Abstract
Molecular beacon (MB) probes are dual-labeled hairpin-shaped oligodeoxyribonucleotides that are extensively used for real-time detection of specific RNA/DNA analytes. In the MB probe, the loop fragment is complementary to the analyte: therefore, a unique probe is required for the analysis of each new analyte sequence. The conjugation of an oligonucleotide with two dyes and subsequent purification procedures add to the cost of MB probes, thus reducing their application in multiplex formats. Here we demonstrate how one MB probe can be used for the analysis of an arbitrary nucleic acid. The approach takes advantage of two oligonucleotide adaptor strands, each of which contains a fragment complementary to the analyte and a fragment complementary to an MB probe. The presence of the analyte leads to association of MB probe and the two DNA strands in quadripartite complex. The MB probe fluorescently reports the formation of this complex. In this design, the MB does not bind the analyte directly; therefore, the MB sequence is independent of the analyte. In this study one universal MB probe was used to genotype three human polymorphic sites. This approach promises to reduce the cost of multiplex real-time assays and improve the accuracy of single-nucleotide polymorphism genotyping.
Keywords: biosensors, DNA recognition, molecular beacons, nucleic acids
Introduction
Real-time assays for the detection of specific nucleic acids are of great importance because they enable instant detection of specific RNA/DNA sequences without the need of separating the probe–analyte hybrid from the unbound probe.[1] Molecular beacon (MB) probe is a state-of-the-art tool for real-time nucleic acid analysis.[2] The traditional MB probe is a stem–loop folded oligonucleotide with a fluorophore at the 5′-end and a quencher at the 3′-end. Hybridization to a complementary single-stranded DNA or RNA switches MB probe into the elongated conformation, in which the fluorophore is remote from the quencher; this results in high fluorescence. The simplicity and the elegancy of MB design has made this probe a popular tool for nucleic acid analysis and has served as an inspiration for a number of related assays.[3] MB probes have been extensively used in real-time PCR assays.[4] In addition MB probes were suggested for RNA monitoring in living cells.[5] Importantly, not only the stem–loop structure brings the fluorophore close to the quencher, but it also improves the probe specificity. MB probes discriminate between two nucleic acid sequences that differ by a single nucleotide in a wider temperature range than linear hybridization probes do.[6] These properties are of particular importance for single-nucleotide polymorphism (SNP) genotyping.
SNPs represent the most abundant class of genetic variations in humans, accounting for 80–90% of the difference between the genomes of two individuals. There are over three million validated SNPs, although the actual number is believed to be in the range of ten million.[7] They are associated with many common genetic disorders, such as sickle cell anemia and muscular dystrophy.[8] SNPs can predispose people to acquired diseases and influence their response to pathogens, chemicals, drugs, vaccines, and other agents. Type 2 diabetes, breast cancer, and Alzheimer’s disease are a few acquired disease states linked to SNPs.[9] Moreover, SNPs can serve as gene markers and provide a “genetic fingerprint” unique for a person or a group of people. Therefore, SNP analysis is important in population-based genetic risk assessment,[9] molecular diagnostics,[10] pharmaceutical drug development,[11] linkage analysis,[12] and identity testing in forensic applications.[13] A number of practical applications require analysis of hundreds of thousands SNPs.[14]
One factor that limits the application of MB probes in multiplex SNP genotyping assays is their high synthetic cost. Indeed, chemical synthesis of a regular MB probe requires conjugation of an oligonucleotide with two organic dyes, a fluorophore and a quencher. Moreover, at least one round of HPLC purification is required. This step removes fluorescent impurities, which cause high background fluorescence and reduce both the sensitivity and the dynamic range of the probe. Taking into account that the analysis of each individual SNP requires two MB probes (each complementary to a specific allele), genotyping thousands of SNPs by MB probes becomes very expensive. We have recently developed a binary approach for nucleic acid recognition.[15] In this approach two oligonucleotide probes form short hybrids with the analyte and generate a fluorescent[15a–c,e] or visible[15d] signal upon complex formation. The approach enables SNP genotyping at room temperature with high specificity because each part of the probe forms a short (8–10 nucleotide) hybrid with the analyte. Each hybrid is extremely sensitive to minor imperfections, such a single-base mispairing. This study demonstrates how a single MB probe can be utilized for the detection of potentially any sequence when used as a part of binary DNA probe (BDP).[15b]
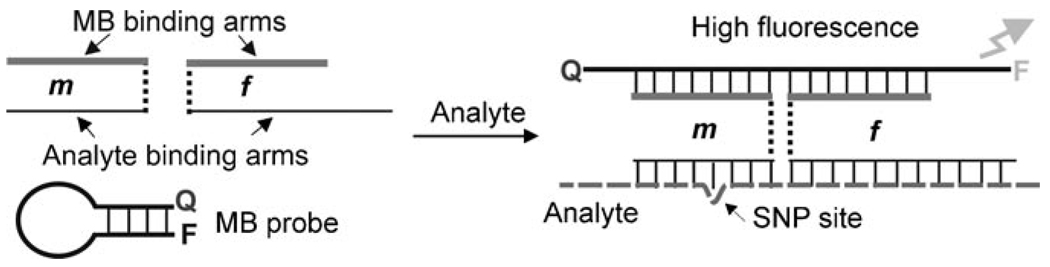
In this approach (Figure 1), oligonucleotide strands m and f contain segments that are complementary to an MB probe (MB-binding arms) and segments that are complementary to a nucleic acid analyte (analyte binding arms). In the absence of the analyte, strands m and f and the MB probe coexist unbound in solution; MB is in the form of a hairpin (Figure 1, left), and the fluorescent signal is low. Addition of a DNA analyte triggers the formation of a quadripartite complex (Figure 1, right). Consequently, the fluorophore (FAM) is separated from the quencher (Q) in this complex resulting in a high fluorescent signal.
Figure 1.
Principal Scheme of indirect binding of MB probe to the analyte by using a binary approach.[15b] The probe consists of an MB probe and the two synthetic oligodeoxyribonucleotides m and f, which co-exist in dissociated state in the absence of a DNA analyte (left). Addition of the specific nucleic acid analyte results in the formation of a quadripartite associate, in which MB probe adopts the open conformation (right). The complex is unstable if there is a mismatch base-pairing in the hybrid of analyte and strand m.
Importantly, strands m and f with the same MB-binding arms can be designed to recognize any analyte simply by varying the analyte-binding arm sequences. In this approach, an optimized MB probe can be synthesized in bulk amounts and utilized efficiently for the analysis of potentially any SNP of interest. Importantly, strands m and f are inexpensive synthetic oligonucleotides that can be used without purification.
Not only does this approach reduce the cost of MB-probe-based assays, but it also improves assay specificity. For example, an MB probe typically requires an elevated temperature (40–65 °C) and precise temperature control for accurate SNP identification.[2, 16] In contrast, binary probes demonstrate high specificity even at room temperature.[15] This is because short probe–analyte hybrids can be tailored to be sensitive to even minor hybrid imperfections, such as a single mismatched base pair. Indeed, the short and delicate hybrid formed by strand m with the analyte is extremely sensitive to a mismatch (Figure 1, right).
We demonstrate the feasibility of using a single optimized MB probe to genotype several human SNP sites. The structure of the MB probe was optimized in terms of signal-to-background ratio (S/B) and reliability of the signal generation. Next, six BDPs were tailored to genotype three polymorphic sites of human genome. We show that the three SNP sites can be genotyped under identical hybridization conditions. The approach demonstrates excellent SNP discrimination at room temperature in real time without the need for precise temperature control.
Results
Optimization of the universal molecular beacon probe
In a traditional MB approach the analyte sequence dictates the MB primary structure because the loop is complementary to the analyte. BDP approach introduces the ability to optimize a MB probe and use it as a universal reporter. The following criteria for MB design were used: 1) the stem structure was chosen to be as short as possible to ensure high hybridization rates.[16] On the other hand, the stem should be stable to provide efficient quenching of the fluorophore. 2) The loop structure was A–T rich to minimize possible non-specific interactions in the reaction mixtures containing multiple analytes. 3) S/B should be as high as possible. 4) The absolute fluorescence of the probe should be high in order to provide a stable, well-reproducible signal.
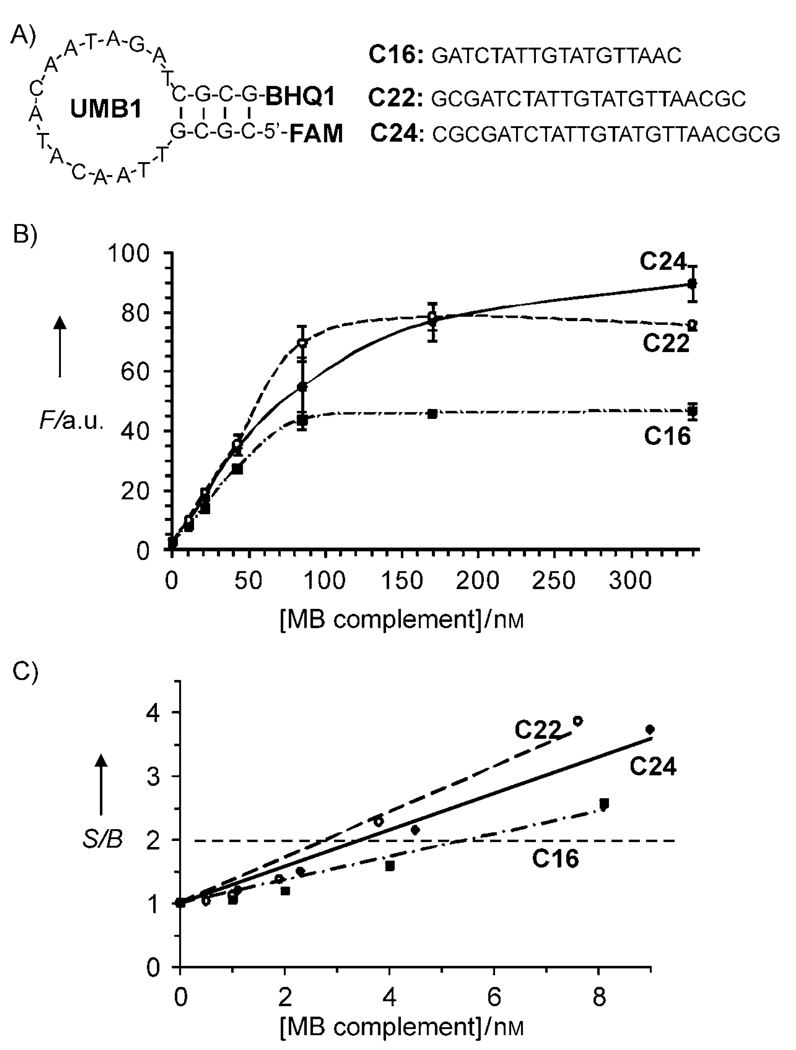
The sequence of UMB (Table 1) forms only one secondary structure (Figure 2A) as predicted by Mfold software.[17] UMB has a short 4-nucleotide stem with four alternating G–C base pairs for the increased stability. The stem Tm was calculated to be ~60 °C, which is high enough for the MB probe to acquire a stable stem–loop conformation at room temperature (20 °C).
Table 1.
Oligonucleotides used in the study[a]
| Name | Sequence | Purification |
|---|---|---|
| UMB | FAM-5′-CGC GTT AAC ATA CAA TAG ATC GCG-BHQ1 |
double HPLC |
| UMB′ | FAM-5′-CGC GTT AAC ATA CAA TAG ATC GCG |
HPLC |
| C18 | 5′-GAT CTA TTG TAT GTT AAC | SD[b] |
| C22 | 5′-GC GAT CTA TTG TAT GTT AAC GC | SD |
| C24 | 5′-CGC GAT CTA TTG TAT GTT AAC GCG | SD |
| BDP rs14-f | 5′-CCT GCT CTG AGG CCA GC/TEG/TAT GTT AAC |
SD |
| BDP rs14m-A | 5′-GAT CTA TTG/TEG/CAG TTT TGC | SD |
| BDP rs14m-G | 5′-GAT CTA TTG/TEG/CAG TTC TGC | SD |
| BDP rs71-f | 5′-ACC TAA TGA CAG ACG T/TEG/TAT GTT AAC |
SD |
| BDP rs71m-A | 5′-GAT CTA TTG/TEG/TCA CTA CAC | SD |
| BDP rs71m-G | 5′-GAT CTA TTG/TEG/TCA CCA CAC | SD |
| BDP rs87-f | 5′-TA GCA GAG TGT GAC AAA/TEG/TAT GTT AAC |
SD |
| BDP rs87m-C | 5′-GAT CTA TTG/TEG/AAA TGT ACT | SD |
| BDP rs87m-T | 5′-GAT CTA TTG/TEG/AAA TAT ACT | SD |
| rs14-G | 5′-ACT GGG CTG ATG TGG GTT CTT TGC AGA ACT G GC TGG CCT CAG AG C AGG GA |
SD |
| rs14-A | 5′-ACT GGG CTG ATG TGG GTT CTT TGC AAA ACT G GC TGG CCT CAG AG C AGG GA |
SD |
| rs71-A | 5′-GAA AGG CAT ATC GTA TTA ACT GTG TAG TGA ACG TCT GTC ATT AGG TTT AGC |
SD |
| rs71-G | 5′-GAA AGG CAT ATC GTA TTA ACT GTG TGG TGA ACG TCT GTC ATT AGG TTT AGC |
SD |
| rs87-C | 5′-ATA CCA CTG CAC TGA AGT ATA AGT ACA TTT TTT GTC ACA CTC TGC TA A CT |
SD |
| rs87-T | 5′-ATA CCA CTG CAC TGA AGT ATA AGT ATA TTT TTT GTC ACA CTC TGC TAA CT |
SD |
Stem-forming nucleotides of MB probe are shown in italics, “/TEG/” indicates triethylene glycol linker inserts; the underlined nucleotides indicate the SNP sites and the positions complementary to SNPs.
SD is standard desalting.
Figure 2.
Design and performance of the universal MB probe (UMB). A) Structures of UMB and complementary oligonucleotides. B) Fluorescent response of UMB in the presence of different concentrations of complementary DNA: UMB complement C24 (●), UMB complement C22 (○) or UMB complement C18 (■). C) Signal-to-background ratio for the complexes of the MB probe in the presence of low concentrations of C24, C22 or C18. The S/B threshold of 2 is shown by the horizontal dashed line. Assay conditions: 100 nm MB, 50 mm Tris–HCl, pH 7.4, 50 mm MgCl2, 20°C.
The S/B of UMB was studied by using three complementary oligonucleotides (Figure 2A), which were chosen to hybridize with all MB nucleotides (C24), with 22-nucleotide-long internal fragment (C22) or with 18 nucleotides (C18). As expected, longer complementary oligonucleotides enabled greater plateau fluorescence because the rigid structure of the double-stranded DNA maximizes the separation of the fluorophore from the quencher. Indeed, C24, C22, and C18 triggered fluorescence response with S/B of ~36, 33, and 19, respectively, at saturated concentrations (Figure 2B). It was found that a twofold fluorescence increase was achieved in the presence of as low as 3–6 nm complementary oligonucleotides (Figure 2C). These values correspond to the typical limit of detection of conventional MB probes.[2]
Design of BDP for the recognition of three human SNP sites
Three polymorphic sites of human genome, namely rs1490413, rs876724, and rs717302 (abbreviated here as rs14, rs87, and rs71, respectively), were selected as model SNPs to be analyzed by UMB and the pairs of strands m and f. These three SNP sites were previously demonstrated to be useful markers for human identification.[18] Six oligonucleotides that mimic both alleles for each of the three sites were used as analytes in this study (Figure 3). Pairs of strands m and f had short (9 nt) MB-binding arms, which ensured little background interaction with UMB in the absence of the analyte.
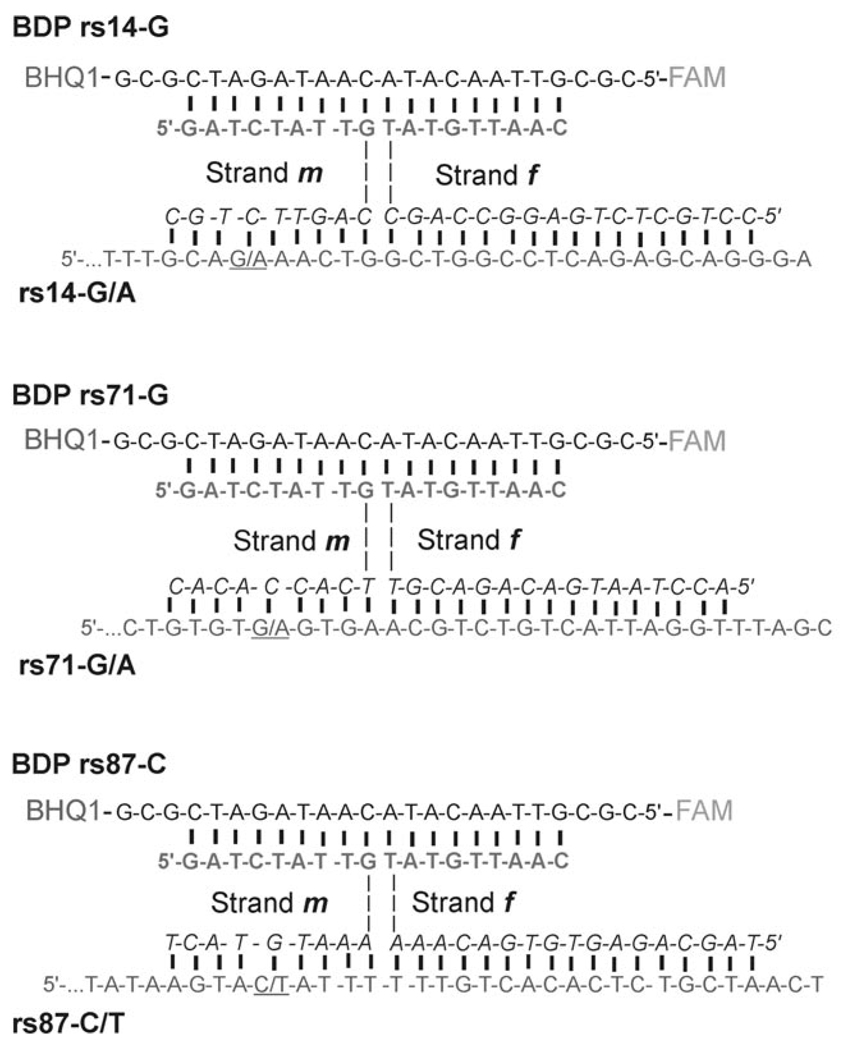
Figure 3.
Structures of BDPs in the complex with cognate analytes. Triethylene glycol linkers are represented as dashed lines. BHQ1 is black hole quencher 1; FAM represents fluorescein residue. Only the 3′-end sequences of each analyte are shown (see Table 1 in the Experimental Section for full sequences). SNP sites are underlined. Analyte-binding arms of strands m and f are in italic.
The analyte-binding arms of strands m and f were chosen to have different affinities to the analyte. Specifically, each f strand contained a long (16–17 nt) analyte-binding arm. This enabled hybridization with high affinity to the fragment adjacent to the SNP site. Therefore, strand f formed a complex with a target containing either of the two alleles. On the contrary, the analyte-binding arm of strand m was short (9 nt) and allele-specific (Figure 3). The short hybrid is stable only if the analyte is fully complementary, thus allowing high fluorescence of the probe only in the presence of one allele. These features contributed to excellent BDP selectivity.
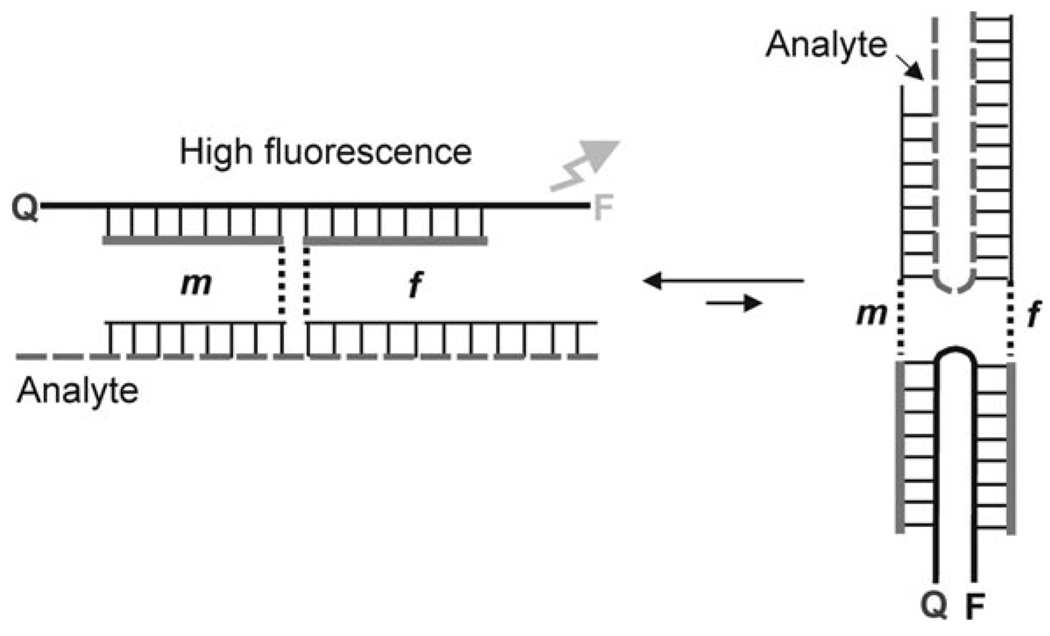
Analyte-binding arms were connected to MB-binding arms by triethylene glycol (TEG) linkers.[19] The introduction of TEG linkers was essential to obtain the highest S/B ratio. When no linkers were used, the analyte-dependent change in fluorescence was significantly lower (data not shown). This observation can be explained by the fact that DNA four-way junctions exist as a mixture of two right-handed antiparallel crosses.[20] In the case of BDP, only one of these conformers contains an MB probe in elongated form, which allows for a substantial fluorescence increase (Figure 4). In general, the ratio between the two conformers depends on the nucleotide composition at the point of strands exchange, which in the case of nucleic acid analysis would be predetermined by the analyte sequence. In practice, however, it is important that the MB probe acquires an elongated conformation in the quadripartite associate independently of the analyte sequence. This is realized in the complexes shown in Figure 3, in which UMB and the analytes acquire the preferred elongated conformations, whereas strands m and f are bent at the point of the TEG inserts.
Figure 4.
Two alternative conformations of binary probe quadripartite complex. The left conformation contains a MB probe in an elongated conformation thus allowing a high fluorescent signal. The right conformation contains fluorophore close to the quencher, which prevents significant increase in fluorescence of the quadripartite complex.
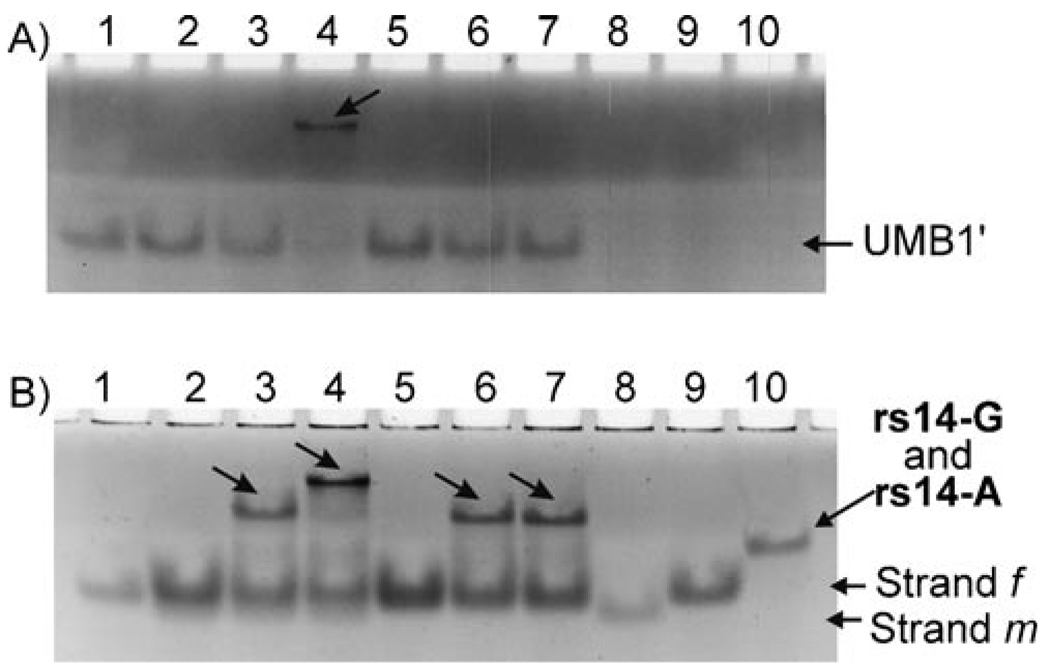
For each SNP site, two m strands specific to each of the two alleles were used in the assay. The ability of strands m and f to form quadripartite complexes with UMB and the complementary analytes was verified by native PAGE. Low-mobility bands, which correspond to the quadripartite complexes (shown in Figure 3) were observed only in the presence of the fully complementary analytes (data not shown). However, these complexes were visualized only after staining the gel with SYBR Gold. We could not visualize the complex by using an intrinsic fluorescence of the MB’s FAM group because of the residual quenching of the fluorescence by BHQ1 even in the open conformation. To trace the UMB-containing complexes in the gel, we employed a singly labeled oligonucleotide hairpin, UMB′, which lacked a quencher at its 3′ terminus (the structure is shown in Table 1). Figure 5 demonstrates that the UMB′-containing associate was formed only when strands m and f of BDP rs14-A and the fully complementary analyte were present (Figure 5A, lane 4, indicated by an arrow). The mobility of the observed band corresponds to the predicted migration of the quadripartite complex composed from UMB′, strands m and f and the analyte. Importantly, the mismatched rs14-G did not produce a fluorescent complex (Figure 5A, lane 3). When the same gel was stained with SYBR Gold, additional associates with low gel mobility were visualized (Figure 5B, lanes 3, 6 and 7, respectively). These associates did not contain UMB′ because they were not visible in the unstained gel. Based on the gel migration rate, the band was attributed to the hybrids of strand f with rs14-A or rs14-G. PAGE analysis of BDP rs71 and BDP rs87 in the presence of the analyzed DNA revealed similar formation of high-molecular-weight associates only in the presence of fully complementary analytes (data not shown).
Figure 5.
Analysis of BDP rs14-A in 12% native polyacrylamide gel by using UMB′ (FAM-5′-CGC GTT AAC ATA CAA TAG ATC GCG). UMB′ (100 nm) was incubated either alone (lane 1) or with different combinations of 300 nm strands m and f and 75 nm analytes rs14-A (matched) or rs14-G (mismatched). Lane 2: UMB′, m and f; lane 3: UMB′, m, f and rs14-G; lane 4: UMB′, m, f, rs14-A; lane 5: UMB′ and f; lane 6: UMB′, f and rs14-G; lane 7: UMB′, f and rs14-A; lanes 8–10 contained pure oligonucleotides m, f or rs14-A, respectively. A) Gel without staining (only UMB′-containing bands are detected). B) The same gel after SYBR Gold staining (all oligonucleotides in the gel are detected). Note, strands m and f were used in fourfold excess over analytes; thus no analyte was observed in its free form in the samples containing strands m and f.
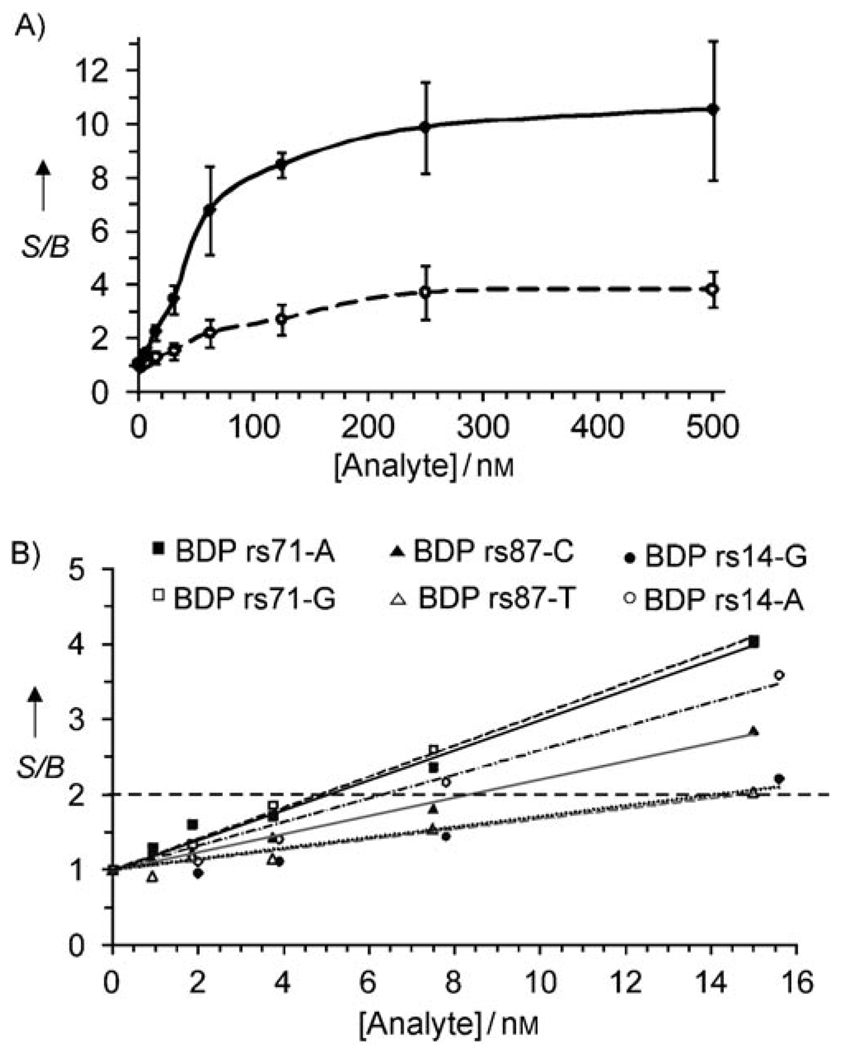
When different concentrations of the fully complementary analytes were added to BDPs, the fluorescence of the probes increased in a dose-dependent manner (Figure 6A, solid curve, for BDP rs14-G as an example). The fluorescence enhancement was approximately tenfold at saturation. This value is ~1.9 times lower than maximal fluorescence enhancement observed for UMB hybridized to the complementary oligonucleotide C16, and comparable with that of other MB probes that are conventionally used for nucleic acid analysis.[2] The mismatched analytes also triggered the concentration-dependent increase in BDPs fluorescence (Figure 6A, dashed curve). However, this increase was significantly lower than that in the presence of the true analyte, thus allowing discrimination between the two alleles. The discrimination factor varied from 3 to 10 depending on the analyte concentration. The discrimination factors can be further improved by introducing conformational constrains in the form of stem loop in the analyte binding arm of strand m.[15b,c,e]
Figure 6.
A) Fluorescence enhancement (S/B) for BDP rs14-G (300 nm strands m and f and 100 nm UMB) in the presence of perfectly matched analyte rs14-G (●) or mismatched analyte rs14-A (○) as functions of the analyte concentrations. Fluorescence emission of the BDP rs14-G at 517 nm in the absence of the analyte is taken as a background. B) Fluorescent enhancement for all BDPs at low analyte concentrations. The S/B threshold of 2 is shown by a horizontal dashed line. Assay conditions: 100 nm UMB; strands: 300 nm for BDP rs14-G (●) or BDP rs14-A (○), 120 nm for BDP rs71-G (□) or BDP rs71-A (■), 600 nm for BDP rs87-C (▲), 1000 nm for BDP rs87-T (△).
To estimate the lowest concentration of an analyte that can be reliably distinguished from the absence of the analyte, we employed twofold fluorescence enhancement as a criterion. It was determined that the BDPs were able to detect as low as 5–15 nm perfectly complementary analytes (Figure 6B). This limit of detection (LOD) is comparable with that of MB approach (3–6 nm, Figure 2C).
Selectivity and specificity of binary DNA probe
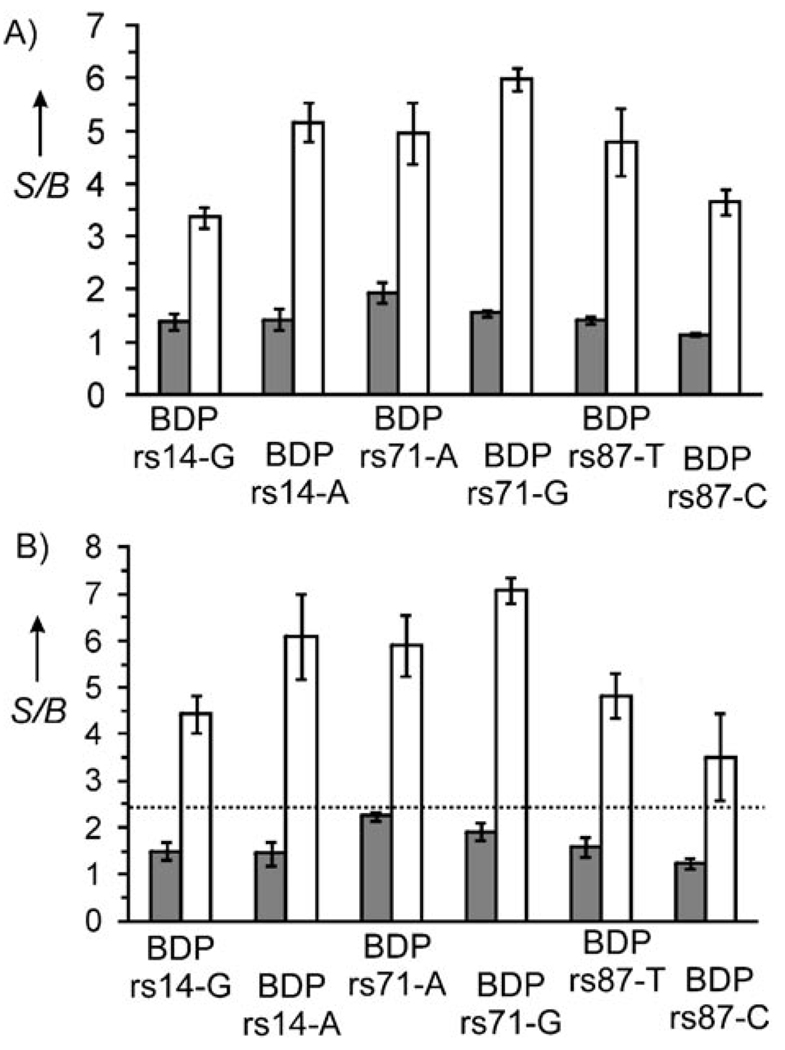
It was found that all six BDPs reliably distinguish fully matched from single-base-mismatched analytes (Figure 7A). Indeed, the fluorescent signal was within the background (S/B ~1) in the presence of mismatched nucleotides (gray bars), whereas addition of the true analyte increased fluorescence 3–6 times (white bars).
Figure 7.
Specificity and selectivity of binary DNA probes. A) Fluorescence enhancement of BDPs in the presence of the correspondent matched (white bars) or mismatched (gray bars) analytes. B) Fluorescence enhancement of BDPs in the presence of all six DNA analytes (white bars) or all DNA analytes except for the correspondent matched analyte (gray bars). The dashed line indicates the signal-threshold level. All experiments were carried out in triplicate. The error bars represent three standard deviations. Assay conditions: 100 nm UMB, 25 nm analytes, 300 nm BDP rs14-G and BDP rs14A, 120 nm BDP rs71-A and BDP rs71-G, 1000 nm BDP rs87-T, 600 nm BDP rs87-C, 50 mm Tris–HCl, pH 7.4, 50 mm MgCl2, 20°C.
The ability of BDPs to detect fully matched DNAs in the presence of other nucleic acids (detection specificity) is demonstrated in Figure 7B. High fluorescent signals were observed only when samples contained all six, including fully matched analytes (white bars). In contrast, the fluorescent signal was within the background for each of the six BDP tested in the presence of five mismatched analytes (no matched analyte added; gray bars).
This result demonstrates the high discriminative power of the binary approach. BDPs are able to report fully matched analytes even in the presence of other single-base-substituted fragments (Figure 7B). Remarkably, positive signals for each of the six probes tested were above any negative signal. This allows implementing a threshold (Figure 7B), which can universally and undeniably distinguish true positive from false positive signals. Importantly, the BDP performance was achieved at room temperature and in the same hybridization buffer. Thus, no tedious optimization of hybridization conditions was necessary.
Discussion
The elegance of the MB design has made this probe one of the most widely used tools for nucleic acid analysis. However, the MB design is not as simple as just the attachment of arbitrary stem-forming nucleotide sequences to the previously designed linear probes. The stem arms can interact with flanking target sequences and change the hybridization specificity. Another problem arising from the presence of a stem is the thermodynamic conformational switch between hairpin and non-hairpin structures. This is known as stem invasion, which is a result of the unwanted intramolecular hybridization between the stem and the loop fragments. Stem invasion can cause a significant fluorescence background due to incomplete quenching of the fluorophore. Optimization of the MB structure for each new analyte to avoid stem invasion complicates MB design. Sometimes the stem invasion cannot be avoided at all, resulting in poor probe performance. Continuous efforts in modernization of MB probes have been addressing this issue.[21] The improvements take advantage of non-natural modifications to the MB probe, which make such MB probes unaffordable for the majority of laboratories.
The BDP approach eliminates the problem of stem invasion because the optimized MB probe can be designed to avoid the undesired folding and used as a universal fluorescent reporter for the analysis DNA/RNA targets with arbitrary sequences. Furthermore, the stem-forming nucleotides of UMB do not interfere with probe hybridization because the UMB does not interact directly with the analyte. Moreover, the stem-forming nucleotides of UMB can participate in hybridization with MB-binding arms of strands m and f. Indeed, the UMB probe designed in this study folds in a single stem–loop structure. The two stem-forming nucleotides are complementary to MB-binding arms of the adaptor strands. We demonstrate that UMB can be used to detect at least six targets. Importantly, the design of the adaptor strands m and f is straightforward: change of the analyte-binding arm sequences was the only modification made for the adjustment of the probe for each new analyte. UMB probe designed in this study represents a first universal reporter, the performance of which needs to be further improved in terms of S/B ratio.
The quadripartite associate formed in the presence of the analyte contains a DNA four-way junction motif (4J). It is a naturally occurring, well-studied structure.[20b] Unlike the three-way junction, in 4J all nucleotides form base pairs at the position of strand exchange and, in the presence of Mg2+, are involved in stacking interactions. Owing to these features, 4J represents a stable and practically useful platform for the association of complex DNA assemblies. Indeed, 4J is the most popular branched structure used by DNA nanotechnology to build objects of desired shapes and functions[22] as well as computational elements.[23] We used the 4J as a basis for the assembly of fluorescent complexes in which each constructing element is specialized to serve a distinct function as follows. Strand f forms a stable complex with the analyte and can unwind its secondary structure if required. Strand m forms a short sensitive to a mismatch hybrid with the SNP site, and is responsible for the probe specificity. MB probe reports the formation of the 4J associate. It is not surprising that splitting the functional roles between the three components led to a superior sensor for nucleic acids.
Indeed, the binary probe achieves high specificity in recognition of closely related targets at room temperature without the need for precise temperature control for accurate allele discrimination. We analyzed the mixtures of three pairs of SNP-containing DNA fragments. Each of the six probes generated high fluorescence only when the analyzed oligonucleotide mixture contained the fully complementary oligonucleotide even in the presence of noncomplementary targets (Figure 7B). For the simplicity of the experimental setup we designed BDPs that are highly specific at ambient temperature (~20 °C). However, optimal temperature can be adjusted to the required value by varying the length of the analyte-binding arm of strand m. Our preliminary data demonstrate that this approach is highly specific even at temperatures as high as 63°C, which makes it perfect for application in real-time PCR assays. The binary probe, therefore, represents an easily adoptable and versatile tool for nucleic acid analysis, the full potential of which is yet to be realized.
Conclusions
This study demonstrates multiple advantages of the MB-based binary probe. First, one MB probe is sufficient for the analysis of many DNA or RNA analytes. This opens the possibility of synthesizing bulk amounts of an optimal MB probe and using it as a universal reporter in various applications. Second, the probe allows accurate SNP genotyping even at room temperature in regular hybridization buffers; this eliminates the need for optimization of hybridization conditions or temperature control for accurate SNP genotyping. These properties, taken together with the real-time signal generation offered by an MB probe, could be of great value in multiplex SNP assays. The branched nature of the quadripartite associate formed by the probe opens a venue for application of DNA nanotechnology in nucleic acid analysis.
Experimental Section
Materials and instrumentation
DNAse/RNAse-free water was purchased from Fisher Scientific, Inc. (Pittsburgh, PA) and used for all buffers and for the stock solutions of oligonucleotides. Molecular beacon was custom-made by TriLink BioTechnologies, Inc. (San Diego, CA). All other oligonucleotides were obtained from Integrated DNA Technologies, Inc. (Coralville, IA). The oligonucleotides were dissolved in water and stored at −20 °C until needed. Stock concentrations of oligonucleotides were calculated by measuring the absorption of the solutions at 260 nm by using a Perkin–Elmer Lambda 35 UV/Vis spectrometer (San Jose, CA). Extinction coefficients of oligonucleotides were calculated by using OligoAnalyzer 3.1 software (Integrated DNA Technologies, Inc.). Molecular beacon was additionally purified by denaturing polyacrylamide gel electrophoresis followed by HPLC purification by using a JASCO XLC HPLC system (Easton, MD). Purification reduced the background fluorescence by at least twice. For HPLC purification, MB was loaded on a Phenomenex Oligo-RP column (150 × 10 mm) and eluted with a linear 3–50% MeCN gradient in 0.05 m LiClO4 over 30 min. The purified molecular beacon was precipitated by 3% LiClO4 in acetone; the pellet was washed with acetone and air dried. Fluorescent spectra were recorded on a Perkin–Elmer LS-55 luminescence spectrometer equipped with a Hamamatsu xenon lamp. Experiments were performed at excitation wavelength of 485 nm. Emission of FAM was monitored at 517 nm. Excitation and emission slits were 5 and 20 nm, respectively. The data were processed by using Microsoft Excel.
MB probe assay
A solution of UMB (100 nm, 120 µL) in 50 mm Tris–HCl, pH 7.4, 50 mm MgCl2 was incubated alone or in the presence of various concentrations of UMB complements (5–500 nm) for 15 min at room temperature (20 °C). Then fluorescent emission spectra of the solutions were recorded, and the fluorescence intensities at 517 nm were plotted against the UMB complements concentration.
BDP assay
A solution of UMB (100 nm, 120 µL) in 50 mm Tris–HCl, pH 7.4, 50 mm MgCl2 was mixed with BDP strands f and m and incubated alone or in the presence of either matched or mismatched analytes for 15 min at RT (20 °C). Then fluorescent emission spectra of the solutions were recorded, and the fluorescence intensities at 517 nm were plotted against the analyte concentrations.
Native PAGE
Nondenaturing gel electrophoresis was carried out at RT by using 12% polyacrylamide gels (acrylamide/bisacrylamide, 19:1) in the reaction buffer containing 50 mm Tris–HCl, pH 7.4, 50 mm MgCl2. Oligonucleotide-containing samples (20 µL) were mixed with loading buffer containing the reaction buffer, 0.05% each of Bromophenol Blue and Xylene Cyanol, and 50% glycerol. The gels were run on an Ellard Instrumentation (Monroe, WA) vertical electrophoresis system at 100 V, stained with SYBR Gold and visualized by using a U:Genius imaging system (SYNGENE, Frederick, MD).
Multiplex detection
Seven analyte mixtures were prepared: one contained all six analytes used in the study; the other six mixtures contained only five out of six possible analytes. The concentration of each analyte in the mixtures was 2.5 µm. A solution of UMB (100 nm) was mixed with strand f and strand m of a particular BDP and incubated alone or in the presence of analyte mixtures (25 nm) for 15 min at RT (20 °C). Each BDP was incubated with the mixture containing all analytes, or with the mixture containing all but the specific matched analyte. The BDP concentration was optimal for its performance: BDP rs14-G and BDP rs14-A (300 nm), BDP rs71-A and BDP rs71-G (120 nm), BDP rs87-C (600 nm), and BDP rs87-T (1000 nm). The fluorescence intensity at 517 nm was measured.
Acknowledgements
D.M.K. is grateful to Fred R. Kramer, Salvatore A. E. Marras and Sanjay Tyagi for useful discussion. This study was supported by UCF Office of Research and Commercialization, College of Science, Chemistry Department and NHGRI R21 HG004060.
References
- 1.a) Morrison LE, Halder TC, Stols LM. Anal. Biochem. 1989;183:231–244. doi: 10.1016/0003-2697(89)90473-9. [DOI] [PubMed] [Google Scholar]; b) Cardullo RA, Agrawal S, Flores C, Zamechnik PC, Wolf DE. Proc. Natl. Acad. Sci. USA. 1988;85:8790–8794. doi: 10.1073/pnas.85.23.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ebata K, Masuko M, Ohtani H, Kashiwasake-Jibu M. Photochem. Photobiol. 1995;62:836–839. doi: 10.1111/j.1751-1097.1995.tb09144.x. [DOI] [PubMed] [Google Scholar]; d) Santangelo P, Nitin N, Bao G. Ann. Biomed. Eng. 2006;34:39–50. doi: 10.1007/s10439-005-9003-6. [DOI] [PubMed] [Google Scholar]; e) Silverman AP, Kool ET. Adv. Clin. Chem. Trends Biotechnol. 2005;23:225–230. doi: 10.1016/j.tibtech.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 2.a) Tyagi S, Kramer FR. Nat. Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]; b) Marras SA, Tyagi S, Kramer FR. Clin. Chim. Acta. 2006;363:48–60. doi: 10.1016/j.cccn.2005.04.037. [DOI] [PubMed] [Google Scholar]; c) Wang K, Tang Z, Yang CJ, Kim Y, Fang X, Li W, Wu Y, Medley CD, Cao Z, Li J, Colon P, Lin H, Tan W. Angew. Chem. 2009;121:870–885. doi: 10.1002/anie.200800370. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2009;121:870–885. [Google Scholar]; Angew. Chem. 2009;121:870–885. [Google Scholar]; d) Li Y, Zhou X, Ye D. Biochem. Biophys. Res. Commun. 2008;373:457–461. doi: 10.1016/j.bbrc.2008.05.038. [DOI] [PubMed] [Google Scholar]; e) Venkatesan N, Seo YJ, Kim BH. Chem. Soc. Rev. 2008;37:648–663. doi: 10.1039/b705468h. [DOI] [PubMed] [Google Scholar]
- 3.a) Du H, Disney MD, Miller BL, Krauss TD. J. Am. Chem. Soc. 2003;125:4012–4013. doi: 10.1021/ja0290781. [DOI] [PubMed] [Google Scholar]; b) Ye Y, Bloch S, Achilefu S. J. Am. Chem. Soc. 2004;126:7740–7741. doi: 10.1021/ja049441z. [DOI] [PubMed] [Google Scholar]; c) Liu J, Lu Y. Methods Mol. Biol. 2006;335:275–288. doi: 10.1385/1-59745-069-3:275. [DOI] [PubMed] [Google Scholar]; d) Grossmann TN, Roglin L, Seitz O. Angew. Chem. 2007;119:5315–5318. doi: 10.1002/anie.200700289. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:5223–5225. doi: 10.1002/anie.200700289. [DOI] [PubMed] [Google Scholar]; e) Lin YW, Ho HT, Huang CC, Chang HT. Nucleic Acids Res. 2008;36:e123. doi: 10.1093/nar/gkn537. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Yamana K, Ohshita Y, Fukunaga Y, Nakamura M, Maruyama A. Bioorg. Med. Chem. 2008;16:78–83. doi: 10.1016/j.bmc.2007.04.053. [DOI] [PubMed] [Google Scholar]; g) Conlon P, Yang CJ, Wu Y, Chen Y, Martinez K, Kim Y, Stevens N, Marti AA, Jockusch S, Turro NJ, Tan W. J. Am. Chem. Soc. 2008;130:336–342. doi: 10.1021/ja076411y. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Matsumoto K, Shinohara Y, Bag SS, Takeuchi Y, Morii T, Saito Y, Saito I. Bioorg. Med. Chem. Lett. 2009;19:6392–6395. doi: 10.1016/j.bmcl.2009.09.060. [DOI] [PubMed] [Google Scholar]; i) Zhang Z, Guo L, Tang J, Guo X, Xie J. Talanta. 2009;80:985–990. doi: 10.1016/j.talanta.2009.08.028. [DOI] [PubMed] [Google Scholar]; j) Nesterova IV, Erdem SS, Pakhomov S, Hammer RP, Soper SA. J. Am. Chem. Soc. 2009;131:2432–2433. doi: 10.1021/ja8088247. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Häner R, Biner SM, Langenegger SM, Meng T, Malinovskii VL. Angew. Chem. 2010;122:1249–1252. doi: 10.1002/anie.200905829. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2010;49:1227–1230. doi: 10.1002/anie.200905829. [DOI] [PubMed] [Google Scholar]; l) Shi C, Gu H, Ma C. Anal. Biochem. 2010;400:99–102. doi: 10.1016/j.ab.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Whitman DF, Dunbar SA. Recent Pat. DNA Gene Sequences. 2008;2:20–26. doi: 10.2174/187221508783406558. [DOI] [PubMed] [Google Scholar]
- 5.a) Bao G, Rhee WJ, Tsourkas A. Annu. Rev. Biomed. Eng. 2009;11:25–47. doi: 10.1146/annurev-bioeng-061008-124920. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tyagi S. Nat. Methods. 2009;6:331–338. doi: 10.1038/nmeth.1321. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet G, Tyagi S, Libchaber A, Kramer FR. Proc. Natl. Acad. Sci. USA. 1999;96:6171–6176. doi: 10.1073/pnas.96.11.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Altshuler D, Brooks LD, Chakravarti A, Collins FS, Daly MJ, Donnelly P.Nature 20054371299–1320.16255080 [Google Scholar]; b) Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, Sherry S, Mullikin JC, Mortimore BJ, Willey DL, Hunt SE, Cole CG, Coggill PC, Rice CM, Ning Z, Rogers J, Bentley DR, Kwok PY, Mardis ER, Yeh RT, Schultz B, Cook L, Davenport R, Dante M, Fulton L, Hillier L, Waterson RH, McPherson JD, Gilman B, Schaffner S, Van Etten WJ, Reich D, Higgins J, Daly MJ, Blumenstiel B, Baldwin J, Stange-Thomann N, Zody MC, Linton L, Lander ES, Altshuler D. Nature. 2001;409:928–933. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 8.a) Roberts RG, Passos-Bueno MR, Bobrow M, Vainzof M, Zatz M. Hum. Mol. Genet. 1993;2:75–77. doi: 10.1093/hmg/2.1.75. [DOI] [PubMed] [Google Scholar]; b) Tabara Y, Osawa H, Kawamoto R, Onuma H, Shimizu I, Miki T, Kohara K. Diabetes. 2009;58:791–795. doi: 10.2337/db07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Burwinkel B, Shanmugam KS, Hemminki K, Meindl A, Schmutzler RK, Sutter C, Wappenschmidt B, Kiechle M, Bartram CR, Frank B. BMC Cancer. 2006;6:268. doi: 10.1186/1471-2407-6-268. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Nat. Genet. 2007;39:17–28. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 9.Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J, Kruglyak L, Stein L, Hsie L, Topaloglou T, Hubbell E, Robinson E, Mittmann M, Morris MS, Shen N, Kilburn D, Rioux J, Nusbaum C, Rozen S, Hudson TJ, Lipshutz R, Chee M, Lander ES. Science. 1998;280:1077–1082. doi: 10.1126/science.280.5366.1077. [DOI] [PubMed] [Google Scholar]
- 10.a) Johnson GC, Todd JA. Curr. Opin. Genet. Dev. 2000;10:330–334. doi: 10.1016/s0959-437x(00)00075-7. [DOI] [PubMed] [Google Scholar]; b) Schork NJ, Fallin D, Lanchbury JS. Clin. Genet. 2000;58:250–264. doi: 10.1034/j.1399-0004.2000.580402.x. [DOI] [PubMed] [Google Scholar]; c) Beaudet AL, Belmont JW. Annu. Rev. Med. 2008;59:113–129. doi: 10.1146/annurev.med.59.012907.101800. [DOI] [PubMed] [Google Scholar]
- 11.Güzey C, Spigset O. Curr. Top. Med. Chem. 2004;4:1411–1421. doi: 10.2174/1568026043387791. [DOI] [PubMed] [Google Scholar]
- 12.a) Risch NJ. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]; b) Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 13.a) Budowle B, van Daal A. Biotechniques. 2008;44:603–608. doi: 10.2144/000112806. [DOI] [PubMed] [Google Scholar]; b) Sobrino B, Brion M, Carracedo A. Forensic Sci. Int. 2005;154:181–194. doi: 10.1016/j.forsciint.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 14.a) Chen X, Sullivan PF. Pharmacogenomics J. 2003;3:77–96. doi: 10.1038/sj.tpj.6500167. [DOI] [PubMed] [Google Scholar]; b) Xiao M, Kwok PY. Genome Res. 2003;13:932–939. doi: 10.1101/gr.987803. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Steemers FJ, Chang W, Lee G, Barker DL, Shen R, Gunderson KL. Nat. Methods. 2006;3:31–33. doi: 10.1038/nmeth842. [DOI] [PubMed] [Google Scholar]; d) Ragoussis J. Annu. Rev. Genomics Hum. Genet. 2009;10:117–133. doi: 10.1146/annurev-genom-082908-150116. [DOI] [PubMed] [Google Scholar]
- 15.a) Kolpashchikov DM. J. Am. Chem. Soc. 2005;127:12442–12443. doi: 10.1021/ja0529788. [DOI] [PubMed] [Google Scholar]; b) Kolpashchikov DM. J. Am. Chem. Soc. 2006;128:10625–10628. doi: 10.1021/ja0628093. [DOI] [PubMed] [Google Scholar]; c) Kolpashchikov DM. ChemBioChem. 2007;8:2039–2042. doi: 10.1002/cbic.200700384. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kolpashchikov DM. J. Am. Chem. Soc. 2008;130:2934–2935. doi: 10.1021/ja711192e. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Gerasimova YV, Cornett E, Kolpashchikov DM. ChemBioChem. 2010;11:811–817. doi: 10.1002/cbic.201000006. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kolpashchikov DM. Chem. Rev. 2010 doi: 10.1021/cr900323b. in press. [DOI] [PubMed] [Google Scholar]
- 16.Tsourkas A, Behlke MA, Rose SD, Bao G. Nucleic Acids Res. 2003;31:1319–1330. doi: 10.1093/nar/gkg212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuker M. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez J, Phillips C, Børsting C, Balogh K, Bogus M, Fondevila M, Harrison CD, Musgrave-Brown E, Salas A, Syndercombe-Court D, Schneider PM, Carracedo A, Morling N. Electrophoresis. 2006;27:1713–1724. doi: 10.1002/elps.200500671. [DOI] [PubMed] [Google Scholar]
- 19.TEG linkers add $40 per linker. The cost for three adaptor strands for genotyping of one SNP site is ~$150 (universal MB cost is not included). This is significantly lower than the cost of two MB probes (~$450 each = $900).
- 20.a) Churchill ME, Tullius TD, Kallenbach NR, Seeman NC. Proc. Natl. Acad. Sci. USA. 1988;85:4653–4656. doi: 10.1073/pnas.85.13.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lilley DMJ. Q. Rev. Biophys. 2000;33:109–159. doi: 10.1017/s0033583500003590. [DOI] [PubMed] [Google Scholar]
- 21.a) Browne KA. J. Am. Chem. Soc. 2005;127:1989–1994. doi: 10.1021/ja046369w. [DOI] [PubMed] [Google Scholar]; b) Kim Y, Yang CJ, Tan W. Nucleic Acids Res. 2007;35:7279–7287. doi: 10.1093/nar/gkm771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a) Shen Z, Yan H, Wang T, Seeman NC. J. Am. Chem. Soc. 2004;126:1666–1674. doi: 10.1021/ja038381e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seeman NC. Mol. Biotechnol. 2007;37:246–257. doi: 10.1007/s12033-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Buranachai C, McKinney SA, Ha T. Nano Lett. 2006;6:496–500. doi: 10.1021/nl052492p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lake A, Shang S, Kolpashchikov DM. Angew. Chem. 2010;122:4561–4564. doi: 10.1002/anie.200907135. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. Engl. 2010;49:4459–4462. doi: 10.1002/anie.200907135. [DOI] [PubMed] [Google Scholar]