; therefore, this model provides an overview of the kinetics of acute hepatic injury as well as healing. Although the initial injury observed in the present study was similar to mice fed CD, feeding WD exacerbated injury 2–7 days following CCl4 treatment, a time at which healing had begun in CD-fed mice. One distinction among the two dietary groups that may account for enhanced injury beginning at day 2 is the lack of appropriate repair and regeneration mechanisms in mice fed WD. Indeed, mRNA expression of collagen αI, a matrix protein involved in hepatic healing, as well as nuclear staining of the regeneration marker Ki67 were markedly diminished in WD-fed mice following CCl4 exposure.
Bioactivation to the trichloromethyl radical via cytochrome P450 2E1 (CYP 2E1) is a requirement for injury in this model 10. To delineate the hepatic capacity for CCl4 bioactivation, mRNA levels of CYP 2E1 were measured and found to be significantly enhanced at day 4; however no statistical differences were detected between dietary treatments (data not shown). Thus, it is not likely that the observed pattern of injury in the WD group can be explained by enhanced bioactivation. On the other hand, one distinction among the two dietary groups that may account for enhanced injury is the lack of appropriate repair and regeneration mechanisms in mice fed WD.
As an organ, the liver has the unique ability of self-restoration. Following massive cellular injury or tissue loss (e.g. partial hepatectomy) a proliferative response is triggered in the remaining healthy hepatocytes, which results in nearly complete recovery of the lost tissue mass within several days to weeks 11. Complete regeneration is heavily dependent on an appropriate induction of the innate immune system. For example, previous studies indicate that leukocyte-derived cytokines such as TNF-α and IL-6 act as potent hepatocyte growth factors 12–14. These findings are supported by additional experiments demonstrating a lack of regeneration in neutropenic mice 15. Deletion of ICAM-1, an adhesion molecule that mediates firm adhesion and immigration of neutrophils into organs, prevented hepatic neutrophil accumulation and similarly prevented regeneration following partial hepatectomy. Kupffer cells, the resident hepatic macrophages, have been shown to play an integral part in production of a cytokine milieu during injury/healing, including CCl4-induced injury. For example, a study conducted by Edwards et al. demonstrated that destruction of Kupffer cells, with GdCl3 significantly attenuated acute (24 h) hepatic injury in response to CCl4{1297}. Moreover, we have shown that destruction of Kupffer cells with GdCl3 as well as inactivation of these cells with glycine blunts fibrogenesis in rats treated chronically with CCl4{8427}. Blunted fibrosis was associated with diminished α-SMA and TGF-β expression. These previous findings suggest that the initial and primary source of noxious mediators of the CCl4 response is derived from Kupffer cells. However, since both stellate and neutrophils can produce a similar cytokine response, a role for these cells cannot be ruled out. In the present study we found that the presence of neutrophils at the site of injury was markedly diminished in mice fed WD. Consistent with previous findings, diminished hepatic neutrophil content in WD-fed mice was associated with reduced ICAM-1 expression along hepatic sinusoids, decreased cytokine expression and blunted proliferation as indexed by immunostaining for Ki67. These findings support the idea that feeding WD blunts regeneration by impairing the innate immune response.
Hepatic stellate cells are perisinusoidal cells located in the space of Disse in close proximity to hepatocytes. Under normal conditions stellate cells are storage sites of vitamin A and fat; however, during insult such as exposure to alcohol or CCl4, they undergo phenotypic transformation to myofibroblast-like cells that produce collagen 16;17. These cells have been identified as the primary source of matrix proteins during hepatic repair and fibrogenesis 18. Leukocytes have been shown to mediate the proliferation and phenotypic transformation of stellate cells via production of cytokines 19;20. In fact, increases in expression of IL-6 and TGF-β have been shown to directly correlate with stellate cell transformation and accumulation of matrix proteins in response to CCl4 17;21. Herein we report that feeding WD attenuated the hepatic capacity to up-regulate the expression of IL-6 as well as collagen αI. Thus, in addition to regeneration, impaired leukocyte accumulation and cytokine production may have also contributed to the observed blunted healing response. In support of the idea that the saturated fatty acid compenent of WD, palmitic acid, could directly influence healing, Abergel et al. reported that exposure of stellate cells to palmitic acid significantly blunted transformation and the potential to produce matrix proteins such as type I collagen 22. The response of stellate cells to saturated fatty acid was believed to be mediated via PPAR-γ
Previous experiments in vitro demonstrated that over-expression of the nuclear receptor PPAR-γ prevented phenotypic transformation and production of collagen by hepatic stellate cells. In support of a role for PPAR-γ in protection against CCl4-induced injury, activation of this pathway with the synthetic agonist pioglitazone prevented necro-inflammation and healing 8. In the present study we found that feeding WD enhanced PPAR-γ expression. Moreover, addition of palmitic acid directly to the medium of cultured C3A hepatocytes also up-regulated PPAR-γ protein levels. This finding was expected since PPAR-γ is involved in lipid storage and has been shown previously to be up-regulated as dietary fat content increases.23 This raises the question of whether components of WD such as saturated fatty acids may act as natural PPAR-γ ligands. A recent study by Sauma et al 24 demonstrated that palmitic and stearic acids, both of which are constituents in the WD formulation used in the present study, stimulated activity of the PPAR-γ response element indicating activation of this nuclear signaling cascade. Moreover, Abergel et al. have shown that palmitic acid added directly to stellate cells in culture can prevent the activated phenotype; these studies also implicated PPAR-γ in the process of reversion to quiescence 22. Thus, although not tested directly, our observations are consistent with the idea that increased PPAR-γ expression due to feeding WD likely contributed to blunted inflammation and healing in CCl4-treated mice. Moreover, our finding of increase PPAR-γ expression in livers of mice fed WD taken together with the previous observations of the direct influence of PPAR-γ on stellate cells support the hypothesis that decreased matrix production in mice fed WD was due to inappropriate stellate cell activation.
In summary, we have demonstrated that feeding WD enhanced CCl4-induced injury, while blunting regeneration and healing. Although the exact mechanisms underlying the altered response to CCl4 remain undetermined, results of the present study suggest that the combined detrimental effects of blunted immunity and impaired regeneration contributed to the observed pathology. These findings underscore the need to elucidate the complex relationship between diet, innate immunity and liver pathology.
Acknowledgments
This work was supported in part by federal funds from the National Heart Lung and Blood Institute (1K01HL084723-01) and the National Institute of Diabetes and Digestive and Kidney Diseases (3P01DK43785-13S1).
Abbreviations
- CCl4
carbon tetrachloride
- CD
Control Diet
- WD
Western Diet
- PPAR
peroxisome proliferator-activated receptor
- TNF-α
tumor necrosis factor alpha
- TGF-β
transforming growth factor beta
- ICAM-1
intercellular adhesion molecule-1
Reference List
- 1.Nanji AA, French SW. Dietary factors and alcoholic cirrhosis. Alcohol Clin Exp Res. 1986;10:271–273. doi: 10.1111/j.1530-0277.1986.tb05088.x. [DOI] [PubMed] [Google Scholar]
- 2.Nanji AA, Zakim D, Rahemtulla A, et al. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatol. 1997;26:1538–1545. doi: 10.1002/hep.510260622. [DOI] [PubMed] [Google Scholar]
- 3.Nanji AA, Rahemtulla A, Daly T, et al. Cholesterol supplementation prevents necrosis and inflammation but enhances fibrosis in alcoholic liver disease in the rat. Hepatol. 1997;26:90–97. doi: 10.1002/hep.510260112. [DOI] [PubMed] [Google Scholar]
- 4.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatol. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Q, Vandeberg JF, Jett C, et al. Arterial endothelial dysfunction in baboons fed a high-cholesterol, high-fat diet. Am J Clin Nutr. 2005;82:751–759. doi: 10.1093/ajcn/82.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera CA, Abrams SH, Tcharmtchi HM, Allman M, Finegold MJ, Smith WC. Feeding A Corn Oil/Sucrose-Enriched Diet Enhances Steatohepatitis in Sedentary Rats. Am J Physiol Gastrointest Liver Physiol. 2005 doi: 10.1152/ajpgi.00229.2005. [DOI] [PubMed] [Google Scholar]
- 7.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–171. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 8.Kon K, Ikejima K, Hirose M, et al. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem Biophys Res Commun. 2002;291:55–61. doi: 10.1006/bbrc.2002.6385. [DOI] [PubMed] [Google Scholar]
- 9.Orfila C, Lepert JC, Alric L, Carrera G, Beraud M, Pipy B. Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem Cell Biol. 2005;123:585–593. doi: 10.1007/s00418-005-0785-2. [DOI] [PubMed] [Google Scholar]
- 10.Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 11.Steer CJ. Liver regeneration. FASEB J. 1995;9:1396–1400. doi: 10.1096/fasebj.9.14.7589980. [DOI] [PubMed] [Google Scholar]
- 12.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerman P, Cote P, Yang SQ, et al. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 15.Selzner N, Selzner M, Odermatt B, Tian Y, van RN, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 16.Zerbe O, Gressner AM. Proliferation of fat-storing cells is stimulated by secretions of Kupffer cells from normal and injured liver. Exp Mol Pathol. 1988;49:87–101. doi: 10.1016/0014-4800(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto H, Lin M. ITO Cell myofibroblastic activation and kupffer cell cytokine gene expression in alcoholic liver fibrosis. In: Wisse E, Knook DL, Wake K, editors. The Netherlands Cells of the hepatic Sinusoid. Kupffer Cell Foundation; 1995. pp. 390–391. [Google Scholar]
- 18.Mak KM, Leo MA, Lieber CS. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984;87:188–200. [PubMed] [Google Scholar]
- 19.Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 20.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275–279. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsukasa H, Nagy P, Evarts RP, Hsia C-C, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-B1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990;85:1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abergel A, Sapin V, Dif N, et al. Growth arrest and decrease of alpha-SMA and type I collagen expression by palmitic acid in the rat hepatic stellate cell line PAV-1. Dig Dis Sci. 2006;51:986–995. doi: 10.1007/s10620-005-9031-y. [DOI] [PubMed] [Google Scholar]
- 23.Inoue M, Ohtake T, Motomura W, et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 24.Sauma L, Stenkula KG, Kjolhede P, Stralfors P, Soderstrom M, Nystrom FH. PPAR-gamma response element activity in intact primary human adipocytes: effects of fatty acids. Nutrition. 2005 doi: 10.1016/j.nut.2005.04.011. [DOI] [PubMed] [Google Scholar]


