Abstract
Background
Ventilatory efficiency (VE/VCO2 slope) and peak oxygen consumption (VO2) provide robust prognostic information in patients with heart failure (HF) undergoing cardiopulmonary exercise testing (CPX). The purpose of the present study is to assess the change in prognostic characteristics of CPX at different time intervals.
Methods and Results
Seven hundred and ninety-one subjects (74% male, mean age: 60.7 ±12.9 years, ejection fraction: 34.6 ±15.0%, ischemic etiology: 51%) underwent CPX and were tracked for major cardiac events over a four year period. All event-free subjects were tracked for at least three years. Mean VE/VCO2 slope and peak VO2 were 35.0 ±10.0 and 16.0 ±6 mlO2•kg−1•min−1, respectively. There were a total of 263 major cardiac events (199 deaths/45 transplants/19 left ventricular assist device implantations). Both continuous and dichotomous expressions of the VE/VCO2 slope and peak VO2 were prognostically significant up to 18 months post CPX. Continuous and dichotomous expressions of the VE/VCO2 slope remained prognostically significant up to 36 months post CPX while peak VO2 was not predictive during the third and fourth year of follow-up. In a multivariate analysis, the VE/VCO2 slope was consistently the superior prognostic marker while peak VO2 added predictive value and was retained in the regression up to 18 months post CPX.
Conclusions
These results indicate that commonly assessed CPX variables retain prognostic value for at least two years. The VE/VCO2 slope is the superior predictor of adverse events throughout follow-up, although peak VO2 provides additive prognostic information during the first two years of follow-up.
Keywords: Expired gas, ventilatory efficiency, aerobic capacity, adverse events
Introduction
Cardiopulmonary exercise testing (CPX) is a well accepted evaluation technique in patients with heart failure (HF), as indicated by position stands put forth by American1 and European2 organizations. The premise for the strong level of support for the application of CPX in this chronic disease population is the robust body of scientific evidence, now spanning more that 25 years, consistently demonstrating the prognostic value provided by variables attained from ventilatory expired gas analysis.3 From CPX, the minute ventilation/carbon dioxide production (VE/VCO2) slope and peak oxygen consumption (VO2) are the most frequently assessed variables for prognostic purposes and, although the former appears to provide superior predictive information, multivariate modeling including both measures is recommended.3
From the entire HF population, it appears that those patients being actively considered for heart transplantation are most often referred for CPX in order to assist in determining whose prognosis is least favorable and therefore in the greatest need of surgical intervention. While clinicians afford a high level of predictive credibility to information obtained from CPX, little consideration is given to the length of time the data provide valid insight into which patients are at greatest risk for adverse events. Our group has attempted to address this issue in the past, finding the prognostic strength of both the VE/VCO2 slope and peak VO2 is diminished greater than one year following CPX.4 This initial investigation was performed in a relatively small cohort (n=258) with a limited amount of hard end-points (45 cardiac related deaths).
While this initial investigation indicated there was a finite period of time that CPX data should be considered for prognostic purposes in patients with HF, additional analysis is needed. Given the fluid nature of this chronic disease, from asymptomatic left ventricular dysfunction to refractory HF, the prognostic applications of CPX should not be considered indefinite. However, the optimal window for applying CPX data in a prognostic context has not been well defined. Clarifying this window would have implications for the frequency with which HF patients should be referred for CPX, and would further optimize the application of this procedure. The purpose of the present investigation was to further define an optimal prognostic window for CPX responses in patients with HF.
Methods
This study was a multi-center analysis including HF patients from the exercise testing laboratories at San Paolo Hospital, Milan, Italy, Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina, USA, LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina, USA, VA Palo Alto Health Care System, Palo Alto, California, USA and Virginia Commonwealth University, Richmond, Virginia, USA. A total of 791 patients with chronic HF were included. Inclusion criteria consisted of a diagnosis of HF5 and evidence of left ventricular dysfunction by two-dimensional echocardiography obtained within one month of data collection. All subjects completed a written informed consent and institutional review board approval was obtained at each institution.
CPX Procedures
Symptom-limited CPX was performed on all subjects and pharmacologic therapy was maintained during exercise testing. Conservative ramping protocols were employed at all centers and ventilatory expired gas analysis was performed using a metabolic cart (Medgraphics CPX-D and Ultima, Minneapolis, MN, Sensormedics Vmax29, Yorba Linda, CA or Parvomedics TrueOne 2400, Sandy, UT). A treadmill and lower extremity ergometer were employed as the exercise mode in 69% and 31% of the tests, respectively. Before each test, the equipment was calibrated in standard fashion using reference gases. Minute ventilation (VE), oxygen uptake (VO2), and carbon dioxide output (VCO2) were acquired breath-by-breath, and averaged over 10-second intervals. Peak VO2 and peak respiratory exchange ratio (RER) were expressed as the highest 10-second averaged sample obtained during the last 20 seconds of testing. VE and VCO2 values, acquired from the initiation of exercise to peak, were input into spreadsheet software (Microsoft Excel, Microsoft Corp., Bellevue, WA) to calculate the VE/VCO2 slope via least squares linear regression (y = mx + b, m=slope). Previous studies have shown that this method of calculating the VE/VCO2 slope to be prognostically optimal.3
Endpoints
In the overall cohort, subjects were followed for major cardiac events event (mortality, LVAD implantation, urgent heart transplantation) via medical chart review. Subjects were followed by the HF programs at their respective institution providing a high likelihood that all events were captured. External means of tracking events, such as the Social Security Death Index, were not utilized in the present study. Any death with a cardiac-related discharge diagnosis was considered an event. Subjects in the overall group not suffering a major cardiac event were tracked for a minimum of three years.
Statistical Analysis
Statistical software packages (SPSS 17.0, Chicago, IL and StudySize 2.0, Vastra Frolunda, Sweden) were used to perform all analyses. Continuous data are reported as mean ± standard deviation. One way analysis of variance (ANOVA) assessed differences in continuous variables between subjects who remained event free or suffered a major cardiac event at six month intervals for the overall group tracked for three years. Post-hoc analysis was performed by Tukey’s honestly significant difference test. Chi-square analysis assessed distribution differences of categorical data amongst groups. Independent t-tests assessed differences in CPX variables between subgroups of subjects who remained event free for four years or suffered a major cardiac event either between 36–42 or 42–48 months. In subjects suffering a major cardiac event, Pearson Product Moment Correlation analysis was used to assess the relationship between both peak VO2 and the VE/VCO2 slope and time to event. A series of Cox proportional hazard models were performed to assess the prognostic value of peak VO2 and the VE/VCO2 slope, both as continuous and dichotomous expressions (VE/VCO2 slope: </≥36; peak VO2: </≥10 ml• kg−1•min−1), during the four year tracking period. The first was a traditional time-to-event analysis with the date of CPX serving as the baseline time point. Subsequent proportional hazard models began follow-up time at succeeding six month intervals past CPX. Subjects suffering an event in the preceding six months were removed from the next analysis. Dichotomous thresholds for both CPX variables were based upon previous investigations demonstrating their prognostic significance.3 Using all cardiac events, a post-hoc power analysis was conducted for both CPX variables (continuous expressions) for all Cox proportional hazard models. Derived hazard ratios and total number of cardiac events were used to calculate power. Kaplan-Meier analysis assessed event free survival of the VE/VCO2 slope and peak VO2 according to the four level Ventilatory6 and Weber7 classification systems, respectively. Multivariate Cox regression (Forward stepwise method; entry and removal value 0.05 and 0.10, respectively) assessed the combined prognostic value of peak VO2 and the VE/VCO2 slope as continuous expressions. Multivariate survival analysis was also used to assess the combined prognostic value of all variables listed in Table 1 in addition to the aforementioned CPX variables. For this latter analysis, major cardiac events were considered for the first three years following CPX. Finally, univariate Cox regression assessed the prognostic value of CPX at each of the five laboratories included in the analysis and based upon mode of exercise as well as a peak RER threshold of </≥1.00. A p-value <0.05 was considered statistically significant for all tests.
Table 1.
Baseline Characteristics and Therapy Distribution
| Overall Group (n=791) |
No Events (n=560) |
0–6 Months (n=76) |
6–12 Months (n=51) |
12–18 Months (n=39) |
18–24 Months (n=30) |
24–30 Months (n=20) |
30–36 Months (n=15) |
|
|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | ||||||||
| Age, years | 60.7 ±12.9 | 61.5 ±11.9 a | 55.7 ±14.5 | 59.7 ±12.6 | 56.1 ±17.5 | 62.9 ±14.2 | 64.8 ±15.6 | 61.7 ±12.5 |
| Sex, % Male | 80 | 80b | 74 | 80 | 72 | 87c | 80 | 93d |
| Etiology, % Isch./Non-Isch. | 51/49 | 51/49 | 49/51 | 56/44 | 46/54 | 54/46 | 53/47 | 62/38e |
| NYHA Class | 2.4 ±0.67 | 2.2 ±0.65f | 2.9 ±0.72 | 2.8 ±0.64 | 2.7 ±0.49 | 2.7 ±0.51 | 2.6 ±0.53 | 2.3 ±0.61g |
| LVEF, % | 34.6 ±15.0 | 38.8 ±14.1h | 23.2 ±11.6 | 25.4 ±13.0 | 26.6 ±12.3 | 27.3 ±12.3 | 31.1 ±16.4 | 33.4 ±13.5 |
| Event Type | ||||||||
| death/transplant/LVAD | 168/44/19 | 0/0/0 | 35/33/8 | 40/7/4 | 35/1/3 | 25/2/3 | 19/1/0 | 14/0/1 |
| Therapy Distribution, % | ||||||||
| ACE Inhibitor | 67 | 64 | 63 | 88i | 83j | 66 | 67 | 58k |
| Diuretic | 59 | 49l | 86 | 80 | 83 | 85 | 81 | 64m |
| Beta-Blocker | 52 | 49 | 64 | 69n | 58 | 53 | 33o | 54 |
No event group younger than 0–6 month event group (p<0.01)
Male % in no event group higher than 0–6 and 12–18 month event groups (p<0.01)
Male % in 18–24 month event group higher than no event, 0–6 month, and 18–24 month event groups (p<0.01)
Male % in 30–36 month event group higher than all others with exception of 18–24 month event group (p<0.05)
Heart failure etiology distribution different in 30–36 month event group compared to no event and 0–6 month event groups (p<0.05)
NYHA class in no event group lower compared to 0–6, 6–12, 12–18 and 18–24 month event groups (p<0.05)
NYHA class in 30–36 month event group lower compared to 0–6 month event group (p<0.05)
Left ventricular ejection fraction higher in the no event group compared to 0–6, 6–12, 12–18 and 18–24 month event groups (p<0.01)
ACE inhibitor use higher in 6–12 month event group compared to all other groups with the exception of the 12–18 month event group (p<0.05)
ACE inhibitor use higher in 12–18 month event group compared to no event, 0–6, 18–24, and 30–36 event groups (p<0.05)
ACE inhibitor use higher in the no event group compared to the 30–36 month event group (p<0.01)
Diuretic use lower in no event group compared to all other groups (p<0.05)
Diuretic use lower in 30–36 month group compared to all other groups suffering an event (p<0.05)
Beta-blocker use higher in 6–12 month group compared to no event and 24–30 month event group (p<0.05)
Beta-blocker use lower in 24–30 month event group compared to all other groups with the exception of the 30–36 month event group (p<0.05)
Results
The clinical and cardiac event characteristics of the 791 subjects included in this analysis are listed in Table 1. Five hundred and sixty subjects remained event-free for three years while the remaining 231 subjects suffered a major cardiac event during that time period (annual event rate: 9.9%). None of the subjects who remained event-free for three years post CPX were lost to follow-up over that time period. One hundred and thirteen subjects were lost to follow up during the fourth year of tracking. Over the first three years of tracking, 168 events were cardiac-related mortality (annual mortality rate: 7.3%). Several significant differences existed according to event status. Of note, NYHA class was significantly lower and LVEF was significantly higher in subjects who were event-free compared to those subjects suffering a major-cardiac event during the first two years following CPX. Both variables were comparable between subjects who were event free and those suffering a major cardiac event during the third year following CPX.
CPX characteristics are listed in Table 2. Peak RER was comparable amongst groups, indicating similar effort. Peak VO2 was significantly higher in subjects who remained event free compared to those suffering a major cardiac event during the first two years following CPX (Figure 1). The VE/VCO2 slope was significantly lower in subjects who remained event free compared to those suffering a major cardiac event during the first two and a half years following CPX. Moreover, the VE/VCO2 slope was significantly higher in subjects suffering a major cardiac event in the first six months compared to subjects suffering a major cardiac event from 6–12, 12–18 and 30–36 months (Figure 2).
Table 2.
CPX Data
| Overall Group (n=791) |
No Events (n=560) |
0–6 Months (n=76) |
6–12 Months (n=51) |
12–18 Months (n=39) |
18–24 Months (n=30) |
24–30 Months (n=20) |
30–36 Months (n=15) |
|
|---|---|---|---|---|---|---|---|---|
| Peak VO2, ml• kg−1•min−1 | 16.0 ±6.4 | 17.4 ±6.6a | 11.4 ±3.7 | 13.1 ±4.7 | 12.9 ±4.1 | 12.4 ±4.4 | 15.2 ±4.3 | 14.9 ±4.6 |
| VE/VCO2 slope | 35.0 ±10.0 | 32.2 ±10.0b | 45.6 ±12.1c | 39.7 ±8.8 | 39.9 ±12.0 | 40.2 ±11.4 | 41.1 ±9.8 | 36.1 ±3.4 |
| Peak RER | 1.09 ±0.17 | 1.09 ±0.17 | 1.08 ±0.18 | 1.13 ±0.19 | 1.08 ±0.13 | 1.06 ±0.11 | 1.07 ±0.12 | 1.12 ±0.18 |
Peak VO2: no event group greater than 0–6, 6–12, 12–18 and 18–24 event groups (p<0.001)
The VE/VCO2 slope: no event group less than 0–6, 6–12, 12–18, 18–24 and 24–30 event groups (p<0.001)
The VE/VCO2 slope: 0–6 month greater than 6–12, 12–18 and 30–36 month event groups (p<0.05)
Figure 1.
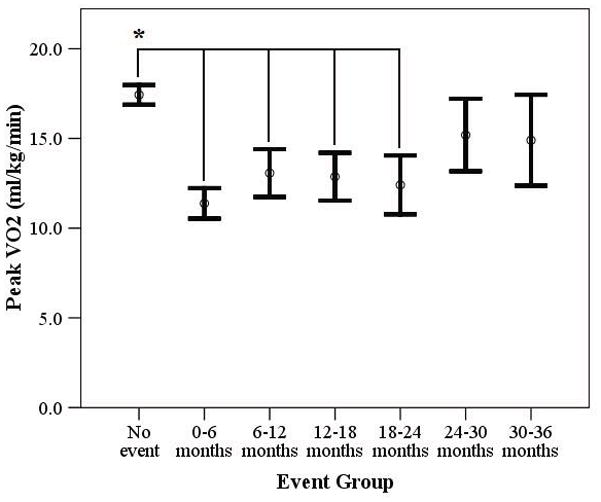
Difference in Peak VO2 According to Event Status
* p<0.001
Figure 2.
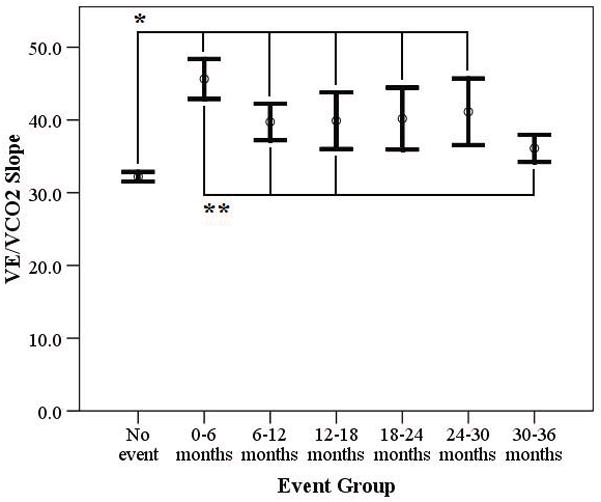
Difference in the VE/VCO2 Slope According to Event Status
*p<0.001
** p<0.05
Four hundred and ninety-nine subjects were tracked and remained event free for 42 months while 13 suffered a major cardiac event between 36 and 42 months. The difference in peak VO2 (No-event: 17.5 ±6.6 vs. Event: 17.8 ±8.7 ml• kg−1 •min−1, p=0.98) and peak RER (No-event: 1.09 ±0.17 vs. Event: 1.12 ±0.18, p=0.56) were not significantly different while the VE/VCO2 slope (No-event: 32.0 ±7.7 vs. Event: 38.0 ±17.8, p=0.008) was significantly higher in subjects suffering an event. Additionally, 428 subjects were tracked and remained event free for 48 months while 19 suffered a cardiac death between 42 and 48 months During the last six months of the fourth year, the differences in peak VO2 (No-event: 17.7 ±6.6 vs. Event: 15.7 ±6.4 ml• kg−1 • min−1, p=0.26), peak RER (No-event: 1.09 ±0.17 vs. Event: 1.12 ±0.19, p=0.48), and the VE/VCO2 slope (No-event: 31.9 ±7.7 vs. Event: 35.3 ±10.1, p=0.06) were not significant according to event status.
In the subjects suffering a major cardiac event over the four year tracking period (n=263), Pearson Product Moment Correlation revealed the VE/VCO2 slope (r = −0.24, p<0.001) and peak VO2 (r = 0.32, p<0.001) were both significantly correlated with time to event.
CPX variables of interest were significant predictors (p<0.05) of adverse events irrespective of: CPX laboratory, exercise mode, or a peak threshold of </≥1.00. In each instance, the VE/VCO2 slope was the superior prognostic marker. Kaplan-Meier analysis revealed the four level Weber7 (Figure 3) and Ventilatory6 (Figure 4) classification systems clearly delineated risk over the three years following CPX.
Figure 3.
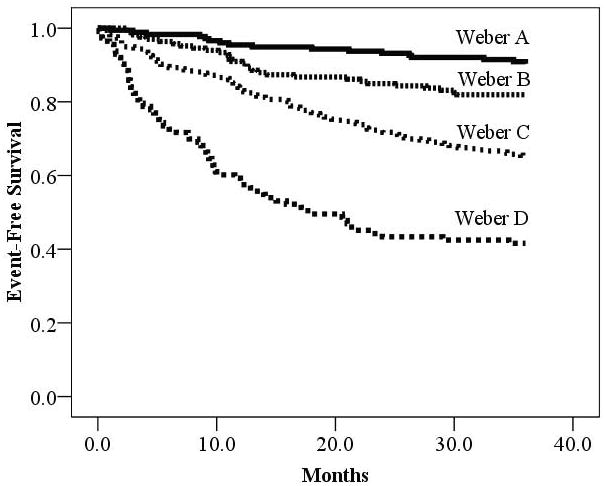
Kaplan-Meier Analysis According to Weber Class
| Weber Class | Meeting criteria | Major Cardiac Events | Percent Event Free |
|---|---|---|---|
| A: >20.0 ml• kg−1•min−1 | 176 | 17 | 90.3% |
| B: 16.0–19.9 ml• kg g−1•min−1 | 166 | 30 | 81.9% |
| C: 10.0–15.9 ml• kg−1•min−1 | 336 | 118 | 64.9% |
| D: <10.0 ml • kg−1•min−1 | 113 | 66 | 41.6% |
| Log rank: 115.7, p<0.001 |
Figure 4.
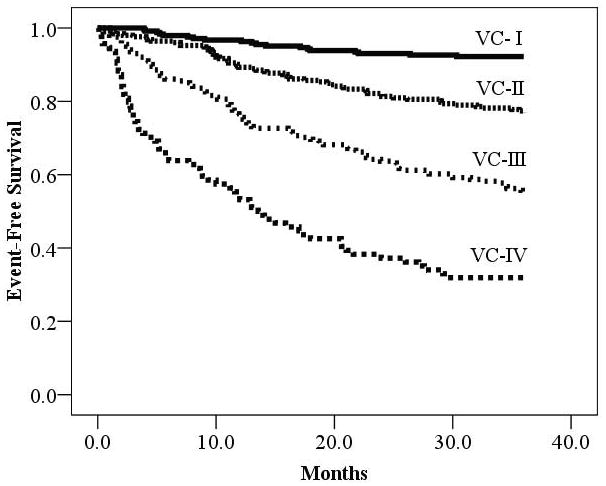
Kaplan-Meier Analysis According to Ventilatory Class
| Ventilatory Class | Meeting criteria | Major Cardiac Events | Percent Event Free |
|---|---|---|---|
| I: ≤29.9 | 244 | 19 | 92.2% |
| II: 30–35.9 | 252 | 58 | 77.0% |
| III: 36–44.9 | 201 | 90 | 55.2% |
| IV: ≥45.0 | 94 | 64 | 31.9% |
| Log rank: 193.9, p<0.001 |
Cox proportional hazards models are listed in Table 3. Both continuous and dichotomous expressions of the VE/VCO2 slope were significant predictors of major cardiac events and cardiac mortality at each six month interval for the first three and a half years following CPX. Power calculations for survival analysis using the VE/VCO2 slope remained above 90% up to 30 months post CPX and dropped to 79% and 61% for the 36 and 42 month post CPX analyses, respectively. The continuous expression of peak VO2 was a significant predictor of major cardiac events and cardiac mortality up to and including the 24 month post CPX hazards model while dichotomous expression was no longer significant following 12 months post CPX. Neither expression of peak VO2 identified subjects at increased risk from 30 months post CPX to the end of tracking. When considering peak VO2 as the prognostic variable, power was above 90% up to 18 months post CPX, dropped to 64% 24 months CPX and declined to <50 thereafter.
Table 3.
Cox Proportional Hazards Models for CPX Variables According to Time Past Baseline Assessment
| Hazard Ratio (95% CI) Continuous | p-value | Hazard Ratio (95% CI) Dichotomous* | p-value | Hazard Ratio (95% CI) Continuous | p-value | Hazard Ratio (95% CI) Dichotomous* | p-value | |
|---|---|---|---|---|---|---|---|---|
| VE/VCO2 slope | ||||||||
| All Cardiac Events | Cardiac Mortality | |||||||
| Overall (263 events; 199 deaths) | 1.06 (1.05–1.07) | <0.001 | 4.1 (3.2–5.3) | <0.001 | 1.05 (1.04–1.06) | <0.001 | 3.5 (2.7–4.7) | <0.001 |
| 6 months post CPX (187 events; 164 deaths) | 1.05 (1.04–1.06) | <0.001 | 3.3 (2.5–4.4) | <0.001 | 1.05 (1.04–1.06) | <0.001 | 3.2 (2.3–4.4) | <0.001 |
| 12 months post CPX (136 events; 124 deaths) | 1.06 (1.04–1.07) | <0.001 | 3.4 (2.4–4.8) | <0.001 | 1.05 (1.04–1.07) | <0.001 | 3.3 (2.3–4.7) | <0.001 |
| 18 months post CPX (95 events; 89 deaths) | 1.06 (1.04–1.07) | <0.001 | 3.4 (2.3–5.1) | <0.001 | 1.06 (1.04–1.07) | <0.001 | 3.5 (2.3–5.3) | <0.001 |
| 24 months post CPX (67 events; 64 deaths) | 1.05 (1.04–1.07) | <0.001 | 3.4 (2.1–5.5) | <0.001 | 1.05 (1.04–1.07) | <0.001 | 3.5 (2.2–5.8) | <0.001 |
| 30 months post CPX (47 events; 45 deaths) | 1.05 (1.02–1.07) | <0.001 | 2.6 (1.5–4.7) | 0.001 | 1.05 (1.02–1.07) | <0.001 | 2.9 (1.6–5.2) | <0.001 |
| 36 months post CPX (32 events; 31 deaths) | 1.05 (1.02–1.08) | 0.001 | 2.4 (1.2–4.8) | 0.01 | 1.05 (1.02–1.08) | 0.001 | 2.5 (1.2–5.1) | 0.01 |
| 42 months post CPX (19 events; 19 deaths) | 1.05 (1.00–1.09) | 0.05 | 2.2 (0.90–5.6) | 0.08 | 1.05 (1.00–1.09) | 0.05 | 2.2 (0.90–5.6) | 0.08 |
| Peak VO2 | ||||||||
| All Cardiac Events | Cardiac Mortality | |||||||
| Overall (263 events; 199 deaths) | 0.88 (0.86–0.91) | <0.001 | 3.2 (2.4–4.2) | <0.001 | 0.91 (0.88–0.93) | <0.001 | 2.3 (1.6–3.2) | <0.001 |
| 6 months post CPX (187 events; 164 deaths) | 0.90 (0.88–0.93) | <0.001 | 2.6 (1.8–3.7) | <0.001 | 0.91 (0.89–0.94) | <0.001 | 2.2 (1.5–3.3) | <0.001 |
| 12 months post CPX (136 events; 124 deaths) | 0.91 (0.88–0.94) | <0.001 | 2.0 (1.3–3.2) | 0.002 | 0.92 (0.88–0.95) | <0.001 | 1.8 (1.1–3.0) | 0.02 |
| 18 months post CPX (95 events; 89 deaths) | 0.92 (0.89–0.96) | <0.001 | 1.7 (0.95–3.1) | 0.07 | 0.93 (0.89–0.96) | <0.001 | 1.7 (0.93–3.2) | 0.08 |
| 24 months post CPX (67 events; 64 deaths) | 0.96 (0.91–1.00) | 0.03 | 1.1 (0.45–2.5) | 0.82 | 0.95 (0.91–1.00) | 0.03 | 1.2 (0.5–2.7) | 0.73 |
| 30 months post CPX (47 events; 45 deaths) | 0.96 (0.91–1.01) | 0.10 | 1.3 (0.53–3.4) | 0.53 | 0.96 (0.91–1.01) | 0.10 | 1.4 (0.56–3.6) | 0.47 |
| 36 months post CPX (32 events; 31 deaths) | 0.97 (0.92–1.03) | 0.34 | 1.6 (0.57–4.7) | 0.36 | 0.98 (0.92–1.04) | 0.44 | 1.7 (0.59–4.9) | 0.32 |
| 42 months post CPX (19 events; 19 deaths) | 0.95 (0.88–1.03) | 0.26 | 2.2 (0.63–7.5) | 0.22 | 0.95 (0.88–1.03) | 0.26 | 2.2 (0.63–7.5) | 0.22 |
Thresholds: VE/VCO2 slope </≥36, Peak VO2 </≥10 ml• kg−1•min−1
Multivariate Cox regression results revealed the VE/VCO2 slope and peak VO2 were both retained in the model when considering all events and up to 18 months post CPX. The VE/VCO2 slope was the superior prognostic marker in each instance while peak VO2 added predictive value (residual chi-square:≥4.2, p<0.05). The VE/VCO2 slope was the only variable retained in the regression from 24–36 months post CPX. Neither the VE/VCO2 slope nor peak VO2 was entered into the multivariate regression at 42 months post CPX. When combining these CPX variables with all variables listed in Table 1, the VE/VCO2 slope remained the strongest prognostic marker (Chi-square: 197.6, p<0.001). Left ventricular ejection fraction, NYHA class, age and peak VO2 added prognostic value and were retained (Residual Chi-square ≥5.0, p<0.05).
Discussion
The prognostic power of CPX in HF is firmly supported by a wealth of original research.6, 8, 9 Peak VO2 and the VE/VCO2 slope are the most thoroughly investigated variables and are therefore afforded a high degree of clinical recognition and acceptance.10 This is particularly true for peak VO2, although evidence now supports the prognostic superiority of the VE/VCO2 slope in the HF population3, a finding confirmed by the present investigation. Historically, prognostic analyses have tracked events following CPX without considering the potential influence time past the initial assessment has on the prognostic ability of peak VO2 and the VE/VCO2 slope. Our group addressed this previously, demonstrating the ability of both CPX variables to accurately predict an increased risk for cardiac mortality and hospitalization was substantially higher within the first year of tracking compared to a longer time frame.4 This initial analysis included a relatively small cohort (<300) and small number of events (<45 deaths, 19 within the first year and 26 over the next five years), limiting our ability to perform a more detailed analysis on the impact time past CPX has on prognostic significance. The present investigation attempted to rectify the limitations of our initial analysis and, to our knowledge, is the first addressing this issue in a detailed manner.
Heart failure is a dynamic condition where clinical status has the potential to dramatically deteriorate over a short time period. It is estimated that 80% of men and 70% of women diagnosed with HF under the age of 65 die within 8 years of their diagnosis.11 It is therefore not surprising that the prognostic value of CPX may not be maintained indefinitely, as disease severity will worsen in a majority of these patients in less than 10 years. Importantly, peak VO2 may lose its prognostic value in patients with HF two years post CPX. The VE/VCO2 slope appears to retain prognostic value for three and a half years following CPX. In the latter half of the fourth year of tracking, the VE/VCO2 slope also appears to begin to lose prognostic value. These observations suggest the following: 1) When peak VO2 and the VE/VCO2 slope are assessed from a prognostic perspective without consideration of time past CPX, both are highly prognostic, which is consistent with the previous research3; 2) The prognostic strength of peak VO2 and the VE/VCO2 slope may be dependent on time past CPX. In combination, peak VO2 and the VE/VCO2 slope can be considered for up to two years following CPX with a high degree of confidence. The VE/VCO2 slope may continue to provide prognostic insight into the fourth year following CPX, although its ability to predict risk for adverse events diminishes after 42 months; 3) Neither peak VO2 nor the VE/VCO2 slope may provide reliable prognostic information during the latter half of the fourth year post CPX. While the analysis was not extended into the fifth year following CPX, it is reasonable to hypothesize the trends of diminished predictive value in the fourth year would continue. It should ld be noted, however, that statistical power began to diminish for the VE/VCO2 slope during the fourth year tracking. The results of the present study during this time period should therefore be viewed as compelling but in need of future studies to address this issue. Even so, attaining a cohort with enough events in a short time interval to demonstrate these CPX variables are not prognostic after an extended period and generate a power of at least 80% may be challenging. In addition, the VE/VCO2 slope maintained prognostic significance for a longer period of time despite the lower event rate into the fourth year. This indicates the lack of prognostic significance for peak VO2 during this period does portend a potentially important clinical message.
In 62 patients with HF, Florea et al.12 performed two CPX evaluations that were at least four months apart (mean: 19 months). In the 22 subjects suffering a major cardiac event, there was a significant reduction in peak VO2 from the first to second test (18.3 vs. 13.9 ml• kg−1•min−1, p<0.05) while there was no change in subjects who were event free (18.1 vs. 20.8 ml• kg−1•min−1). The difference in peak VO2 between groups according to event status was not significant during the baseline CPX but differed at the follow-up evaluation (p<0.001). The VE/VCO2 slope increased in subjects suffering a major cardiac event in this analysis (44.4 vs. 50.0) but did not reach statistical significance. The difference in the VE/VCO2 slope in event-free subjects was more stable between the two tests (34.3 vs. 35.9) and was significantly lower compared to the group suffering an adverse event at both the baseline and follow-up tests (p<0.001). This previous investigation underscores that fact that CPX responses vary with time and tend to worsen among subjects who subsequently suffer an adverse event. This is particularly true for peak VO2, which was significantly reduced at follow-up in subjects suffering an event. The VE/VCO2 slope appears to be more stable among subjects eventually suffering an adverse event, exhibiting a significantly higher value at both baseline and follow-up. This stability also appears to be the case for subjects who were event-free, where the VE/VCO2 slope was significantly lower and comparable between baseline and follow-up assessments. The ability of the latter CPX variable to reflect longitudinal disease severity with greater stability during a cross sectional analysis may be a primary reason for its prognostic superiority in comparison to peak VO2.3 Even so, the prognostic window for the VE/VCO2 slope following CPX is not indefinite and subjects initially presenting with a more favorable response will eventually have a higher incidence of adverse events. This is reflected by the statistically significant, albeit weak, correlation between CPX variables and time to event in the present study (i.e. CPX response improves in subjects suffering an event at a longer time point from the assessment).
Numerous scientific statements from respected national/international organizations support the use of CPX for prognostic purposes in patients with HF.1, 13–15 Moreover, the American Heart Association guidelines for the diagnosis and management of patients with HF recommends the use of CPX to identify high risk individuals being considered for heart transplantation or other advanced therapies.16 None of these documents however, address the length of time CPX data maintains prognostic value, an important consideration given the fluid nature of HF etiology and disease progression. Given the results of the present investigation, we propose the following: 1) As a conservative estimate, the current results support the prognostic utility of peak VO2 and the VE/VCO2 slope for up to two years following the exercise assessment; 2) These variables should be assessed in combination over this time period, with progressively higher VE/VCO2 slope values in combination with progressively lower peak VO2 values (VC-IV and Weber D), portending the greatest risk for major cardiac events; 3) Irrespective of the time frame, subjects initially presenting with a poor CPX response should be considered at high risk for adverse events and monitored accordingly; 4) Repeating the CPX every two years in patients with HF who initially have a peak VO2 and VE/VCO2 slope that places them in the intermediate (Weber B/C and VC-II/III) risk categories is warranted. 5) Patients who are in Weber A and VC-I classes on initial CPX tend to remain at extremely how risk for a longer period. Repeating a CPX every three to four years may therefore be acceptable in this latter category.
While the overall number of subjects and events in the present investigation is relatively large for this area of study, removing preceding events at six-month intervals diminishes the ability to perform extensive multivariate analyses. Ten events per predictor variable is a minimal recommended threshold for survival analyses.17 This threshold was surpassed in all of the univariate analyses and either met or surpassed it for the multivariate analyses during the first three and a half years following CPX. Even so, statistical power diminished during third and fourth year of tracking, limiting the strength of conclusions that can be drawn from this portion of the analysis. Moreover, other variables included in standardized prognostic models, such as the Seattle Heart Failure model18 were not available in the present data set. However, while these variables are certainly valuable, previous investigations have demonstrated that CPX variables, particularly the VE/VCO2 slope, are amongst the strongest prognostic markers avaialble.6, 8 This assertion was confirmed in the present study, demonstrating the VE/VCO2 slope remained the single best predictor of adverse events over the entire three-year period when all baseline variables listed in Table 1 were considered. Nevertheless, future investigations should determine if the time-dependent predictive trends for CPX variables in the present study extend to other important clinical measures. Changes in the clinical management of the subjects included in this analysis during the follow-up period were not tracked. It is therefore possible that the addition of medications, such as beta-blockade, or devices, such as an implantable cardioverter defibrillator, defibrillator, would alter prognosis and thus diminish the predictive ability of the CPX variables assessed. Previous research, however, has found both peak VO2 and the VE/VCO2 slope remain prognostic irrespective of beta-blocker use.3, 6 In addition, analysis of the prognostic value of CPX has produced consistent results for more than 25 years, supporting the hypothesis that an abnormal CPX response portends a higher adverse event risk irrespective of treatment strategies. Lastly, there is a referral bias that should be considered with respect to the characteristics of patients referred for CPX that likely differ from the overall HF population.
In conclusion, peak VO2 and the VE/VCO2 slope are well-established prognostic markers in patients with HF. While the present investigation affirms the overall prognostic strength of both peak VO2 and the VE/VCO2 slope, there appears to be a time constraint on their predictive utility, reflecting the variable nature of HF severity over time. Depending on the baseline CPX response, clinicians may want to consider repeating CPX over a two to four year time period to more accurately identify change in adverse event risk.
Acknowledgments
Supported in part by NIH grants R37AG18915 and P60AG10484
Footnotes
Conflict of Interests
No conflicts to disclose
Reference List
- 1.Gibbons RJ, Balady GJ, Timothy BJ, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testinng Guidelines) J Am Coll Cardiol. 2002;40:1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, Vanhees L. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: Interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur J Cardiovasc Prev Rehabil. 2006;13:485–94. doi: 10.1097/00149831-200602000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–69. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Impact of time past exercise testing on prognostic variables in heart failure. International Journal of Cardiology. 2006;106:88–94. doi: 10.1016/j.ijcard.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 5.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JGF, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Heidenreich PA, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF. ACC/AHA Key Data Elements and Definitions for Measuring the Clinical Management and Outcomes of Patients With Chronic Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 6.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a Ventilatory Classification System in Patients With Heart Failure. Circulation. 2007;115:2410–7. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT, Janicki JS, McElroy PA. Determination of aerobic capacity and the severity of chronic cardiac and circulatory failure. Circulation. 1987;76:VI40–VI45. [PubMed] [Google Scholar]
- 8.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21:154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 9.Corra U, Mezzani A, Bosimini E, Scapellato F, Imparato A, Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J. 2002;143:418–26. doi: 10.1067/mhj.2002.120772. [DOI] [PubMed] [Google Scholar]
- 10.Arena R, Myers J, Guazzi M. The clinical importance of cardiopulmonary exercise testing and aerobic training in patients with heart failure. Brazilian Journal of Physical Therapy. 2008;12:75–87. [Google Scholar]
- 11.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 12.Florea VG, Henein MY, Anker SD, Francis DP, Chambers JS, Ponikowski P, Coats AJS. Prognostic value of changes over time in exercise capacity and echocardiographic measurements in patients with chronic heart failure. European Heart Journal. 2000;21:146–53. doi: 10.1053/euhj.2000.1737. [DOI] [PubMed] [Google Scholar]
- 13.Piepoli MF, Corra U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, Vanhees L. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part II: How to perform cardiopulmonary exercise testing in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:300–11. doi: 10.1097/00149831-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Palange P, Ward SA, Carlsen KH, Casaburi R, Gallagher CG, Gosselink R, O’Donnell DE, Puente-Maestu L, Schols AM, Singh S, Whipp BJ. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29:185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: Prediction of Survival in Heart Failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]


