Abstract
Artemisinin is a plant derived anti-malarial drug that has relatively low toxicity in humans and is activated by heme and/or intracellular iron leading to intracellular free radical formation. Interestingly, artemisinin has displayed anti-cancer activity with artemisinin dimers being more potent than monomeric artemisinin. Intracellular iron uptake is regulated by the transferrin receptor (TfR), and the activity of artemisinin depends on the availability of iron. We examined the level of TfR in prostate cancer (PCa) tumor cells, synthesized two new artemisinin dimers, and evaluated the effect of dihydroartemisinin (DHA) and artemisinin dimers ON-2Py and 2Py on proliferation and apoptosis in PCa cells. TfR was expressed in the majority of PCa bone and soft tissue metastases, all twenty-four LuCaP PCa xenografts, and PCa cell lines. After treatment with DHA, ON-2Py, or 2Py all PCa cell lines displayed a dose dependent decrease in cell number. 2Py was the most effective at decreasing cell number. An increase in apoptotic events and growth arrest was observed in the C4-2 and LNCaP cell lines. Growth arrest was observed in PC-3 cells, but no significant change was observed in DU 145 cells. Treatment with 2Py resulted in a loss of the anti-apoptotic protein survivin in all four cell lines. 2Py treatment also decreased androgen receptor and PSA expression in C4-2 and LNCaP cells with a concomitant loss of cell cycle regulatory proteins Cyclin D1 and c-Myc. This study demonstrates the potential use of artemisinin derivatives as therapeutic candidates for PCa and warrants the initiation of pre-clinical studies.
Keywords: Artemisinin, apoptosis, cell cycle, androgen receptor, prostate cancer, dimer
Introduction
The American Cancer Society estimates that during 2008 approximately 28,660 men died of metastatic prostate cancer (PCa) in the USA. While chemotherapeutic strategies show some promise, there is no effective therapy that substantially prolongs survival for hormone refractory PCa. A number of studies have suggested that artemisinin may be useful in the treatment of solid tumors including PCa [1–4].
Artemisinin has been used as an anti-malarial drug for a number of years now [3, 5, 6]. It has a defined mechanism of action, with artemisinin and its derivatives effecting heme-mediated decomposition of the endoperoxide bridge in artemisinin to produce carbon-centered free radicals [7]. This heme-catalyzed excess of reactive oxygen species induces apoptosis through a mitochondrial mediated pathway, ER-stress induction or blocking cell cycle kinetics [2, 8, 9]. These cellular effects, in combination with relatively low toxicity in humans [3], make artemsinin an attractive candidate drug for the treatment of solid tumors.
The levels of TfR can determine the intracellular levels of holo-transferrin (Fe(III)), the iron (III) transport protein in the blood that plays an important role in the electro-catalytic reduction of artemisinin, which can catalyze the cleavage of the endoperoxide bridge in artemisinin [10]. Therefore, we set out to determine the expression of the transferrin receptor (TfR) in human PCa metastases, xenografts and cell lines. Furthermore, artemisinin dimers have shown potent anti-cancer activities both in vitro and in vivo [3, 11–13], and a relatively small number of articles have been published on the effectiveness of artemisinin and its derivatives on inhibiting the growth of PCa cells in vitro and in vivo [1–3, 8].
Therefore, we synthesized two artemisinin dimers (2Py-ON and 2Py), and tested their ability to induce apoptosis and/or proliferation in PCa cell lines in vitro. In summary, the aim of this study was to determine the levels of the TfR in PCa and to elucidate the effects of artemisinin, 2Py-ON and 2Py on growth inhibition and apoptosis in the C4-2, DU 145, LNCaP and PC-3 cells in vitro.
Materials and Methods
Preparation of 2Py
Preparation of dimer hydrazide (Dimer-NHHH2): The trioxane dimer acid (500 mg, 0.8 mmol, prepared by Posner’s method [3]) was treated with tetrafluorophenol (412 mg, 2.56 mmol) in the presence of EDCI-HCl (3-(N,N’-Dimethylamino)propyl)ethyl carbodiimide hydrochloride, 416 mg, 1.6 mmol) and triethylamine (0.5mL, 4 mmol) in dichloromethane (16 mL) at room temperature. After stirring for overnight, anhydrous hydrazine (94 µL, 3.2 mmol) in dry DMF (0.5 mL) was added. The solution was additionally stirred for 1 h. The reaction was quenched with water, and extracted with chloroform. The organic layer was dried over magnesium sulfate, filtered, and concentrated. The residue was purified on silica-gel with dichloromethane:methanol (40:1), and recrystallized in methanol. Isolated yield, 348 mg (68%); 1H NMR (500 MHz, CDCl3) δ 6.97 (bs, 1H), 5.30 (s, 1H), 5.17 (s, 1H), 4.16 (dd, J=10.5, 6.5 Hz, 1H), 4.10 (dd, J=11.0, 6.5 Hz, 1H), 3.70 (bs, 1H), 2.75 (m, 1H), 2.56 (m, 1H), 2.50–2.42 (m, 1H), 2.32 (td, J=14.0, 3.5 Hz, 2H), 2.21 (m, 1H), 2.07–1.97 (m, 2H), 1.97–1.89 (m, 1H), 1.89–1.72 (m, 4H), 1.68–1.56 (m, 4H), 1.55–1.33 (m, 12H, including two singlets at δ 1.44 and 1.40), 1.32–1.18 (m, 4H), 0.96 (d, J=6.0 Hz, 3H), 0.94 (d, J=6.5 Hz, 3H), 0.85 (d, J=7.5 Hz, 3H), 0.83 (d, J=7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 177.8, 103.8, 103.3, 89.6, 88.3, 81.4, 81.1, 71.9, 52.7, 52.2, 44.9, 44.3, 42.8, 37.7, 37.4, 36.9, 36.6, 34.7, 34.5, 34.0, 33.1, 30.3, 30.2, 26.3, 26.2, 25.0, 24.9, 24.7, 20.4, 20.3, 13.7, 12.9; LRMS (ESI), m/z [(M + H+)+] 635.4.
To a solution of trioxane dimer hydrazide (50 mg, 0.078 mmol) in methanol (1 mL) was added 2-pyridinecarboaldehyde (7.5 µL, 0.08 mmol) at 20–25 °C. After stirring for 4 h to overnight, the reaction mixture was filtered or chromatographed then recrystallized. Dimer–2Py; Isolated yield; 54 mg, 96% as an amorphous solid (E:Z=4.5:1): 1H NMR (500 MHz, CD3OD) δ 8.54 (d, J=5.0 Hz, 2H), 8.27 (d, J=8.0 Hz, 1H), 8.16 (s, 1H), 7.88 (dd, J=7.5, 6.5 Hz, 2H), 7.41 (dd, J=5.5, 5.0 Hz, 2H), 5.45 (s, 1H), 5.27 (s, 1H), 4.16–4.23 (m, 1H), 4.11–4.05 (m, 1H), 2.80 (m, 1H), 2.69 (m, 1H), 2.63 (m, 1H), 2.30–2.13 (m, 3H), 2.07–1.77 (m, 8H), 1.73–1.63 (m, 3H), 1.60–1.15 (m including singlets at δ 1.31 and 1.19, 16H), 1.02–0.85 (m, 14H); 1H NMR (500 MHz, CD3OD, Z) δ 8.19 (d, J=8.0 Hz), 7.01 (s), 5.43 (s), 5.36 (s), 1.37 (s), 1.23 (s); 13C NMR (125 MHz, CD3OD) δ 176.2, 154.8, 150.1, 148.4, 138.7, 126.0, 122.4, 104.78, 104.76, 90.5, 89.9, 82.5, 82.3, 76.9, 74.8, 54.2, 54.1, 46.3, 46.1, 43.4, 38.7, 38.5, 37.7, 37.6, 35.9, 35.8, 34.5, 33.6, 31.7, 26.4, 26.37, 26.1, 26.0, 25.9, 20.8, 20.7, 13.8, 13.6; LRMS (ESI), m/z [(M + H+)+] 724.7.
Preparation of Dimer-ON-2Py
To a mixture of dimer alcohol (500 mg, 0.8 mmol, prepared by Posner’s method [12]), triphenylphosphine (1.73 g, 3.2 mmol), N-hydroxy phthalimide in chloroform (12 mL) was slowly added a solution of DEAD (Diethyl azidocarboxylate, 0.52 mL, 3.2 mmol) in chloroform (5 mL) at room temperature. The mixture was stirred for overnight, and quenched with water. The solution was extracted with chloroform, dried over magnesium sulfate, and concentrated. The residue was purified on silica-gel with hexanes:EtOAc (4:1 to 3:1) to give the corresponding product. Isolated yield, 446 mg (74%); 1H NMR (300 MHz, CDCl3) δ 7.85–7.78 (m, 2H), 7.76–7.69 (m, 2H), 5.40 (s, 1H), 5.34 (s, 1H), 4.50–4.18 (m, 5H), 2.75(q, J=6.6 Hz, 1H), 2.65 (q, J=6.6 Hz, 1H), 2.42–2.22 (m, 3H), 2.22–2.08 (m, 1H), 2.04–1.15 (m, 16H including singlet at δ 1.37), 0.96 (dd, J=6.0, 3.6 Hz, 6H), 0.91 (dd, J=7.5, 2.1 Hz, 6H). The dimer phthalimide (202mg, 0.27 mmol) was treated with hydrazine monohydrate (40 µL, 0.81 mmol) in ethanol (7 mL) at room temperature. 1 hour later, the reaction mixture was filtered. After concentration, the residue was purified on silica-gel by eluting with dichloromethane-methanol (45:1) to give the corresponding aminoxy dimer in 98% yield (189 mg); 1H NMR (300 MHz, CDCl3) δ 5.38 (s, 1H), 5.34 (s, 1H), 5.31(s, 1H), 4.36 (m, 1H), 4.16 (m, 1H), 3.97 (d, J=4.6 Hz, 2H), 2.79–2.55 (m, 2H), 2.33(t, J=14.0 Hz, 2H), 2.20–1.30 (m, 19H including singlet at δ 1.41), 0.96 (d, J=6.0 Hz, 6H), 0.86 (dd, J=7.2, 2.1Hz, 6H); LRMS (ESI), m/z [(M + H+)+] 622.5. To a solution of aminoxy dimer artemisinin (50 mg, 0.08 mmol) in methanol (2 mL) was added pyridine-2-carboaldehyde (8 µL, 0.08 mmol) at room temperature. After stirring for 4 h to overnight, the solvent was removed then purified on silica-gel with hexanes-EtOAc (4:1). Isolated yield, 47 mg (83%); 1H NMR (500 MHz, CD3OD) δ 8.54 (d, J=5.0 Hz, 1H), 8.15 (s, 1H), 7.92 (d, J=7.5Hz, 1H), 7.85 (td, J=7.5, 1.5 Hz, 1H), 7.40 (ddd, J=7.5, 5.0, 1.0 Hz, 1H), 5.49 (s, 1H), 5.33 (s, 1H), 4.45–4.32 (m, 3H), 4.24 (m, 1H), 2.66 (q, J=7.5 Hz, 1H), 2.57 (q, J=7.5 Hz, 1H), 2.40–2.20 (m, 3H), 2.10–1.73 (m, 8H) 1.72–1.11 (m, 19H including two singlets at δ 1.37 and 1.33), 0.96 (d, J=6.0 Hz, 3H), 0.92–0.86 (m, 9H); 13C NMR (125 MHz, CD3OD) δ 153.1, 150.4, 149.3, 138.7, 125.8, 122.3, 104.8, 104.4, 90.8, 90.2, 82.5, 82.45, 78.6, 75.4, 73.9, 54.1, 53.9, 46.2, 45.9, 38.7, 38.6, 37.8, 37.7, 36.2, 35.8, 32.2, 32.0, 31.1, 30.9, 26.4, 26.2, 26.1, 26.0, 25.9, 20.1, 13.8, 13.4.
Immunohistochemistry
To assess TfR expression in PCa metastases human tissue microarrays of formalin fixed paraffin-embedded tissues from 22 rapid autopsy patients were used for immunohistochemical (IHC) analyses [14]. To assess TfR expression in twenty-four LuCaP PCa xenografts (developed at the University of Washington) animals were implanted with each of the LuCaP PCa xenograft lines subcutaneously, and the resultant tumors were formalin fixed and embedded in paraffin as described previously [15, 16]. Five-micron sections of the human tissue microarrays and subcutaneous LuCaP tumors were deparaffinized, and antigen retrieval was performed in 10 mM citrate buffer (pH 6) at 120 °C. The sections were then incubated with 3% H2O2, blocked with avidin/biotin blocking solution (Vector Laboratories Inc., Burlingame, CA) and incubated in a 5% chicken/goat/horse serum solution. The sections were stained with 5 µg/mL mouse anti-human TfR antibody (Zymed Laboratories, Inc., South San Francisco, CA). Negative control slides were incubated with rabbit IgG (Vector Laboratories Inc.) or mouse anti-MOPC21 (generated in house from a hybridoma obtained from ATCC, Manassas, VA) at the same concentration as the primary antibody. All slides were then incubated with horse anti-mouse biotinylated secondary antibody (1:150) (Vector Laboratories Inc.) and developed using the Vectastain ABC kit (Vector Laboratories Inc.) and stable DAB (Invitrogen Corp., Carlsbad, CA), counterstained with hematoxylin, and dehydrated and mounted with Cytoseal XYL (Richard Allan Scientific, Kalamazoo, MI). Immunostaining was assessed using the following 4-point categorical compositional scale: 0=no staining, 1=faint/equivocal or focal staining, 2=definite staining of a minority of cells, and 3=definite staining of a majority of cells. The immunostain results were determined by consensus by CM and FVL (listed authors). Statistical analysis of IHC comparing bone, liver and lymph node metastases on tissue microarrays was described previously [14].
Cell Culture and Reagents
PC-3, LNCaP, C4-2 and DU 145 cells were maintained in RPMI 1640 with L-glutamine (Invitrogen Corp.) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA). The compounds Dihydroartemisinin (DHA) and its two derivatives Dimer-2Py (2Py) and Dimer-ON-2Py (ON-2Py) were synthesized by Dr. Tomikazu Sasaki and Dr. Byung Ju Kim, human holo-transferrin was obtained from Fortune Biologicals Inc. (Gaithersburg, MD).
Immunocytochemistry
LNCaP and C4-2 cells were cultured on Lab-Tek Chamber Slides (Nalge Nunc Naperville, IL). After treatment in RPMI 1640 with 10% fetal bovine serum (FBS) (Atlanta Biologicals) with DMSO or 15 µM 2Py for 48 h, cells were fixed and incubated with rabbit polyclonal anti-human β-catenin antibody (5 µg/mL Santa Cruz Biotechnology Inc. Santa Cruz, CA) or control rabbit IgG (5 µg/mL Vector laboratories Inc. Burlingame, CA) and then with goat-anti-rabbit Alexa Fluor 488 at a dilution of 1:400 respectively, and mounted with ProlongGold anti-fade reagent w/DAPI (Invitrogen Corp.).
Crystal Violet Assay
PC-3, LNCaP and C4-2 cells were grown in 24 well plates to 50% confluence in RPMI 1640 with 10% FBS and treated with DHA, 2Py or ON-2Py for 24, 48 or 72 hours. Cells were fixed in 10% buffered formalin, washed with PBS, incubated with 1% crystal violet (Sigma-Aldrich, St. Louis, MO), solubilized with 1% Triton X-100 and analyzed for absorbance at 590 nm on a Tecan GENios Plus (Tecan Group Ltd., Zurich, Switzerland).
MTT Assay
PC-3, C4-2, LNCaP and DU 145 cells were grown in 24 well plates to 50% confluence in RPMI 1640 with 10% FBS and treated with 2Py (controls were treated with vehicle alone) and/or 12.4 µM iron saturated human holo-transferrin for 48 hours. Cells were then incubated with MTT, formazan crystals were solubilized in DMSO and absorbance was measured at 590 nM on a Tecan GENios Plus (Tecan Group Ltd.).
Western Blot Analysis
Whole cell lysates were prepared as we described previously [17]. Protein levels were determined using the Bio-Rad DC Protein Assay kit (BioRad Laboratories, Hercules, CA). Western blotting was performed as described previously [2]. Antibodies to β-catenin, c-Myc, Cyclin D1, androgen receptor, and prostate specific antigen (PSA) were from Epitomics (Burlingame, CA). Antibodies to survivin, bcl-2, and bax were from Cell Signaling Technology (Danvers, MA), to the transferrin receptor (clone H68.4) were from Zymed (Grand Island, NY), and to β-actin were from Sigma (St. Louis, MO).
Apoptosis and viability assays
Apoptotic events were described as a percentage of total events with hypodiploid DNA assessed by propidium iodide (PI) incorporation. Cells were harvested by trypsinization, permeabilized with a hypotonic fluorochrome solution (50 µg/mL PI, 3.4 mmol/L sodium citrate, 1 mmol/L Tris, 0.1 mmol/L EDTA and 0.1% Triton X-100) and incubated on ice for 10 min prior to analysis. Viability assays were performed on the same samples. Cells were harvested by trypsinization, permeabilized with a hypotonic fluorochrome solution (50 µg/mL PI, 3.4 mmol/L sodium citrate, 1 mmol/L Tris, 0.1 mmol/L EDTA) and incubated on ice for 5 min prior to analysis. Samples were run on a BD FACScan (BD Biosciences, San Jose, CA). 5000 events were gated on PI intensity and analyzed using Cell Quest software (BD Biosciences).
Statistical analysis
For the in vitro experiments significance of differences was evaluated using paired Student's t tests as appropriate, with p values ≤0.05 indicating statistical significance.
Results
Transferrin receptor (TfR) expression in PCa metastases, xenografts and cell lines
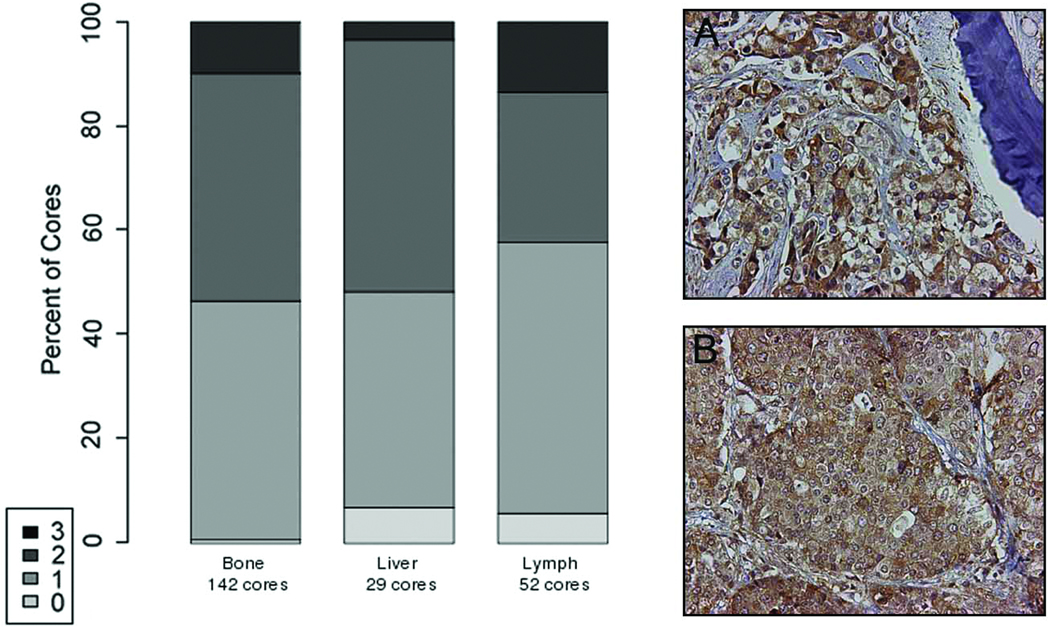
The activity of artemisinin depends on the availability of iron and intracellular iron uptake depends on the presence of the TfR. Therefore we examined TfR expression in PCa metastases, xenografts and cell lines. We observed no significant difference in TfR protein expression between PCa bone, liver, and lymph node metastases by immunohistochemical analysis. In PCa bone, liver, and lymph node metastases the expression pattern of the TfR was cytoplasmic with the majority of tumor cells expressing the TfR. Intense staining was only observed in a minority of cases (Figure 2). Cytoplasmic TfR expression was also observed in all twenty-four PCa LuCaP xenografts and in the C4-2, DU 145, LNCaP and PC-3 cell lines by immunohistochemistry (data not shown). TfR was also observed in C4-2, DU 145, LNCaP and PC-3 cells by Western analysis with elevated levels in the DU 145 and PC-3 cell lines (data not shown).
Figure 2. Immunohistochemical analysis of transferrin receptor (TfR) expression.
in human PCa bone (Panel A) and lymph node (Panel B) metastases from 22 patients (200-fold magnification). There was no evidence that TfR staining intensity varies between bone and soft tissue metastases. Specific immunostaining was assessed on a four-point scale: 3 = intense, 2 = defined, 1 = faint, and 0 = absent.
Effect of Dihydroartemisinin (DHA), ON-2Py, and 2Py on Cell number
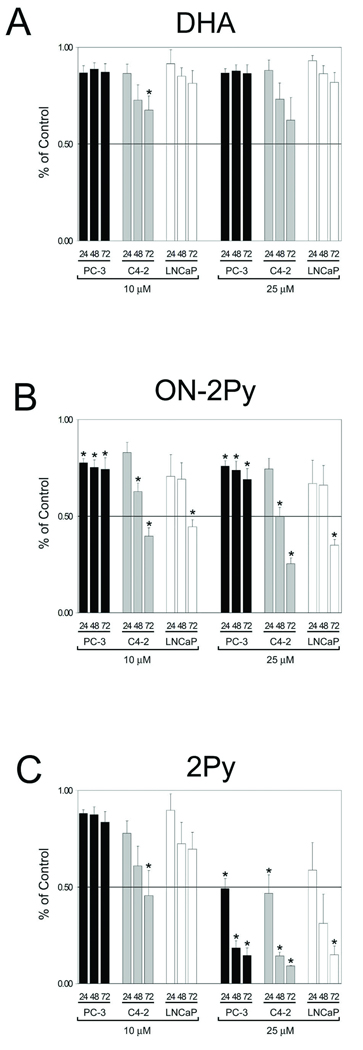
Cell number was initially assessed by crystal violet assay (Figure 3). DHA had no significant effect on reducing cell number in C4-2, LNCaP or PC-3 cells with the exception of one data point for C4-2 cells after 72 hours of treatment under the conditions we used in this study (Figure 3A). ON-2Py at both 10 and 25 µM concentrations had a significant effect on reducing cell number for all three cell lines at the 72 hour time point. This decrease in cell number was more evident in the C4-2 and LNCaP cells. ON-2Py was the most effective compound at decreasing cell number at the 10 µM concentration (Figure 3B). However, the most significant decreases in cell number was seen using 25 µM 2Py which significantly decreased all three cell lines to ~15% of control cell number after 72 hours (Figure 3C). The IC50 values calculated for 2Py at the 48 hour time point were 16.24 µM, 28.53 µM, 9.59 µM, and 17.11 µM for C4-2, DU 145, LNCaP, and PC-3 cells respectively.
Figure 3. Cell number as assessed by crystal violet assay in LNCaP, C4-2 and PC-3 cells.
Cells were brought to 50% confluence and then cultured in RPMI 1640 with 10% FBS medium with DMSO, 10, or 25 µM DHA, ON-2Py or 2Py for 24, 48, and 72 h. Relative cell number was assessed by crystal violet assay (n = 4). Results are expressed as the mean ± SD. * indicates significant difference from control (p < 0.05).
To determine if the effects of 2Py were related to the levels of transferrin available C4-2, DU 145, LNCaP, and PC-3 cells were treated with 5, 10, or 15 µM concentrations of the artemisinin derivative with or without iron saturated human holo-transferrin for 48 hours. Cell number was measured by an MTT assay. While there were subtle differences in cell number in all cases, there was no significant effect of holo-transferrin on cell number. Furthermore, 2Py had a limited effect on decreasing cell number in DU 145 cells when compared to the other cell lines (data not shown).
Effect of 2Py on apoptosis and cell cycle
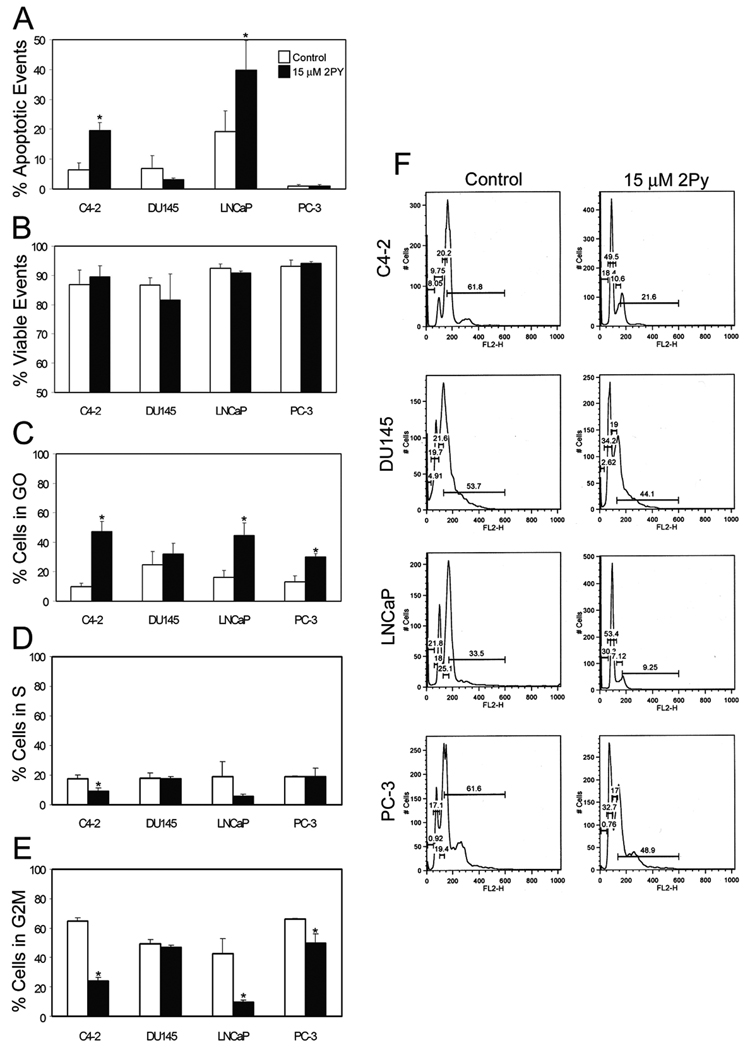
DU 145 and PC-3 cells had no statistically significant increase in apoptotic events with 2Py treatment; however, both C4-2 and LNCaP cells had an increase in apoptotic events in response to 15 µM 2Py treatment (Figure 4A). Membrane integrity (viability) was not altered between doses (Figure 4B). As the loss in cell number in the PC-3 cell line could not be attributed to apoptosis we examined the effect of 2Py on cell cycle kinetics. 15 µM 2Py increased the percentage of cells in the G0 phase of the cell cycle in all cell lines, and significantly increased it in the C4-2, LNCaP, and PC-3 lines (Figure 4C). The same dose of 2Py significantly decreased the number of cells in G2M and S phase in the C4-2, LNCaP and PC-3 cells, with no effect on the DU 145 cells (Figure 4D).
Figure 4. Effect of 2Py on apoptosis and cell cycle in LNCaP, C4-2, PC-3 and DU 145 cells.
Cells were brought to 50% confluence and then cultured in RPMI 1640 with 10% FBS medium with DMSO or 15 µM 2Py for 48 h. The cells were trypsinized and stained with propidium iodide and analyzed on a flow cytometer. The percentage of apoptotic events (Panel A), cell viability (Panel B), and the percentage of cells in G0 and G2M and S phase of the cell cycle (Panels C, D and E) were determined (n=3). Histograms of cell cycle distribution in control and 2Py treated cells (Panel F).
Apoptosis-associated protein expression
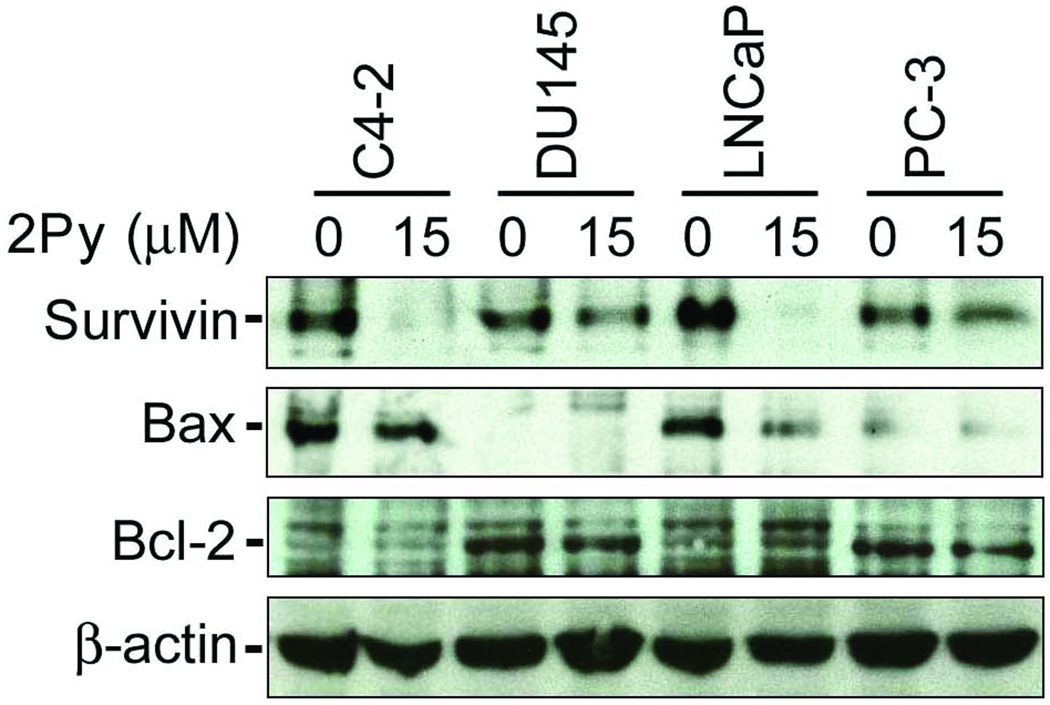
2Py had a limited effect on DU 145 cell number. 2Py altered cell cycle kinetics, but did not induce apoptosis in PC-3 cells while it induced apoptosis and altered cell cycle kinetics in C4-2 and LNCaP cells. To determine why there was a differential response to 2Py in each of the PCa lines we examined the expression of apoptosis-associated proteins. 2Py significantly decreased survivin expression in all four PCa cell lines, with a more pronounced effect in the more sensitive C4-2 and LNCaP cells. As expected the pro-apoptotic Bax expression levels were high in C4-2 and LNCaP cells and low in the DU 145 and PC-3 cell lines, while the anti-apoptotic protein BCL-2 levels were low in C4-2 and LNCaP cells and higher in DU 145 and PC-3 cells. We saw no significant change in Bax or BCL-2 levels after 2Py treatment (Figure 5).
Figure 5. Effect of 2Py on survivin, Bcl-2, and Bax in LNCaP, C4-2, PC-3 and DU 145 cells.
Cells were brought to 50% confluence and then cultured in RPMI 1640 with 10% FBS medium with DMSO or 15 µM 2Py for 48 h, cell lysates were isolated and western blotting analysis performed using antibodies to survivin (n=4), Bax (n=3), and Bcl-2 (n=3), with β-actin as control.
Proliferation-associated protein expression
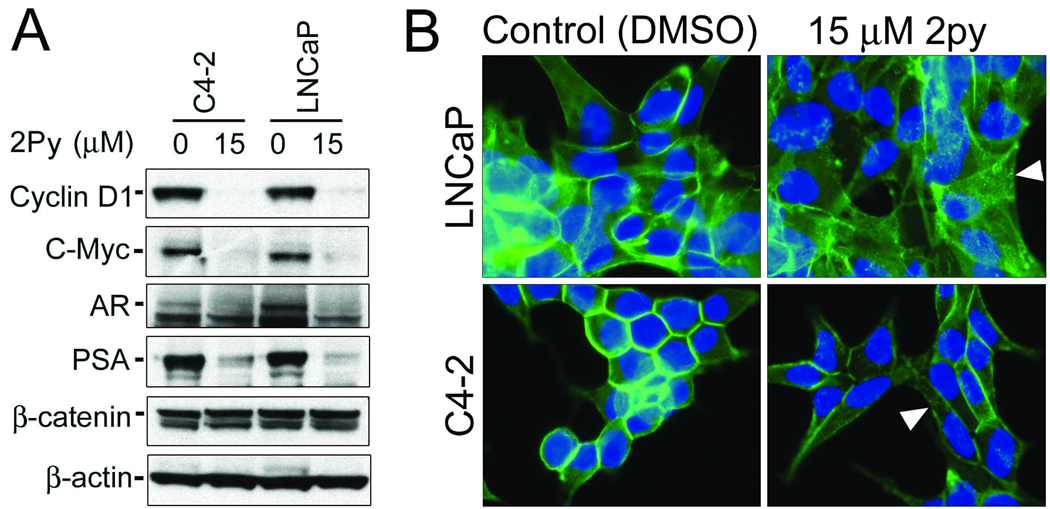
Previously both Cyclin D1 and c-Myc were shown to have a role in regulating cell cycle kinetics in PCa [18, 19]. We observed a dramatic decrease in both Cyclin D1 and c-Myc expression levels in C4-2 and LNCaP cells treated with 2Py (Figure 6A). Cyclin D1 and c-Myc can be regulated by β-catenin, so we examined the effect of 2Py on β-catenin expression in the C4-2 and LNCaP cells. 2Py did not alter β-catenin expression in both cell lines (Figure 6A). Furthermore, using immunocytochemistry we also determined that there was no change in the nuclear localization of β-catenin in the C4-2 and LNCaP cells after treatment with 2Py. There was however, an increase in punctuate β-catenin cytoplasmic staining in both LNCaP and C4-2 cells after treatment with 2Py (Figure 6B).
Figure 6. Effect of 2Py on C-Myc, Cyclin D1, AR, PSA, and β-catenin expression in LNCaP and C4-2 cells.
(A) Cells were brought to 50% confluence and then cultured in RPMI 1640 with 10% FBS medium with DMSO or 15 µM 2Py for 48 h, cell lysates were isolated and Western blotting analysis performed using antibodies to Cyclin D1 (n=3), c-Myc (n=3), AR (n=3), PSA (n=3), and β-catenin (n=2) with β-actin as control (n=3). (B) Immunocytochemical analysis of β-catenin in LNCaP and C4-2 cells cultured in 10% FBS medium with DMSO or 15 µM 2Py for 24 h. Arrows indicate cytoplasmic relocalization of β-catenin after 2Py treatment.
The effect of 2Py on the Androgen receptor (AR) and prostate specific antigen (PSA)
The AR, when present, is known to play a major role in PCa tumor growth, furthermore β-catenin is known to interact with and promote AR activity in PCa cells [20]. We determined the effect of 2Py on the levels of expression of the AR and the androgen receptor regulated protein PSA in the C4-2 and LNCaP cells. 2Py decreased AR and PSA expression in both cell lines (Figure 6A).
Discussion
Artemisinin has been used as an anti-malarial drug with limited toxicity for a number of years [3, 5]. The activity of artemisinin in PCa cells is dependent on a heme-catalyzed reaction and the TfR is required to maintain intracellular iron levels. We analyzed TfR expression in PCa bone and soft tissue metastases from our PCa rapid autopsy program at the University of Washington. Nearly all of the metastatic cores examined had TfR expression with some tumors expressing very high levels. The expression of the TfR in the patient samples as well as the LuCaP xenografts and PCa cell lines suggested that artemisinin may have potential efficacy in this solid tumor type. The high levels of TfR expression in the PCa metastases are in contrast to TfR levels that are essentially undetectable in normal tissues [21, 22].
Artemisinin has been used at high doses in vivo inhibiting PCa tumor cell growth and at up to 300 µM in vitro blocking PCa cell proliferation [8]. While high doses of artemisinin are well tolerated in patients treated for malaria we synthesized two more potent derivatives 2Py-ON and 2Py. These compounds are based on artemisinin dimers reported by Posner et al [3, 12], as artemisinin dimers have shown significantly higher anti-cancer activities, compared to the corresponding monomers. To determine the efficacy of artemisinin in vitro we treated PCa cell lines with lower concentrations of dihydroartemisinin. At lower concentrations the dimer 2Py was more effective than artemisinin at reducing PCa cell number in vitro for all PCa cell lines tested. Furthermore the IC50 values calculated for 2Py show that C4-2, LNCaP and PC-3 cells were more responsive to 2Py than DU 145 cells. Interestingly, 2Py (15 µM) was more potent than artemisinin in LNCaP cells, were it altered cell cycle kinetics and increased apoptotic events, whereas Willoughby et al. showed that a higher dose of artemisinin (300 µM) altered cell cycle kinetics, but did not increase apoptotic events in LNCaP cells [8].
The addition of holo-transferrin to cells can increase the effect of dihydroartemisinin in vitro by increasing intracellular levels of holo-transferrin (Fe(III)) in the cells [23]. The addition of holo-transferrin in combination with 2Py had no additive or synergistic effect on the PCa cells. We speculate that 2Py does not act solely through free intracellular Fe(II) or that the levels of transferrin present in the PCa cells is limited, thereby restricting the availability of intracellular holo-transferrin (Fe(III)) [2]. PCa cells are known to accumulate a large amount of iron [24], and the level of intracellular labile iron in those cells may not be affected by added holo-transferrin. Furthermore, Efferth et al., reported that the modulation effects of added iron on the efficacy of artesunate vary significantly among different cell lines [25].
Artemisinin derived compounds have been shown to induce apoptosis through a mitochondrial mediated pathway. The apoptotic effect of 2Py on the C4-2 and LNCaP cells may be due to their predisposition to apoptosis via a mitochondrial mediated pathway, with both cell lines having low levels of the anti-apoptotic protein Bcl-2 and higher levels of the pro-apoptotic protein Bax. Survivin is undetectable in terminally differentiated adult tissues and is a key regulator of cell survival and cell cycle progression and has been described as a molecular target in PCa [26, 27]. Interestingly, treatment with 2Py decreased the anti-apoptotic survivin protein levels in all of the PCa cell lines.
Willoughby et al. have described the molecular actions of artemisinin as disrupting Sp1 interactions with the cyclin dependent kinase-4 (CDK4) promoter thereby inhibiting CDK4 expression causing cell cycle arrest [8]. The enzymes that regulate G1-to-S-phase transition are CDK2, -4, and -6. CDK4 and CDK6, complex with D-type cyclins, phosphorylating the retinoblastoma protein (pRb) promoting proliferation [28]. Suzuki et al. have shown that survivin interacts with Cdk4, leading to Cdk2/Cyclin E activation and Rb phosphorylation. As a result of Survivin/Cdk4 complex formation, the CDK inhibitor p21 can be released from its complex with Cdk4 and interact with mitochondrial procaspase 3 to suppress cell death [29]. Therefore, the loss of survivin, Cyclin D1, and possibly CDK4 could considerably decrease proliferation and promote a pro-apoptotic phenotype in PCa cells.
C-Myc is a growth regulatory transcription factor strongly implicated in prostate carcinogenesis. It is often amplified in advanced PCa and is involved in androgen independent growth of PCa [30, 31]. Furthermore, the overexpression of c-Myc and Cyclin D1, as well as pRb, promotes G1-S transition and proliferation. Therefore, it is likely that the loss of c-Myc protein in 2Py treated cells also has a role in decreasing proliferation in PCa cells.
Since Cyclin D1 and c-Myc are downstream targets of β-catenin in PCa, we examined protein levels and nuclear localization of β-catenin in the LNCaP and C4-2 cell lines after 2Py treatment. β-catenin protein levels and nuclear localization were not altered significantly with 2Py treatment suggesting the loss of Cyclin D1 and c-Myc expression is not due to changes in β-catenin expression levels or nuclear localization [32]. We did however observe an increase in punctuate cytoplasmic staining after 2Py treatment in the C4-2 and LNCaP cells. This loss of membrane-associated β-catenin and increase in punctuate cytoplasmic staining may relate to the initial loss of cell-cell contact in the early stages of anoikis, before apoptosis occurs in these two cell lines.
The loss of AR and PSA expression in the C4-2 and LNCaP cells is a novel finding for an artemisinin derivative. PSA expression is androgen-dependent and the promoter for the PSA gene is regulated by the AR [33]. It is speculated that 2Py causes a decline in AR expression which subsequently results in the decrease of PSA levels. The AR promotes both androgen dependent and castration resistant PCa tumor growth therefore the deleterious effect of 2Py on AR expression and PSA expression in the AR expressing lines is a potentially significant finding and requires further investigation.
In summary, we have determined that at lower concentrations 2Py is more effective than artemisinin at causing cell cycle arrest and apoptosis in PCa cells in vitro. Furthermore, the loss of survivin and the AR in response to 2Py treatment is a significant finding and warrants further studies. The continued development of more analogs of artemisinin may result in a more potent compound with greater activity leading to the development of more effective therapies for PCa.
Figure 1. Structures of dihydroartemisinin (DHA), ON-2Py and 2Py.
Acknowledgements
We would like to sincerely thank the patients who were willing to take part in the PCa rapid autopsy series and their families. We would also like to acknowledge Drs. Celestia Higano, Paul Lange, Bruce Montgomery, Daniel Lin, William Ellis, Martine Roudier, Lawrence True and Beatrice Knudsen and the rapid autopsy teams, and Alex Dowell and Lisha Brown for their scientific advice. This material is based upon work supported in part by a career development award from the Pacific Northwest Prostate Cancer SPORE grant (P50CA097186) to CM and the Richard M. Lucas Foundation. The work was also supported in part by a grant to TS from the Washington Technology Center and Artemisia BioMedical, Inc. B.G was supported by a Brady Natural Products grant, by Department of Medicinal Chemistry start-up funding to D.G, and Grant ES0006-033 to the Center for Ecogenetics and Environmental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 2.Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290–298. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Posner GH, McRiner AJ, Paik IH, Sur S, Borstnik K, Xie S, et al. Anticancer and antimalarial efficacy and safety of artemisinin-derived trioxane dimers in rodents. J Med Chem. 2004;47:1299–1301. doi: 10.1021/jm0303711. [DOI] [PubMed] [Google Scholar]
- 4.Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Duffy PE, Mutabingwa TK. Drug combinations for malaria: time to ACT? Lancet. 2004;363:3–4. doi: 10.1016/S0140-6736(03)15230-0. [DOI] [PubMed] [Google Scholar]
- 6.Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 7.Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 8.Willoughby JA, Sr, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284:2203–2213. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockwin LH, Han B, Yu SX, Hollingshead MG, ElSohly MA, Gul W, et al. Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int J Cancer. 2009;125:1266–1275. doi: 10.1002/ijc.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai HH, Cai J, Yang PH. Electrochemical activity of holotransferrin and its electrocatalysis-mediated process of artemisinin. Bioorg Med Chem Lett. 2009;19:863–866. doi: 10.1016/j.bmcl.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Galal AM, Gul W, Slade D, Ross SA, Feng S, Hollingshead MG, et al. Synthesis and evaluation of dihydroartemisinin and dihydroartemisitene acetal dimers showing anticancer and antiprotozoal activity. Bioorg Med Chem. 2009;17:741–751. doi: 10.1016/j.bmc.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Posner GH, Paik IH, Sur S, McRiner AJ, Borstnik K, Xie S, et al. Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high stability and efficacy. J Med Chem. 2003;46:1060–1065. doi: 10.1021/jm020461q. [DOI] [PubMed] [Google Scholar]
- 13.Alagbala AA, McRiner AJ, Borstnik K, Labonte T, Chang W, D'Angelo JG, et al. Biological mechanisms of action of novel C-10 non-acetal trioxane dimers in prostate cancer cell lines. J Med Chem. 2006;49:7836–7842. doi: 10.1021/jm060803i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrissey C, True LD, Roudier MP, Coleman IM, Hawley S, Nelson PS, et al. Differential expression of angiogenesis associated genes in prostate cancer bone, liver and lymph node metastases. Clin Exp Metastasis. 2008;25:377–388. doi: 10.1007/s10585-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 15.Brubaker KD, Brown LG, Vessella RL, Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer. 2006;6:15. doi: 10.1186/1471-2407-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey E, Brown LG, Kiefer JA, Quinn JE, Pitts TE, Blair JM, et al. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–1718. doi: 10.1158/0008-5472.CAN-04-2033. [DOI] [PubMed] [Google Scholar]
- 17.Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004;91:151–160. doi: 10.1002/jcb.10679. [DOI] [PubMed] [Google Scholar]
- 18.Kokontis J, Takakura K, Hay N, Liao S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994;54:1566–1573. [PubMed] [Google Scholar]
- 19.Chen Y, Martinez LA, LaCava M, Coghlan L, Conti CJ. Increased cell growth and tumorigenicity in human prostate LNCaP cells by overexpression to cyclin D1. Oncogene. 1998;16:1913–1920. doi: 10.1038/sj.onc.1201719. [DOI] [PubMed] [Google Scholar]
- 20.Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- 21.Niitsu Y, Kohgo Y, Nishisato T, Kondo H, Kato J, Urushizaki Y, et al. Transferrin receptors in human cancerous tissues. Tohoku J Exp Med. 1987;153:239–243. doi: 10.1620/tjem.153.239. [DOI] [PubMed] [Google Scholar]
- 22.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995;91:41–46. doi: 10.1016/0304-3835(94)03716-v. [DOI] [PubMed] [Google Scholar]
- 24.Guntupalli JN, Padala S, Gummuluri AV, Muktineni RK, Byreddy SR, Sreerama L, et al. Trace elemental analysis of normal, benign hypertrophic and cancerous tissues of the prostate gland using the particle-induced X-ray emission technique. Eur J Cancer Prev. 2007;16:108–115. doi: 10.1097/01.cej.0000228409.75976.b6. [DOI] [PubMed] [Google Scholar]
- 25.Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D, et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37:998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 26.Hansen JB, Fisker N, Westergaard M, Kjaerulff LS, Hansen HF, Thrue CA, et al. SPC3042: a proapoptotic survivin inhibitor. Mol Cancer Ther. 2008;7:2736–2745. doi: 10.1158/1535-7163.MCT-08-0161. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 28.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki A, Ito T, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, et al. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346–1353. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]
- 30.Williams K, Fernandez S, Stien X, Ishii K, Love HD, Lau YF, et al. Unopposed c-MYC expression in benign prostatic epithelium causes a cancer phenotype. Prostate. 2005;63:369–384. doi: 10.1002/pros.20200. [DOI] [PubMed] [Google Scholar]
- 31.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–1731. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed V, Mak P, Du C, Balaji KC. Beta-catenin mediates alteration in cell proliferation, motility and invasion of prostate cancer cells by differential expression of E-cadherin and protein kinase D1. J Cell Biochem. 2008;104:82–95. doi: 10.1002/jcb.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]