Abstract
Whether physical functioning in patients with rheumatoid arthritis (RA) differs from that in patients with ankylosing spondylitis (AS) is presently uncertain. Such a comparison poses challenges, not only because the two diseases differ in the domains of functioning affected, but also because of the different instruments used to measure functional limitations. Limiting our analysis to studies using similar self-report questionnaires, we examined published observational studies of unselected cohorts of patients with RA and patients with AS to compare and contrast the severity of functional limitations. Available studies from a few direct comparisons, and mostly indirect comparisons, suggested that patients with RA are generally more severely limited in physical functioning throughout the disease course than patients with AS. Since most studies did not adjust adequately for potentially important confounders, such as age, gender, comorbidity, and disease duration, reported differences in functional disability between patients with RA and patients with AS must be interpreted cautiously.
Keywords: Rheumatoid arthritis, ankylosing spondylitis, physical function, functional limitation
Introduction
Rheumatoid arthritis (RA) and ankylosing spondylitis (AS) are two of the most prevalent types of chronic inflammatory arthritis. Because these diseases involve different joint areas, follow dissimilar courses, and preferentially affect different patient populations, they are expected to impact different domains of physical function. In this review, we outline the main instruments used to measure physical function in RA and AS, examine the challenges in comparing these two diseases, and compare the degree of functional limitations in RA and AS.
Physical functioning, a patient-centered measure, is an important component of health status. According to the World Health Organization International Classification of Functioning, Disability and Health (ICF), limitations in physical functioning is viewed as a complex interaction of the whole person (body functions and structures, activities and participation) with societal and environmental influences (contextual factors) (1). To simplify the use of the 1400 classification categories, disease-specific ICF core sets have been proposed that are considered to be the minimum set representative of certain diseases. Core sets for RA and AS have been described (2, 3) but need further validation.
The important role of physical functioning is reflected by its inclusion as a component of response criteria for RA and AS clinical trials (4, 5). Furthermore, physical functioning contributes to disease burden, as discussed by Kiltz and van der Heijde in this issue (6). The degree of functional limitation predicts health care costs in both RA and AS (7–16), and premature mortality in RA (17–20).
Methods used to measure functional limitations
Two approaches used to assess physical function are patient self-report questionnaires and performance measures. Self-report instruments ask respondents to rate their usual ability to perform tasks, typically activities of daily living. As subjective measures, self-report instruments rely on the subjects’ perceived difficulty in performing a task, which can be influenced by mood. Some investigators have proposed that direct observation of the performance of a task is a more “objective” measure of physical functioning. However, performance measures, such as timed chair stands or walking speed, may be effort-dependent, and therefore influenced by mood or other confounders. Moreover, the tasks tested in performance measures may not be relevant in everyday life, and more often measure capacity to perform a specific task than the person’s usual abilities. Because studies that assess physical function in the rheumatic diseases use self-report instruments almost exclusively, our review is focused on these measures. Physical performance measures in RA have been demonstrated to have good face validity, reliability, and prognostic utility (18, 21); however, we did not include them due to their undetermined applicability to patients with AS.
Physical function should be distinguished from impairments, such as joint deformities or limitations in the range of motion of peripheral or axial joints. Physical functioning evaluates one’s ability to accomplish purposeful tasks. While impairments can be one cause of functional limitations, measures of deformity or flexibility are not measures of physical function. Assessing functional limitations through its downstream consequences, such as work disability, is also limited because many factors other than functional limitations impact these measures.
Main instruments used to measure physical function
Comparison of functional limitations between RA and AS requires that the same measures be used. Because much of the literature on functional limitations in patients with AS uses AS-specific instruments, such as the Bath Ankylosing Spondylitis Functional Index, the Dougados Functional Index, or the Revised Leeds Disability Questionnaire, these studies cannot be used for comparisons. We focus on studies using the Health Assessment Questionnaire (HAQ) and its derivatives, and the Physical Functioning scale of the Short Form-36 (SF-36).
Health Assessment Questionnaire
The HAQ is one of the most widely used patient-oriented questionnaires of physical function (22). The HAQ Disability Index includes 20 items covering 8 domains of functioning (dressing and grooming, arising, eating, walking, hygiene, reach, grip, and usual activities), rated on a 4-level response (without any difficulty, with some difficulty, with much difficulty, and unable to do). The score can range from 0 to 3, with higher scores indicating more limitations.
Health Assessment Questionnaire for Spondyloarthropathies
A HAQ for the Spondyloarthropathies (HAQ-S) was devised to address concerns that the HAQ did not include all domains of functioning relevant to patients with spondylitis (23). The HAQ-S adds 2 domains to the original HAQ, consisting of 5 items that assess posture-associated activities and driving. Item scoring and possible range (0–3) are the same as the original HAQ. The HAQ-S is useful because the original HAQ can be calculated from it, thereby allowing comparison across diseases.
Modified Health Assessment Questionnaire
The modified HAQ (MHAQ) is an abridged version of the HAQ and contains only 8 of the original 20 questions (24). The score is the mean of responses to the eight questions, with a range from 0 to 3 (or 1 to 4). Although faster to complete, the MHAQ is more limited by floor effects than the original HAQ. Floor effects may arise when fewer questions are presented, because limitations in some omitted functions are not captured.
Hannover Functional Status Questionnaire
The Hannover Functional Status Questionnaire (FFbH) is a functional disability self-report questionnaire both highly correlated (r=0.87) and transformable to the HAQ (25). The FFbH assesses limitations with activities of daily living, and the total score represents the percent of full function (range from 0 to 100).
Short Form 36
The Short Form 36 (SF-36) is a generic 36-item health status questionnaire that is structured into an 8-scale profile (26). The SF-36 contains scales on physical functioning, vitality, pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health. The physical functioning scale contains 10 items on ability to perform activities of daily living, with a range from 0 (worst) to 100 (best).
Challenges in comparing functional limitations between RA and AS
Despite the similarities that RA and AS share as chronic systemic inflammatory rheumatic diseases, they have many substantive differences. RA and AS differ in the pattern of involved joints and therefore would be expected to impact different domains of physical functioning. RA affects primarily small peripheral joints, while AS involves predominantly the axial skeleton and entheses. Accordingly, RA would be expected to impact functions that depend on use of the hands, wrists, and feet, such as dressing and grooming, eating, gripping, and walking. In contrast, AS would be expected to affect primarily functions that depend on whole body or head movement, such as bending, crouching, reaching up, and driving. Comparison of the impact of each disease on physical functioning is therefore more challenging than would be the case if similar distributions of joints were affected.
Moreover, the reported severity of functional limitations depends on the types of functions assessed in different questionnaires. If a questionnaire predominately assesses functions mediated by peripheral joints, and functions mediated by the axial skeleton are underrepresented, then patients with conditions that involve peripheral joints may register more limitations, simply as a result of the spectrum of activities assessed. The HAQ has been noted to focus more on hand and upper extremity functions, and this focus may contribute to higher scores among patients with RA compared to patients with AS. AS-specific questionnaires, such as the HAQ-S, were developed to address these differences in the spectrum of affected functions across diseases. Differences in gender and age between patients with RA and AS also confound comparisons of crude scores. Women and older individuals with more comorbid diseases tend to report more limitations than men or those without comorbidity; these factors also favor higher scores in RA than AS. For proper comparisons, results that are stratified or adjusted for gender and age are needed.
Because functional limitations tend to increase with the duration of RA and AS, measures of functional limitations should also be stratified by duration of disease for optimal comparisons. Reporting of mean (or median) scores for a cohort of patients with a wide range of durations of disease is less informative than reporting scores in narrow bands of duration. Furthermore, the causes of functional limitations vary depending on where the patient lies in the disease course. Physical functioning in early RA is likely measuring impairment from active synovitis (a reversible cause of functional limitations), while physical functioning in late RA is likely measuring impairment from both chronic damage of the joints, tendons, and muscles (an irreversible cause) and disease activity (27–29). The relative impact of symptoms and damage on functioning may differ in patients with RA and AS. In a study of patients with AS, Landewe and colleagues reported that functional impairment was determined independently by both disease activity and radiographic damage of the cervical and lumbar spine (30).
With these considerations, we searched the English-language literature for observational studies of unselected cohorts of patients with RA or AS that reported results for either the HAQ (or HAQ-S) or SF-36 physical function score. We excluded studies that focused on particular subsets of patients (e.g. early RA cohorts, treatment registries, or interventional studies) because these samples introduced additional confounders (e.g. disease duration or disease activity) that further complicated comparisons between RA and AS. We included all AS studies but only RA studies of at least 200 subjects. Because no studies reported rates of progression of functional limitation in AS over time, we did not include RA studies that reported these results. Although our search was intended to be complete, we cannot guarantee that we identified all relevant studies, particularly RA studies in which physical function scores might have been incidentally noted. We organized studies into two types: those that directly compared physical functioning between patients with RA and patients with AS recruited using the same procedures, and those that reported physical functioning in patients with RA or AS patients alone. We tabulated the latter studies for indirect comparisons.
Studies of direct comparisons
Three of the six studies that directly compared functional limitations between patients with RA and patients with AS examined subjects in the German national rheumatic disease databank (Table I). Zink and colleagues abstracted data of outpatients with inflammatory rheumatic diseases at 21 arthritis centers in Germany between 1993 and 1997 (31). Among 52,444 patients with RA and 8,776 patients with AS, patients with RA were nearly 13 years older than patients with AS. More women than men had RA, but significantly more men than women had AS. In both disease groups, functional limitations were associated with age, gender, and disease duration. In this study, severe functional disability was reported as <50% of full functional capacity on the FFbH. For each age/gender subgroup, a larger proportion of patients with RA than patients with AS had severe functional disability, although many proportions were only slightly larger in patients with RA. A larger proportion of older patients with RA or AS experienced severe functional disability than their younger counterparts. For the same age range, women systematically reported more severe functional disability than men. Because the measure of functional limitations was dichotomized (high versus low), this study does not provide information on the relative distribution of limitations between RA and AS among patients with milder degrees of limitation.
Table I.
Comparison of functional limitations in patients with rheumatoid arthritis and ankylosing spondylitis at the same study sites.
| Reference | Year | Patients no. |
Age* yrs |
Men % |
Disease duration, yrs* |
HAQ* | SF-36 Physical Function* |
|---|---|---|---|---|---|---|---|
| Zink et al.31 | 2000 | ||||||
| RA | 52444 | 57.1 ± 13.4 | 23 | 8.7 ± 9.5 | Age ≤ 40: (M) 8.4; (W) 10.6† | ||
| Age 41–50: (M) 15.9; (W) 18.9† | |||||||
| Age 1–60: (M) 20.6; (W) 27.9† | |||||||
| Age 61–70: (M) 24.3; (W) 36.5† | |||||||
| Age > 70: (M) 31.1; (W) 49.9† | |||||||
| AS | 8776 | 43.9 ± 12.7 | 69 | 14.7 ± 11.2 | Age ≤ 40: (M) 7.7; (W) 8.8† | ||
| Age 41–50: (M) 12.8; (W) 16.2† | |||||||
| Age 51–60: (M) 16.8; (W) 21.3† | |||||||
| Age 61–70: (M) 19.0; (W) 35.2† | |||||||
| Age > 70: (M) 28.4; (W) 44.2† | |||||||
| Huscher et al.32 | 2006 | ||||||
| RA | 4351 | 53 | 21 | 18.8† | |||
| AS | 827 | 46 | 62 | 16.2† | |||
| Zink et al.33 | 2006 | ||||||
| RA | 9627 | 60 | 23 | 11.2 | 22.0†; 53.6§ | ||
| AS | 1378 | 48.7 | 63 | 15.5 | 15.7†; 51.5§ | ||
| Kjeken et al.34 | 2006 | ||||||
| RA | 1041 | 61.5 ± 15.1 | 22 | 14.1 ± 11.3 | 1.6±0.55¶ | ||
| AS | 152 | 46.9 ± 13.1 | 58 | 15.2 ± 12.3 | 1.4±0.43¶ | ||
| Chorus et al.35 | 2003 | ||||||
| RA | 1056 | 49.0 ± 8.3 | 28 | 11.9 ± 9.1 | (M) 52.7±27.4; (W) 47.9±26.3 | ||
| AS | 658 | 43.5 ± 9.4 | 70 | 12.3 ± 8.0 | (M): 67.8±24.1; (W): 61.3±23.1 | ||
| Salaffi et al.36 | 2009 | ||||||
| RA | 469 | 57.5 ± 14.3 | 28 | 6.1 ± 4.2 | 41.8±20.6 | ||
| AS | 164 | 51.7 ± 9.2 | 81 | 8.2 ± 4.6 | 52.6±21.2 |
HAQ: Health Assessment Questionnaire; SF-36 PF: Short Form 36 Physical Functioning subscale; FFbH: Hannover Functional Status Questionnaire; M: Men; W: Women.
All values are mean ± standard deviation, unless otherwise noted
Proportion of patients with <50% full functional capacity on FFbH
Proportion of patients with HAQ >1.7 (transformed values from FFbH scores)
Proportion of patients with HAQ >1 (transformed values from FFbH scores)
Modified Health Assessment Questionnaire (range 1–4).
Using the same database, Huscher and colleagues studied costs of illness in patients <65 years of age (32). Patients with RA were on average older than patients with AS (Table I). A larger proportion of patients with AS had disease duration >10 years (61.9% versus 42.3%, respectively). In an unadjusted comparison, a slightly higher proportion of patients with RA reported moderate functional disability, as represented by a HAQ score >1.7.
The third analysis of the German Collaborative Arthritis Centres databank compared quality of life and treatment among patients with psoriatic arthritis, RA and AS (33). As in the previous studies, patients with RA were older, more likely women, and had a shorter disease duration than patients with AS (Table I). In unadjusted comparisons, the proportion of patients with severe functional disability (represented by a score of <50 on the FFbH) was slightly higher among those with RA than AS. About one-half of patients in each group had a HAQ >1.0.
In a Norwegian study (34), patients with RA and AS from a medical center-based register were surveyed regarding their satisfaction with medical care. Patients with RA were older and more likely women than patients with AS (Table I). Mean MHAQ scores were significantly higher among patients with RA than patients with AS.
Chorus and colleagues compared health-related quality of life in patients with RA and patients with AS from a Dutch nationwide register (35). Data were from random samples of patients age 16–59 in 1996–1997 (Table I). Mean disease duration was slightly higher in patients with AS. The mean physical functioning subscale score of the SF-36 was 15 points lower (indicating worse functioning) among men with RA than men with AS. Similarly, women with RA averaged 16 points lower than women with AS.
In an Italian study, Salaffi and colleagues compared health-related quality of life between patients with RA and patients with AS who were randomly selected from among outpatients at a university rheumatology clinic (36). Patients with RA were older and had a shorter disease duration than patients with AS (Table I). Mean scores on the physical functioning subscale of the SF-36 were lower for patients with RA than AS, indicating greater functional limitations in patients with RA.
Collectively, these studies suggest patients with RA have worse functioning than patients with AS. The differences were pronounced in studies that compared mean scores of the SF-36, while differences were only slight when disease groups were compared based on the proportion with severe disability on the FFbH. This difference could be due to either the measure used or the choice to dichotomize the results. This choice limits the comparisons that can be made, as we do not know if more patients with AS had little or no functional limitations, and more patients with RA had mild or moderate limitations. The comparisons are also confounded by differences in age, gender and disease duration, the influence of which was addressed in only some studies.
Studies of indirect comparisons
Inferences from indirect comparisons should be made cautiously because of heterogeneity among the cohorts. Studies evaluated patients of different time periods, geographical areas, practice settings, and demographic characteristics (Table II and Table III). Overall, HAQ scores were generally higher in patients with RA (HAQ of 1) than patients with AS (HAQ of 0.4). Among patients with AS, scores on the HAQ-S were higher (HAQ-S of 0.8) than scores on the HAQ, but still lower than the HAQ scores of patients with RA. Assessed with the physical functioning subscale of the SF-36, patients with RA generally scored lower than patients with AS, indicating worse functioning.
Table II.
Functional limitations with the Health Assessment Questionnaire in patients with rheumatoid arthritis and ankylosing spondylitis evaluated at different study sites.
| Reference | Year | Patients no. |
Age * yrs |
Men % |
Disease duration, yrs* |
HAQ* | MHAQ* | HAQ-S* |
|---|---|---|---|---|---|---|---|---|
| RA Sherrer et al.47 | 1986 | 681 | 62 ± 13 | 28 | 22 ± 12 | 1.4 | ||
| Sharp et al.48 | 1991 | 292 | 53.8 | 33 | 9.4 | 1.20 | ||
| Hawley et al.49 | 1991 | 624 | 58.6 ± 14.0 | 27 | 11.5 ± 9.3 | 1.3 ± 0.78 | ||
| Hochberg et al.50 | 1992 | 325 | 56.4 ± 13.2 | 22 | – | 1.3 | ||
| Gardiner et al.51 | 1993 | 208 | 55.4 ± 12.7 | 20 | 12.3 ± 9.6 | 1.78 ± 0.75** | ||
| Reisine et al.52 | 1995 | 392 | 48 ± 10 | 28 | 8.7 ± 7 | 0.50 ± 0.44 | ||
| Bendtsen et al.53 | 1995 | 222 | 63.1 | 24 | 17.8 | 1.37¶¶ | ||
| Reisine et al.54 | 1995 | 696 | 57 ± 10 | 21 | 15 ± 9 | 1.2 ± 0.71 | ||
| Houssien et al.55 | 1997 | 200 | 58.9 ± 13.3 | 26 | 11.3 ± 10.4 | 1.3 ± 0.9 | ||
| Kvien et al.56 | 1998 | 1030 | 62.3 ± 14.9 | 21 | 12.9 ± 11.4 | 1.70¶ | ||
| Chorus et al.57 | 2000 | 1056 | 49.0 | 28 | 11.9 | 1.0 | ||
| Westhoff et al.58 | 2000 | 273 | 60.7 ± 12.1 | 23 | 17 (2–77)§ | 1.96†† | ||
| Fransen et al.59 | 2002 | 803 | 59 ± 13 | 29 | 7 (2, 14)† | 1.0 (0.38,1.63)† | ||
| Yelin et al.60 | 2002 | 1269 | 56.7 ± 14.0 | 23 | 11.1 ± 10.2 | 1.2 ± 0.8 | ||
| Lajas et al.11 | 2003 | 201 | 64.3 ± 11.8 | 22 | 7.7 (3.3,14.3)† | 1.025 (0.37,1.5)†, ††† | 2.3 ± 0.8¶ | |
| Dadoniene et al.61 | 2003 | 201◊ | 55.9 ± 10.0 | 17 | 11.9 ± 9.5 | |||
| Russak et al.62 | 2003 | 291 | 57 ± 14 | 15 | 12.7 ± 9.9 | 0.73 ± 0.69 | ||
| Krishnan et al.63 | 2004 | 6436 | 58.5† | 26 | 8.0 (2.3,16.7)† | 1.13 (0.5,1.8)† | ||
| Escalante et al.64 | 2004 | 776 | 57† | 30 | 8† | 1.89 ± 0.70¶ | ||
| Wolfe et al.65 | 2004 | 14038 | – | – | – | 1.09 ± 0.72 | 0.51 ± 0.49 | |
| Pincus et al.66 | 2004 | 1416 | 56.0 | 23 | 11.7 | 0.6 | ||
| Jawaheer et al.67 | 2004 | 1097 | 41.0 ± 13.1 | 23 | 14.3 ± 11.1 | 1.0 ± 0.8 | ||
| Sokka et al.68 | 2004 | 1095 | 62.4 ± 0.4 | 29 | 11.3 ± 0.3 | 0.83 ± 0.02 | ||
| Lacaille et al.69 | 2004 | 581 | 48 | 21 | 9.8 | 1.1 | ||
| Leeb et al.70 | 2005 | 207 | 59.0 ± 12.9 | 24 | 7.23 ± 8.24 | 0.62 ± 0.66 | ||
| Marra et al.71 | 2005 | 313 | 61.5 ± 25.9 | 22 | 13.9 ± 11.4 | 1.10 ± 0.77 | ||
| Krishnan et al.72 | 2005 | 1530 | 55.4 ± 14.9 | 28 | – | 0.25 | ||
| Armstrong et al.73 | 2005 | 253 | 62.0 ± 11.2 | 28 | 13.4 ± 10.2 | 1.86 ± 0.78 | ||
| Aletaha et al.74 | 2005 | 767 | 54.1 ± 14.9 | 20 | 8.1 ± 10.6 | 0.875 (0.25,1.5)† | ||
| Cole et al.75 | 2005 | 278 | 51 ± 13 | – | 8.7 ± 10 | 1.17 ± 0.70 | ||
| Solomon et al.76 | 2005 | 359 | 56 (25–90)‡ | 16 | 10 (0.6–58)† | 1.17 ± 0.96 | ||
| Wolfe et al.77 | 2005 | 669 | 58.0 ± 13.5 | 27 | 12.5 ± 10.5 | 1.06 ± 0.75 | 0.49 ± 0.51 | |
| Cranney et al.78 | 2005 | 520 | 58.5 ± 11.2 | 30 | 15.5 ± 12.8 | 0.97 ± 0.72 | ||
| Baddoura et al.79 | 2006 | 298 | 51.5 ± 14.7 | 22 | 8.9 ± 8.7 | 0.62 ± 0.65 | ||
| Koh et al.80 | 2006 | 401 | 57 ± 10.9 | 14 | 11 ± 13 | 0.56 ± 0.66*** | ||
| Rupp et al.81 | 2006 | 307 | 58.1 ± 13.4 | 29 | 6.4 ± 7.6 | 0.46 ± 0.48†† | ||
| Ariza-Ariza et al.82 | 2006 | 300 | 59.6 ± 13.3 | 18 | 10.3 ± 8.7 | 1.2 ± 0.9 | ||
| Fronseca et al.83 | 2007 | 491 | 57 ± 13.3 | 15 | 12.7 ± 10.5 | 1.2 ± 0.8 | ||
| Treharne et al.84 | 2007 | 348 | 61.4 ± 11.7 | 28 | 13.1 ± 11.0 | 1.40 ± 0.93 | ||
| Panoulas et al.85 | 2007 | 400 | 63. 1§ | 27 | 10 (4,18)† | 1.5 (0.63, 2.13)† | ||
| Scott et al.86 | 2007 | 321 | 60 | 24 | 9 (1–48)† | 1.5 ± 0.8 | ||
| Soderlin et al.87 | 2007 | 594 | 64 | 27 | 17 | 1.1 | ||
| Bansback et al.88 | 2007 | 308 | 61.4 ± 13.7 | 22 | 14 ± 11.6 | 1.11 ± 0.77 | ||
| Shanahan et al.89 | 2008 | 497 | 51.8 | 30 | 10.7 (0–48)† | 0.63§ | 1.55¶ | |
| Uhlig et al.40 | 2008 | 914 | 58.7 ± 13.4 | 21 | 13.6 ± 10.5 | |||
| Momohara et al.90 | 2008 | 5497 | 55.7 ± 13.2 | 18 | 8.0 ± 8.4 | 0.78 ± 0.72§§ | ||
| Bodur et al.91 | 2008 | 562 | 52.1 ± 12.6 | 21 | 9.8 ± 8.1 | 0.74 ± 0.75◊◊ | ||
| Khanna et al.92 ten | 2008 | 307 | 59.4 ± 13.2 | 17 | 14.1 ± 11.5 | 0.84 ± 0.75 | ||
| Klooster et al.93 | 2008 | 472 | 59.6 ± 14.2 | 30 | 10.5 ± 11.2 | 1.1 ± 0.7†† | ||
| Sokka et al.94 | 2009 | 6004 | 56.2 | 21 | 11.2 | 0.9 | ||
| Lee et al.95 | 2009 | 7413 | 60.3 ± 12.8 | 24 | 12† | 0.4 ± 0.5 | ||
| AS Daltroy et al.23 | 1990 | 44 | 38.5 ± 11.9 | 75 | 13.7 ± 9.8 | 0.38 ± 0.49 | 0.49 ± 0.50 | |
| Hidding et al.96 | 1994 | 144 | 42.5 ± 10.4 | 78 | 4.0 (0–32)§ | 0.31 (0–1.46)§ | ||
| Cury et al.97 | 1995 | 15 | 34 | – | 14 | 1.2 (0.1–2.1)‡ | ||
| Ward et al.98 | 1999 | 216 | 47.4 ± 13.8 | 67 | 20.0 ± 13.7 | 0.375 (0.125,0.875)† | 0.5 (0.2, 1.0)† | |
| Ward16 | 2002 | 241 | 47.1 ± 13.8 | 69 | 19.8 ± 13.8 | 0.69 ± 0.6 | ||
| Heikkila99 | 2002 | 65 | 49 ± 11 | 68 | 23 ± 11 | 0.99 ± 0.63 | ||
| Ward et al.46 | 2005 | 326 | 55.0 ± 10.7 | 74 | 31.7 ± 10.2 | 0.80 ± 0.6 | ||
HAQ: Health Assessment Questionnaire; MHAQ: Modified Health Assessment Questionnaire; HAQ-S: Health Assessment Questionnaire for the Spondyloar-thropathies; SF-36 PF: Short Form 36 Physical Functioning subscale
All values are mean ± standard deviation, unless otherwise noted
Median with interquartile range
Mean with total range
Median (with total range, if available)
MHAQ range 1 – 4
Vilnius cohort
British adaptation of the HAQ; outpatient cohort
HAQ score transformed from Hannover Functional Status Questionnaire score
Dutch adaptation of the HAQ
Japanese adaptation of the HAQ
Swedish adaptation of the HAQ
Turkish adaptation of the HAQ
Chinese adaptation of the HAQ
Spanish adaptation of the HAQ.
Table III.
Functional limitations with the Short Form 36 in patients with rheumatoid arthritis and ankylosing spondylitis evaluated at different study sites.
| Reference | Year | Patients no. |
Age yrs* |
Male % |
Disease Duration, yrs* |
SF-36 Physical Function |
|---|---|---|---|---|---|---|
| RA Ruta et al.100 | 1998 | 233 | 56 ± 14 | 19 | 13 ± 13 | 31 ± 29 |
| Fransen et al.59 | 2002 | 803 | 59 ± 13 | 29 | ≠7 (2, 14)† | 55 (33.3, 80)† |
| Dadoniene et al.61 | 2003 | 201◊ | 55.9 ± 10.0 | 17 | 11.9 ± 9.5 | 35.2 ± 25.2 |
| Wolfe et al.65 | 2004 | 14038 | – | – | – | 47.1 ± 28.4 |
| Escalante et al.64 | 2004 | 776 | 57† | 30 | 8† | 35.6 ± 27.9 |
| Koh et al.80 | 2006 | 401 | 57 ± 10.9 | 14 | 11 ± 13 | 64.5 ± 28.1*** |
| Rupp et al.101 | 2006 | 882 | 59.8 ± 14.8 | 28 | 8.9 ± 9.8 | 49.0 ± 27.2 |
| Soderlin et al.89 | 2007 | 594 | 64 | 27 | 17 | 49 |
| Uhlig et al.40 | 2008 | 914 | 58.7 ± 13.4 | 21 | 13.6 ± 10.5 | 54.5 |
| Alishiri et al.102 | 2008 | 411 | 46.8 ± 12 | 13 | 6.3 ± 5.7 | 50.9 ± 26.4 |
| AS Ward103 | 1999 | 175 | 51.1 ± 14.0 | 68 | 23.7 ± 14.3 | 66 ± 27 |
| Dagfinrud et al.104 | 2004 | 314 | 43.7 ± 12.3 | 63 | 13.3 ± 11.3 | 71 ± 23 |
| Turan et al.105 | 2007 | 46 | 39.2 ± 11.5 | 80 | 13.9 ± 10.4 | 62.4 ± 25.9 |
| Zhu et al.12 | 2008 | 145 | 40 ± 11.1 | 79 | 10 ± 7.9 | 65 |
| Vesovic-Potic et al.106 | 2009 | 74 | 48.5 ± 10.3 | 78 | 15.2 ± 8.8 | 64.4 ± 16.7 |
SF-36 PF: Short Form 36 Physical Functioning subscale
All values are mean ± standard deviation, unless otherwise noted
Median with interquartile range
Vilnius cohort
Chinese adaptation of the SF-36.
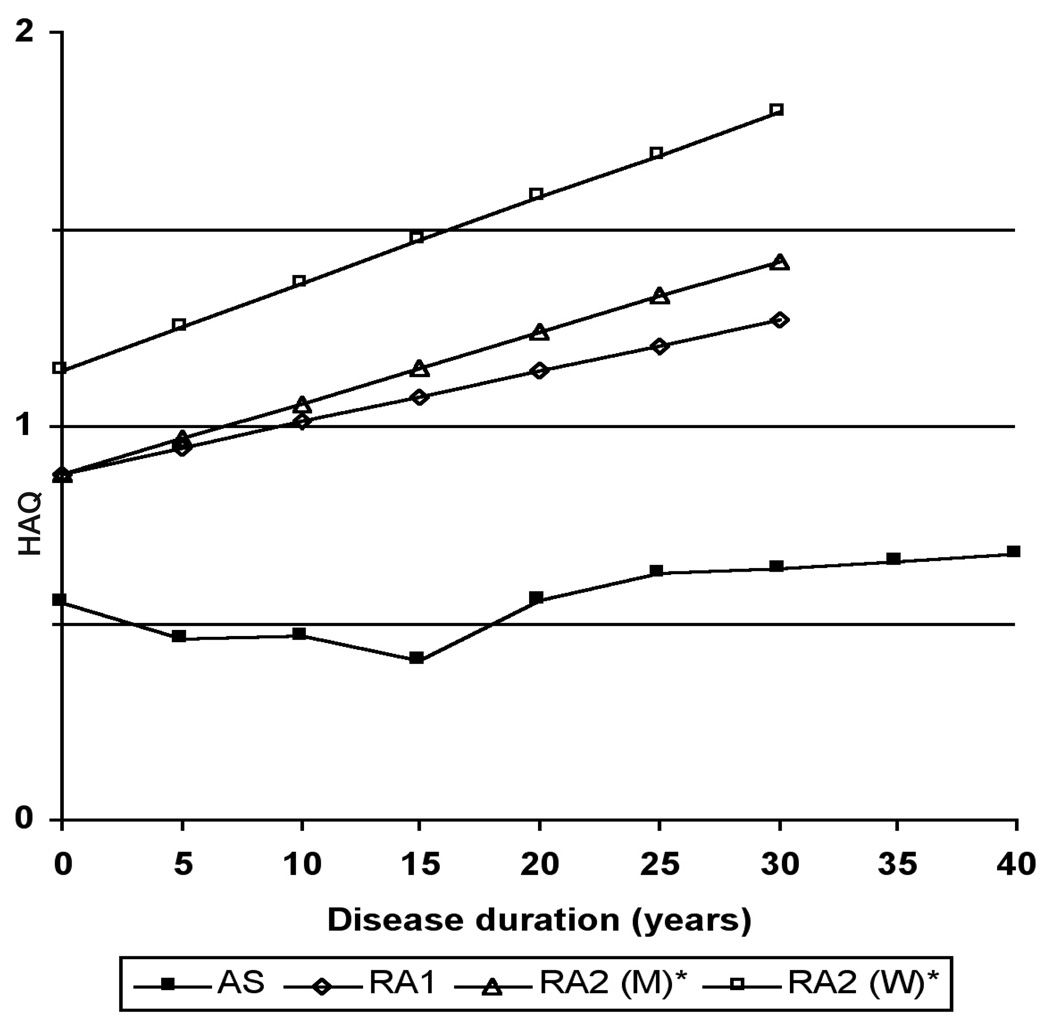
HAQ and SF-36 physical function scores for patients with RA tended to be lower among more recent cohorts.This observation supports some studies that suggest RA has become milder (37–43). Two studies have reported HAQ scores in a large number of patients with RA, stratified by duration of RA (44, 45). Wolfe and Cathey abstracted data from patients in the Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS) database from 1976 and 1988. Of the 1274 patients, mean (± standard deviation) age was 54.8±14.2 years, and 376 were men (29.5%). In a subsequent study by Wolfe, data from 1843 patients from 1974 and 1999 was abstracted from the same database. Demographic characteristics were similar to the earlier study. In these studies, the HAQ scores at onset of RA ranged from 0.9 to 1.1, and were progressively higher among patients with longer durations of RA (Fig. 1). Women had higher scores than men across all durations of RA.
Fig. 1.
Comparison of mean Health Assessment Questionnaire scores by disease duration between rheumatoid arthritis and ankylosing spondylitis.
HAQ: Health Assessment Questionnaire
AS: Ankylosing Spondylitis Cohort (unpublished data)
RA1: Rheumatoid Arthritis Cohort 1 44
RA2: Rheumatoid Arthritis Cohort 2 45
M: Men
W: Women
*Values represent group medians
To provide comparable data for AS, we analyzed data from the PSOAS (Prospective Study of Outcomes in AS) cohort. In this cross-sectional analysis, we computed mean HAQ scores for patients, grouped into 5 year bands of duration of AS. Among the 702 patients with AS, 508 (72.4%) were men. The mean HAQ was 0.55±0.62 at onset, and scores were only slightly higher in subgroups with longer duration of AS. At 20 years of AS, mean HAQ was 0.56±0.55, and at 40 years of AS the mean HAQ was 0.67±0.67. In this indirect comparison of HAQ stratified by duration of disease, functional limitations appeared to be greater among patients with RA than patients with AS throughout the disease course.
Comparisons across studies should be interpreted cautiously. Patients with AS were generally younger than patients with RA, and age has been shown to be an important independent predictor of functional decline. Given the older age of those with RA, they will likely have more comorbidities which could also contribute to functional limitations. Women predominate among those with RA and men predominate among those with AS. Women have been noted to report greater functional limitations than men in RA (45) and AS (46), although this observation may be due to ascertainment bias or confounding rather than a true gender difference.
Conclusions
Given the differences between RA and AS, comparison of functional limitations between these two diseases is challenging. In general, the evidence suggests that functional limitations are more severe in patients with RA than in patients with AS. However, this conclusion is tempered by several caveats. The scope of functional limitations in RA and AS is determined by differences in the distribution of involved joints. Few studies have directly compared functional limitations in these patients, and few have adjusted for potential confounding by age, gender, comorbidity, or disease duration. Therefore, perceived differences should be viewed cautiously. In future studies, we may learn that after controlling for differences in age, comorbidity, duration of disease, disease severity, and treatment, patients with RA have worse functional disability than patients with AS. Functional limitations are one component of the burden of disease, and have prognostic importance. Knowledge of differences in functional limitations between patients with RA and AS is useful for future healthcare resource planning. Functional limitation is also a modifiable outcome of disease, and provides a measure of progress in developing and disseminating effective treatments.
Acknowledgments
This study was supported in part by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
References
- 1.World Health Organization. Geneva: WHO; International Classification of Functioning, Disability and Health: ICF. 2001 Available at www3.who.int/icf/icftemplate.cfm.
- 2.Stucki G, Cieza A. The International Classification of Functioning, Disability and Health (ICF) Core Sets for rheumatoid arthritis: a way to specify functioning. Ann Rheum Dis. 2004;63 Suppl. II:ii40–ii45. doi: 10.1136/ard.2004.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Echteld I, Cieza A, Boonen A, et al. Identification of the most common problems by patients with ankylosing spondylitis using the International Classification of Functioning, Disability and Health. J Rheumatol. 2006;33:2475–2483. [PubMed] [Google Scholar]
- 4.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001;44:1876–1886. doi: 10.1002/1529-0131(200108)44:8<1876::AID-ART326>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Kiltz U, Van der Heijde D. Health-related quality of life in patients with rheumatoid arthritis and in patients with ankylosing spondylitis. Clin Exp Rheumatol. 2009;27 Suppl. 55:S107–S110. [PubMed] [Google Scholar]
- 7.Allaire SH, Prashker MJ, Meenan RF. The costs of rheumatoid arthritis. Pharma coeconomics. 1994;6:513–522. doi: 10.2165/00019053-199406060-00005. [DOI] [PubMed] [Google Scholar]
- 8.Clarke AE, Levinton C, Joseph L, et al. Predicting the short term direct medical costs incurred by patients with rheumatoid arthritis. J Rheumatol. 1999;26:1068–1175. [PubMed] [Google Scholar]
- 9.van Jaarsveld CH, Jacobs JW, Schrijvers AJ, Heurkens AH, Haanen HC, Bijlsma JW. Direct cost of rheumatoid arthritis during the first six years: a cost-of-illness study. Br J Rheumatol. 1998;37:837–847. doi: 10.1093/rheumatology/37.8.837. [DOI] [PubMed] [Google Scholar]
- 10.Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum. 2003;48:2750–2762. doi: 10.1002/art.11439. [DOI] [PubMed] [Google Scholar]
- 11.Lajas C, Abasolo L, Bellajdel B, et al. Costs and predictors of costs in rheumatoid arthritis: a prevalence-based study. Arthritis Rheum. 2003;49:64–70. doi: 10.1002/art.10905. [DOI] [PubMed] [Google Scholar]
- 12.Zhu TY, Tam LS, Lee VW, et al. Costs and quality of life of patients with ankylosing spondylitis in Hong Kong. Rheumatology (Oxford) 2008;47:1422–1425. doi: 10.1093/rheumatology/ken287. [DOI] [PubMed] [Google Scholar]
- 13.Ara RM, Packham JC, Haywood KL. The direct healthcare costs associated with ankylosing spondylitis patients attending a UK secondary care rheumatology unit. Rheumatology (Oxford) 2008;47:68–71. doi: 10.1093/rheumatology/kem296. [DOI] [PubMed] [Google Scholar]
- 14.Kobelt G, Andlin-Sobocki P, Maksy-Mowych WP. Costs and quality of life of patients with ankylosing spondylitis in Canada. J Rheumatol. 2006;33:289–295. [PubMed] [Google Scholar]
- 15.Boonen A, van der Heijde, Landewe R, et al. Direct costs of ankylosing spondylitis and its determinants: an analysis among three European countries. Ann Rheum Dis. 2003;62:732–740. doi: 10.1136/ard.62.8.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward MM. Functional disability predicts total costs in patients with ankylosing spondylitis. Arthritis Rheum. 2002;46:223–231. doi: 10.1002/1529-0131(200201)46:1<223::AID-ART498>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Pincus T, Callahan LF, Vaughn WK. Questionnaire, walking time and button test measures of functional capacity as predictive markers for mortality in rheumatoid arthritis. J Rheumatol. 1987;14:240–251. [PubMed] [Google Scholar]
- 18.Pincus T, Brooks RH, Callahan LF. Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med. 1994;120:26–34. doi: 10.7326/0003-4819-120-1-199401010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Callahan LF, Pincus T, Huston JW, 3rd, Brooks RH, Nance EP, Jr, Kaye JJ. Measures of activity and damage in rheumatoid arthritis: depiction of changes and prediction of mortality over five years. Arthritis Care Res. 1997;10:381–394. doi: 10.1002/art.1790100606. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1530–1542. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 21.Pincus T, Callahan LF. Rheumatology function tests: grip strength, walking time, button test and questionnaires document and predict longterm morbidity and mortality in rheumatoid arthritis. J Rheumatol. 1992;19:1051–1057. [PubMed] [Google Scholar]
- 22.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 23.Daltroy LH, Larson MG, Roberts NW, Liang MH. A modification of the Health Assessment Questionnaire for the spondyloar- thropathies. J Rheumatol. 1990;17:946–950. [PubMed] [Google Scholar]
- 24.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 25.Lautenschlager J, Mau W, Kohlmann T, et al. Comparative evaluation of a German version of the Health Assessment Questionnaire and the Hannover Functional Capacity Questionnaire. Z Rheumatol. 1997;56:144–155. doi: 10.1007/s003930050030. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 27.Aletaha D, Smolen J, Ward MM. Measuring function in rheumatoid arthritis. Identifying reversible and irreversible components. Arthritis Rheum. 2006;54:2784–2792. doi: 10.1002/art.22052. [DOI] [PubMed] [Google Scholar]
- 28.Drossaers-Bakker KW, de Buck M, van Zeben D, Zwinderman AH, Breedveld FC, Hazes JM. Long-term course and out-come of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum. 1999;42:1854–1860. doi: 10.1002/1529-0131(199909)42:9<1854::AID-ANR9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001;44:2009–2017. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.LandewÉ R, Dougados M, Mielants H, van der Tempel, van der Heijde Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis. 2009;68:863–867. doi: 10.1136/ard.2008.091793. [DOI] [PubMed] [Google Scholar]
- 31.Zink A, Braun J, Listing J, Wollen-Haupt J. Disability and handicap in rheumatoid arthritis and ankylosing spondylitis—results from the German rheumatological database. German Collaborative Arthritis Centers. J Rheumatol. 2000;27:613–622. [PubMed] [Google Scholar]
- 32.Huscher D, Merkesdal S, Thiele K, Zeidler H, Schneider M, Zink A for the German Collaborative Arthritis Centres. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65:1175–1183. doi: 10.1136/ard.2005.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zink A, Thiele K, Huscher D, et al. for the German Collaborative Arthritis Centres. Healthcare and burden of disease in psoriatic arthritis. A comparison with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33:86–90. [PubMed] [Google Scholar]
- 34.Kjeken I, Dagfinrud H, Mowinckel P, Uhlig T, Kvien TK, Finset A. Rheumatology care: involvement in medical decisions, received information, satisfaction with care, and unmet health care needs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 2006;55:394–401. doi: 10.1002/art.21985. [DOI] [PubMed] [Google Scholar]
- 35.Chorus AM, Miedema HS, Boonen A, van der Linden S. Quality of life and work in patients with rheumatoid arthritis and ankylosing spondylitis of working age. Ann Rheum Dis. 2003;62:1178–1184. doi: 10.1136/ard.2002.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward MM. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983–2001. Arthritis Rheum. 2004;50:1122–1131. doi: 10.1002/art.20158. [DOI] [PubMed] [Google Scholar]
- 38.Porter DR, Capell HA, Mcinnes I, et al. Is rheumatoid arthritis becoming a milder disease? Or are we starting second-line therapy in patients with milder disease? Br J Rheumatol. 1996;35:1305–1308. doi: 10.1093/rheumatology/35.12.1305. [DOI] [PubMed] [Google Scholar]
- 39.Welsing PM, Fransen J, van Riel PL. Is the disease course of rheumatoid arthritis becoming milder? Time trends since 1985 in an inception cohort of early rheumatoid arthritis. Arthritis Rheum. 2005;52:2616–2624. doi: 10.1002/art.21259. [DOI] [PubMed] [Google Scholar]
- 40.Uhlig T, Heiberg T, Mowinckel P, Kvien TK. Rheumatoid arthritis is milder in the new millennium: health status in patients with rheumatoid arthritis 1994–2004. Ann Rheum Dis. 2008;67:1710–1715. doi: 10.1136/ard.2007.084673. [DOI] [PubMed] [Google Scholar]
- 41.Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum. 2005;52:1009–1119. doi: 10.1002/art.20941. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan E, Fries JF. Reduction in long-term functional disability in rheumatoid arthritis from 1977 to 1998: a longitudinal study of 3035 patients. Am J Med. 2003;115:371–376. doi: 10.1016/s0002-9343(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan E, Fries JF. Rheumatoid arthritis: radiographic progression is getting milder. J Rheumatol. 2005;32:195. [PubMed] [Google Scholar]
- 44.Wolfe F, Cathey MA. The assessment and prediction of functional disability in rheumatoid arthritis. J Rheumatol. 1991;18:1298–1306. [PubMed] [Google Scholar]
- 45.Wolfe F. A reappraisal of HAQ disability in rheumatoid arthritis. Arthritis Rheum. 2000;43:2751–2761. doi: 10.1002/1529-0131(200012)43:12<2751::AID-ANR15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Ward MM, Weisman MH, Davis JC, Reveille JD. Risk factors for functional limitations in patients with long-standing ankylosing spondylitis. Arthritis Rheum. 2005;53:710–717. doi: 10.1002/art.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherrer YS, Bloch DA, Mitchell DM, Young DM, Fries JF. The development of disability in rheumatoid arthritis. Arthritis Rheum. 1986;29:494–500. doi: 10.1002/art.1780290406. [DOI] [PubMed] [Google Scholar]
- 48.Sharp JT, Wolfe F, Mitchell DM, Bloch DA. The progression of erosion and joint space narrowing scores in rheumatoid arthritis during the first twenty-five years of disease. Arthritis Rheum. 1991;34:660–668. doi: 10.1002/art.1780340606. [DOI] [PubMed] [Google Scholar]
- 49.Hawley DJ, Wolfe F. Pain, disability, and pain/disability relationships in seven rheumatic disorders: a study of 1,522 patients. J Rheumatol. 1991;18:1552–1557. [PubMed] [Google Scholar]
- 50.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 51.Gardiner PV, Sykes HR, Hassey GA, Walker DJ. An evaluation of the Health Assessment Questionnaire in long-term longitudinal follow-up of disability in rheumatoid arthritis. Br J Rheumatol. 1993;32:724–728. doi: 10.1093/rheumatology/32.8.724. [DOI] [PubMed] [Google Scholar]
- 52.Reisine S, Mcquillan J, Fifield J. Predictors of work disability in rheumatoid arthritis patients. A five-year followup. Arthritis Rheum. 1995;38:1630–1637. doi: 10.1002/art.1780381115. [DOI] [PubMed] [Google Scholar]
- 53.Bendtsen P, Bjurulf P, Trell E, Lind-Strom F, Larsson JE. Cross-sectional assessment and subgroup comparison of functional disability in patients with rheumatoid arthritis in a Swedish health-care district. Disabil Rehabil. 1995;17:94–99. doi: 10.3109/09638289509166634. [DOI] [PubMed] [Google Scholar]
- 54.Reisine S, Fifield J. Health insurance problems among insured rheumatoid arthritis patients. Arthritis Care Res. 1995;8:155–160. doi: 10.1002/art.1790080307. [DOI] [PubMed] [Google Scholar]
- 55.Houssien DA, Mckenna SP, Scott DL. The Nottingham Health Profile as a measure of disease activity and outcome in rheumatoid arthritis. Br J Rheumatol. 1997;36:69–73. doi: 10.1093/rheumatology/36.1.69. [DOI] [PubMed] [Google Scholar]
- 56.Kvien TK, Kaasa S, Smedstad LM. Performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. II. A comparison of the SF-36 with disease-specific measures. J Clin Epidemiol. 1998;51:1077–1086. doi: 10.1016/s0895-4356(98)00099-7. [DOI] [PubMed] [Google Scholar]
- 57.Chorus AM, Miedema HS, Wevers CJ, van der Lindens Labour force participation among patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;59:549–554. doi: 10.1136/ard.59.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westhoff G, Listing J, Zink A. Loss of physical independence in rheumatoid arthritis: interview data from a representative sample of patients in rheumatologic care. Arthritis Care Res. 2000;13:11–22. [PubMed] [Google Scholar]
- 59.Fransen J, Uebelhart D, Stucki G, Langenegger T, Seitz M, Michel BA. The ICIDH-2 as a framework for the assessment of functioning and disability in rheumatoid arthritis. Ann Rheum Dis. 2002;61:225–231. doi: 10.1136/ard.61.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yelin E, Trupin L, Wong B, Rush S. The impact of functional status and change in functional status on mortality over 18 years among persons with rheumatoid arthritis. J Rheumatol. 2002;29:1851–1857. [PubMed] [Google Scholar]
- 61.Dadoniene J, Uhlig T, Stropuviene S, Venalis A, Boonen A, Kvien TK. Disease activity and health status in rheumatoid arthritis: a case-control comparison between Norway and Lithuania. Ann Rheum Dis. 2003;62:231–235. doi: 10.1136/ard.62.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russak SM, Sherbourne CD, Lubeck DP, et al. Validation of rheumatoid arthritis-related quality of life instrument, the CSHQ-RA. Arthritis Rheum. 2003;49:798–803. doi: 10.1002/art.11478. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan E, Tugwell P, Fries JF. Percentile benchmarks in patients with rheumatoid arthritis: Health Assessment Questionnaire as a quality indicator (QI) Arthritis Res Ther. 2004;6:R505–R513. doi: 10.1186/ar1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escalante A, Del Rincon I, Cornell JE. Latent variable approach to the measurement of physical disability in rheumatoid arthritis. Arthritis Rheum. 2004;51 doi: 10.1002/art.20404. 399-07. [DOI] [PubMed] [Google Scholar]
- 65.Wolfe F, Michaud K, Pincus T. Development and validation of the Health Assessment Questionnaire II: a revised version of the Health Assessment Questionnaire. Arthritis Rheum. 2004;50:3296–3305. doi: 10.1002/art.20549. [DOI] [PubMed] [Google Scholar]
- 66.Pincus T, Keysor J, Sokka T, Krishnan E, Callahan LF. Patient questionnaires and formal education level as prospective predictors of mortality over 10 years in 97% of 1416 patients with rheumatoid arthritis from 15 United States Private Practices. J Rheumatol. 2004;31:229–234. [PubMed] [Google Scholar]
- 67.Jawaheer D, Lum RF, Amos CI, Gregersen PK, Criswell LA. Clustering of disease features within 512 multicase rheumatoid arthritis families. Arthritis Rheum. 2004;50:736–741. doi: 10.1002/art.20066. [DOI] [PubMed] [Google Scholar]
- 68.Sokka T, Hakkinen A, Krishnan E, Hannonen P. Similar prediction of mortality by the health assessment questionnaire in patients with rheumatoid arthritis and the general population. Ann Rheum Dis. 2004;63:494–497. doi: 10.1136/ard.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacaille D, Sheps S, Spinell JJ, Chalmers A, Esdaile JM. Identification of modifiable work-related factors that influence the risk of work disability in rheumatoid arthritis. Arthritis Rheum. 2004;51:843–852. doi: 10.1002/art.20690. [DOI] [PubMed] [Google Scholar]
- 70.Leeb BF, Andel I, Leder S, Leeb BA, Rintelen B. The patient’s perspective and rheumatoid arthritis disease activity indexes. Rheumatology (Oxford) 2005;44:360–365. doi: 10.1093/rheumatology/keh484. [DOI] [PubMed] [Google Scholar]
- 71.Marra CA, Woolcott JC, Kopec JA, et al. A comparison of generic, indirect utility measures (the HU12, HU13, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med. 2005;60:1571–1582. doi: 10.1016/j.socscimed.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan E, Hakkinen A, Sokka T, Hannonen P. Impact of age and comorbidities on the criteria for remission and response in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1350–1352. doi: 10.1136/ard.2005.037903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armstrong C, Swarbrick CM, Pye SR, O’Neill TW. Occurrence and risk factors for falls in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1602–1604. doi: 10.1136/ard.2004.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aletaha D, Nell VK, Stamm T, et al. Acute phase reactants add little to composite activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–R806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole JC, Motivala SJ, Khanna D, Lee JY, Paulus HE, Irwin MR. Validation of single-factor structure and scoring protocol for the Health Assessment Questionnaire-Disability Index. Arthritis Rheum. 2005;53:536–542. doi: 10.1002/art.21325. [DOI] [PubMed] [Google Scholar]
- 76.Solomon A, Christian BF, Dessein PH, Stanwix AE. The need for tighter rheumatoid arthritis control in a South African public health care center. Semin Arthritis Rheum. 2005;35:122–131. doi: 10.1016/j.semarthrit.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Wolfe F, Michaud K, Pincus T, Furst D, Keystone E. The Disease Activity Score is not suitable as the sole criterion for initiation and evaluation of anti-tumor necrosis factor therapy in the clinic. Arthritis Rheum. 2005;52:3873–3879. doi: 10.1002/art.21494. [DOI] [PubMed] [Google Scholar]
- 78.Cranney AB, Harrison A, Ruhland L, et al. Driving problems in patients with rheumatoid arthritis. J Rheumatol. 2005;32:2337–2342. [PubMed] [Google Scholar]
- 79.Baddoura R, Haddad S, Awada H, et al. Severity of rheumatoid arthritis: the SEVERA study. Clin Rheumatol. 2006;25:700–704. doi: 10.1007/s10067-005-0136-7. [DOI] [PubMed] [Google Scholar]
- 80.Koh ET, Leong KP, Tsou IY, et al. Tan Tock Seng Hospital Rheumatoid Arthritis (TTSH) Study Group. The reliability, validity and sensitivity to change of the Chinese version of SF-36 in oriental patients with rheumatoid arthritis. Rheumatology (Oxford) 2006;45:1023–1028. doi: 10.1093/rheumatology/kel051. [DOI] [PubMed] [Google Scholar]
- 81.Rupp I, Boshuizen HC, Dinant HJ, Jacobi CE, van den Bos GA. Disability and health-related quality of life among patients with rheumatoid arthritis: association with radiographic joint damage, disease activity, pain, and depressive symptoms. Scand J Rheumatol. 2006;35:175–181. doi: 10.1080/03009740500343260. [DOI] [PubMed] [Google Scholar]
- 82.Ariza-Ariza R, Hernandez-Cruz B, Carmona L, Dolores Ruiz-Montesinos M, Ballina J, Navarro-Sarabia F Costs and Quality of Life in Rheumatoid Arthritis Study Group. Assessing utility values in rheumatoid arthritis: a comparison between time trade-off and the EuroQoL. Arthritis Rheum. 2006;55:751–756. doi: 10.1002/art.22226. [DOI] [PubMed] [Google Scholar]
- 83.Fronseca JE, Cavaleiro J, Teles J, et al. Contribution for new genetic markers of rheumatoid arthritis activity and severity: sequencing of the tumor necrosis factor-alpha gene promoter. Arthritis Res Ther. 2007;9:R37. doi: 10.1186/ar2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Treharne GJ, Douglas KM, Iwaszko J, et al. Polypharmacy among people with rheumatoid arthritis: the role of age, disease duration and comorbidity. Musculoskeletal Care. 2007;5:175–190. doi: 10.1002/msc.112. [DOI] [PubMed] [Google Scholar]
- 85.Panoulas VF, Millionis HJ, Douglas KM, et al. Association of serum uric acid and cardiovascular disease in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1466–1470. doi: 10.1093/rheumatology/kem159. [DOI] [PubMed] [Google Scholar]
- 86.Scott DL, Khoshaba B, Choy EH, Kingsley GH. Limited correlation between the Health Assessment Questionnaire (HAQ) and EuroQoL in rheumatoid arthritis: questionable validity of deriving quality adjusted life years from HAQ. Ann Rheum Dis. 2007;66:1534–1537. doi: 10.1136/ard.2007.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soderlin MK, Lindroth Y, Jacobsson LT. Trends in medication and health-related quality of life in a population-based rheumatoid arthritis register in Malmo, Sweden. Rheumatology (Oxford) 2007;46:1355–1358. doi: 10.1093/rheumatology/kem143. [DOI] [PubMed] [Google Scholar]
- 88.Bansback N, Marra C, Tsuchiya A, et al. Using the Health Assessment Questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57:963–971. doi: 10.1002/art.22885. [DOI] [PubMed] [Google Scholar]
- 89.Shanahan EM, Smith M, Roberts-Thomson L, Esterman A, Ahern M. Influence of rheumatoid arthritis on work participation in Australia. Intern Med J. 2008;38:166–173. doi: 10.1111/j.1445-5994.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 90.Momohara S, Inoue E, Ikari K, et al. Risk factors for wrist surgery in rheumatoid arthritis. Clin Rheumatol. 2008;27:1387–1391. doi: 10.1007/s10067-008-0928-7. [DOI] [PubMed] [Google Scholar]
- 91.Bodur H, Ataman S, Akbulu L, et al. Characteristics and medical management of patients with rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol. 2008;27:1119–1125. doi: 10.1007/s10067-008-0877-1. [DOI] [PubMed] [Google Scholar]
- 92.Khanna D, Pope JE, Khanna PP, et al. The minimally important difference for the fatigue visual analog scale in patients with rheumatoid arthritis followed in an academic clinical practice. J Rheumatol. 2008;35:2339–2343. doi: 10.3899/jrheum.080375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.ten Klooster PM, Taal E, van de Laar MA. Rasch analysis of the Dutch Health Assessment Questionnaire disability index and the Health Assessment Questionnaire II in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:1721–1728. doi: 10.1002/art.24065. [DOI] [PubMed] [Google Scholar]
- 94.Sokka T, Toloza S, Cutolo M, et al. for the Quest-RA Group. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA Study. Arthritis Res Ther. 2009;11:R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SJ, Chang H, Yazici Y, Greenberg JD, Kremer JM, Kavanaugh A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United Sates observational cohort study. J Rheumatol. 2009;36:1161–1167. doi: 10.3899/jrheum.080889. [DOI] [PubMed] [Google Scholar]
- 96.Hidding A, de Witte L, van der Linden S. Determinants of self-reported health status in ankylosing spondylitis. J Rheumatol. 1994;21:275–278. [PubMed] [Google Scholar]
- 97.Cury SE, Ferraz MB, Dos Santos JQ, et al. The use of focus group interview in the evaluation of patients with ankylosing spondyli-tis. Br J Rheumatol. 1995;34:150–155. doi: 10.1093/rheumatology/34.2.150. [DOI] [PubMed] [Google Scholar]
- 98.Ward MM, Kuzis S. Validity and sensitivity to change of spondylitis-specific measures of functional disability. J Rheumatol. 1999;26:121–127. [PubMed] [Google Scholar]
- 99.Heikkila S, Viitanen JV, Kautiainen H, Kauppi M. Functional long-term changes in patients with spondyloarthropathy. Clin Rheumatol. 2002;21:119–122. doi: 10.1007/s10067-002-8270-y. [DOI] [PubMed] [Google Scholar]
- 100.Ruta DA, Hurst NP, Kind P, Hunter M, Stubbings A. Measuring health status in British patients with rheumatoid arthritis: reliability, validity and responsiveness of the short form 36-item health survey (SF-36) Br J Rheumatol. 1998;37:425–436. doi: 10.1093/rheumatology/37.4.425. [DOI] [PubMed] [Google Scholar]
- 101.Rupp I, Boshuizen HC, Roorda LD, Dinant HJ, Jacobi CE, Van Den Bos G. Poor and good health outcomes in rheumatoid arthritis: the role of comorbidity. J Rheumatol. 2006;33:1488–1495. [PubMed] [Google Scholar]
- 102.Alishiri GH, Bayat N, Fathi Ashtiani A, Tavallaii SA, Assari S, Moharamzad Y. Logistic regression models for predicting physical and mental health-related quality of life in rheumatoid arthritis patients. Mod Rheumatol. 2008;18:601–608. doi: 10.1007/s10165-008-0092-6. [DOI] [PubMed] [Google Scholar]
- 103.Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res. 1999;12:247–255. [PubMed] [Google Scholar]
- 104.Dagfinrud H, Mengshoel AM, Hagen KB, Loge JH, Kvien TK. Health status of patients with ankylosing spondylitis: a comparison with the general population. Ann Rheum Dis. 2004;63:1605–1610. doi: 10.1136/ard.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turan Y, Duruoz MT, Cerrahoglu L. Quality of life in patients with ankylosing spondylitis: a pilot study. Rheumatol Int. 2007;27:895–899. doi: 10.1007/s00296-007-0315-8. [DOI] [PubMed] [Google Scholar]
- 106.Vesovic-Potic V, Mustur D, Stanislav-Ljevic D, Ille T, Ille M. Relationship between spinal mobility measures and quality of life in patients with ankylosing spondylitis. Rheumatol Int. 2009 Jan 27; doi: 10.1007/s00296-008-0759-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]