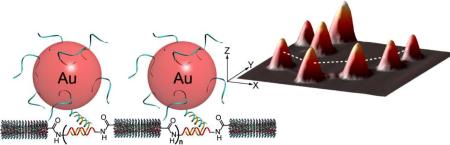
Abstract
Despite many advances in carbon nanotube (CNT) research, several issues continue to plague the field with regard to the construction of well-defined hybrid CNT materials. Regiospecific covalent functionalization, non-specific surface absorption, and carbon nanotube aggregation/bundling present major difficulties when working with these materials. In this communication, we circumvent these problems and report a new addressable hybrid material composed of single-walled carbon nanotubes terminally linked by oligonucleotides into a nanowire motif. We show that the oligonucleotides junctions are addressable and can be targeted by gold nanoparticles.
The synthesis of well-defined hybrid carbon nanotubebiomaterials remains a significant challenge at the frontier of nanotechnology and nanoparticle constructs are having a major impact in bio-diagnostics.1 The regiospecific covalent functionalization of single walled carbon nanotubes (SWCNTs) with oligonucleotides could provide new hybrid materials with sensing and self-assembly properties for the precise positioning of biomolecules and nanoparticles. To date, most methods for the functionalization of SWCNTs with oligonucleotides have relied on non-covalent surface adsorption,2,3 and those using covalent attachment have suffered from a lack of regioselectivity due to defect sites on the SWCNT surface created during strong oxidation procedures.4,5,6 Furthermore, SWCNT aggregation/bundling3,4,6 and nonspecific surface absorption has also been a serious limitation that is seldom addressed in previous studies. Oligonucleotides have been shown to undergo adsorption to the surface of SWCNTs and this property has formed the basis for SWCNT purification methods.7–10 Previous studies have elegantly demonstrated that DNA detection can be performed at the single molecule level between two DNA linked CNT's however the use of ultrahigh resolution electron beam lithography and reproducibility are still major concerns for the mass production of reliable sensory devices.11,12 In this communication we report the synthesis of a new nanostructured material composed of unbundled SWCNTs that are regiospecifically and covalently linked at their termini with oligonucleotides of a defined sequence. By utilizing a SWCNT surface protection step we are able to simultaneously circumvent the problem of regiospecific SWCNT covalent attachment and non-specific surface adsorption/aggregation.13 Additionally, we show that the oligonucleotide junctions are addressable and can be used to position gold nanoparticles in a specific location along the nanostructures.
Figure 1 shows the reaction sequence for SWCNT oxidation, shielding, DNA conjugation, and gold nanoparticle targeting. In a typical experiment HiPco SWCNTs were acid treated in a mixture of concentrated sulfuric and nitric acid (3:1, 98% and 70%, respectively), subjected to sonication for 35 min. at 40 °C, and then oxidized with a mixture of concentrated sulfuric acid and hydrogen peroxide (4:1 98% and 30%, respectively, 30 min.) to give carboxylic acid functionalized nanotube ends and to remove extraneous carbon particles.14 In addition, studies have shown that under mild oxidation conditions the optical and electronic properties of CNTs can be preserved.15–18 For Raman spectra of CNTs before and after oxidation see figure S1. Previous studies have demonstrated that the chemical shortening of SWCNTs by mixtures of strong acids leads to oxidation at the nanotube ends and some sidewall defect sites.14 The oxidized nanotubes were incubated with Triton X-100/PEG (Mn = 10,000) in an aqueous solution and sonicated for 4 h in an ice bath. This process results in a stable dispersion of SWCNTs wrapped with surfactant and polymer to prevent unwanted functionalization of the nanotube sidewalls and non-specific surface adsorption.19,20 The oxidized termini of the shielded SWCNTs were then selectively coupled to the DNA strands (1) bearing a nucleophilic primary amine at the 5' and 3' ends. Efficient coupling was achieved using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS) resulting in SWCNTs linked by DNA (1) at their termini in a nanowire configuration.13 Next, the resulting DNA-SWCNT nanowires were used to sequence specifically address gold nanoparticles to the DNA junctions connecting adjacent nanotubes.
Figure 1.
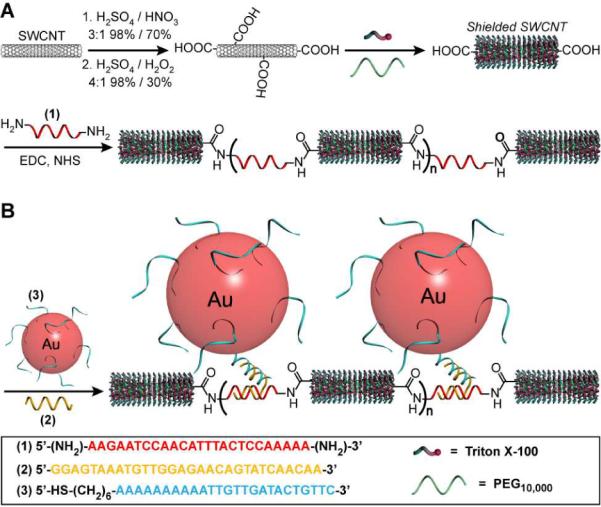
Nanowire assembly and characterization. (A) DNA-CNT nanowire assembly showing oxidation, shielding, and nanotube-DNA conjugation. (B) DNA-CNT nanowire and AuNP-probe hybridization for DNA junction visualization.
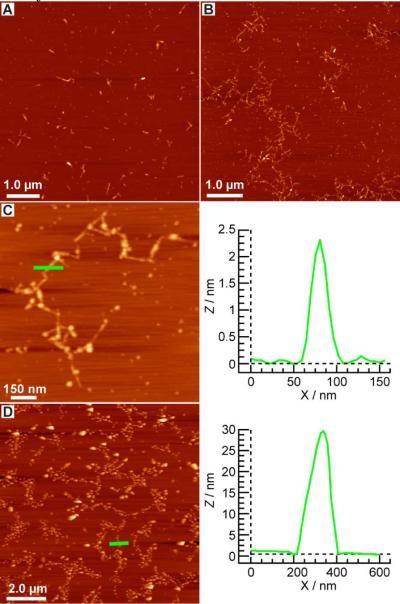
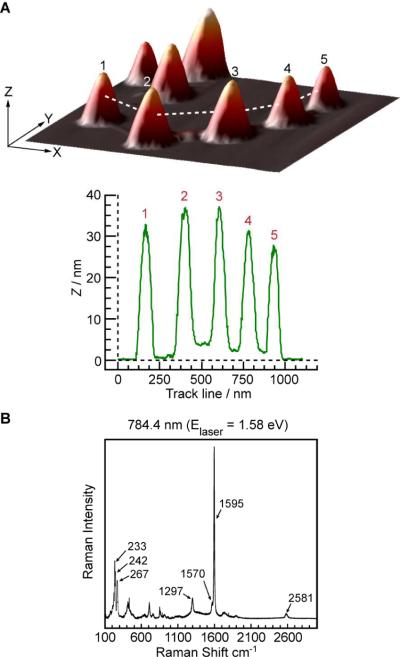
Characterization of the DNA-SWCNT nanowires was performed using AFM and confocal Raman spectroscopy before and after gold nanoparticle hybridization (Figure 2 and 3). Oxidized shielded SWCNTs are dispersed and separated from adjacent nanotubes after sonication as evidenced by AFM, with average lengths of ~200 nm (Figure 2A). In contrast, DNA-SWCNT nanowires show the presence of contiguous nanotubes with terminal connections mediated by DNA junctions (1) and individual nanotube lengths of ~200 nm (Figure 2B,C). To confirm the nature of the nanowire DNA junctions we utilized DNA (2) and AuNP probes (3), which showed AuNPs bound at each DNA junction by AFM, confirming the presence of DNA-SWCNT nanowires (Figure 2D and Figure 3A). AuNP probes were chosen due to their large diameter (~30 nm) compared to shielded-SWCNTs (1–5 nm) providing an unambiguous probe for identification of the DNA junctions. Confocal Raman spectroscopy was performed on the mica deposited AFM samples and confirmed the presence of CNT's (Figure 3B). In addition, experiments were carried out with ~10 nm AuNP probes which showed similar results to the 30 nm particles above (See figure S2). Control experiments using non-complimentary DNA confirmed the sequence specificity of our AuNP targeting strategy (See figure S2).
Figure 2.
AFM images of oxidized CNTs and DNA-CNT nanowires. (A) Oxidized shielded carbon nanotube AFM image after sonication. (B,C) AFM images of DNA-conjugated CNT nanowires with terminal connections mediated by DNA junctions (1). Height profile of a single shielded CNT within a nanowire indicated in the image of (C) by a green line. (D) AFM image of DNA-conjugated CNT nanowires with bound AuNP probes (3) and DNA (2) (1nM). Height profile of a single AuNP probe bound to a DNA nanowire junction indicated in the image by a green line.
Figure 3.
Nanowire characterization. (A) DNA-CNT nanowire characterization by AFM utilizing DNA (2) and AuNP-probes (3). (B) Confocal Raman spectra of the AFM sample shown in A verifying the presence of SWCNTs.
The potential use of this new material for applications beyond gold nanoparticle addressing are considerable. The assembly of the SWCNT networks linked by oligonucleotides could serve as a novel sensing domain for applications such as DNA detection or biosensing and efforts in this area are underway. In addition to gold nanoparticles, the potential for addressing enzymes such as alkaline phosphatase, glucose oxidase or DNAzymes may provides many new avenues for the construction of hybrid biocatalytic-CNT devices. Importantly, this new material could have implications for the mass production of new bio-diagnostic sensory devices.
Supplementary Material
Acknowledgment
This work was supported by NSF ECCS-0731100 and ARO W911NF-07-D-0004. D.M.C. is grateful for an NIH-NIGMS postdoctoral fellowship (1-F32-GM087028-01A1).
Footnotes
Supporting Information Available: Full experimental details and Figures S1 and S2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- (2).Han X, Li Y, Deng Z. Angew. Chem. Int. Ed. Engl. 2007;46:7481–7484. doi: 10.1002/anie.200701748. [DOI] [PubMed] [Google Scholar]
- (3).Chen Y, Liu H, Ye T, Kim J, Mao C. J. Am. Chem. Soc. 2007;129:8696–8697. doi: 10.1021/ja072838t. [DOI] [PubMed] [Google Scholar]
- (4).Dwyer C, Guthold M, Falvo M, Washburn S, Superfine R, Erie D. Nanotechnology. 2002;13:601–604. [Google Scholar]
- (5).Williams KA, Veenhuizen PTM, de la Torre B, Eritja R, Dekker C. Nature. 2002;420:761. doi: 10.1038/420761a. [DOI] [PubMed] [Google Scholar]
- (6).Li S, He P, Dong J, Guo Z, Dai L. J. Am. Chem. Soc. 2005;127:14–15. doi: 10.1021/ja0446045. [DOI] [PubMed] [Google Scholar]
- (7).Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. Nat. Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- (8).Zheng M, Jagota A, Strano MS, Santos AP, Barone P, Chou SG, Diner BA, Dresselhaus MS, McLean RS, Onoa GB, Samsonidze GG, Semke ED, Usrey M, Walls DJ. Science. 2003;302:1545–1548. doi: 10.1126/science.1091911. [DOI] [PubMed] [Google Scholar]
- (9).Tu X, Manohar S, Jagota A, Zheng M. Nature. 2009;460:250–253. doi: 10.1038/nature08116. [DOI] [PubMed] [Google Scholar]
- (10).Johnson RR, Johnson AT, Klein ML. Nano. Lett. 2008;8:69–75. doi: 10.1021/nl071909j. [DOI] [PubMed] [Google Scholar]
- (11).Guo X, et al. Science. 2006;311:356–359. doi: 10.1126/science.1120986. [DOI] [PubMed] [Google Scholar]
- (12).Guo X, Gorodetsky AA, Hone J, Barton JK, Nuckolls C. Nat. Nanotechnol. 2008;3:163–167. doi: 10.1038/nnano.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Weizmann Y, Lim J, Chenoweth DM, Swager TM. Nano. Lett. 2010;10:2466–2469. doi: 10.1021/nl1008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Liu J, Rinzler AG, Dai H, Hafner JH, Bradley RK, Boul PJ, Lu A, Iverson T, Shelimov K, Huffman CB. Science. 1998;280:1253. doi: 10.1126/science.280.5367.1253. others. [DOI] [PubMed] [Google Scholar]
- (15).Johnston DE, Islam MF, Yodh AG, Johnson AT. Nat. Mater. 2005;4:589–592. doi: 10.1038/nmat1427. [DOI] [PubMed] [Google Scholar]
- (16).D'Este M, De Nardi M, Menna E. Eur. J. Org. Chem. 2006:2517–2522. [Google Scholar]
- (17).Menna E, Negra FD, Fontana MD, Meneghetti M. Phys. Rev. B. 2003;68:193412, 1–4. [Google Scholar]
- (18).Han ZJ, Ostrikov K. App. Phys. Lett. 2010;96:233115, 1–3. [Google Scholar]
- (19).Chen RJ, Bangsaruntip S, Drouvalakis KA, Kam NW, Shim M, Li Y, Kim W, Utz PJ, Dai H. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4984–4989. doi: 10.1073/pnas.0837064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rastogi R, Kaushal R, Tripathi SK, Sharma AL, Kaur I, Bharadwaj LM. J. Colloid. Interface. Sci. 2008;328:421–428. doi: 10.1016/j.jcis.2008.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.