Abstract
Context
Chronic starvation is characterized by GH resistance and obesity by decreased GH secretion. In both extremes, IGF-1 levels may be low and androgen levels abnormal.
Objective
To investigate the determinants of IGF-1 and GH across the weight spectrum in women.
Design
Cross-sectional
Setting
Clinical Research Center
Study Participants
32 women: 11 with anorexia nervosa, 11 normal-weight, and 10 obese women of comparable mean age
Intervention
None
Main Outcome Measures
Pooled hourly overnight serum samples assayed for IGF-1, GH, estradiol, testosterone, SHBG, insulin, free fatty acids, and trunk fat
Results
Free testosterone was higher in obese women and lower in women with anorexia nervosa than in normal-weight women and was the only independent (and positive) predictor of IGF-1 levels, accounting for 14% of the variability (p=0.032) in the group as a whole. This relationship was stronger when obese women were excluded, with free testosterone accounting for 36% of the variability (p=0.003). Trunk fat accounted for 49% of the variability (p<0.0001) of GH, with an additional 7% of the variability attributable to estradiol (p=0.042) in the group as a whole but was not a significant determinant of GH secretion when obese women were excluded.
Conclusions
Free testosterone is a significant determinant of IGF-1 levels in women across the body weight spectrum. In contrast, GH secretion is differentially regulated at the extremes of the weight spectrum.
Keywords: Anorexia nervosa, GH, IGF-1, androgens
INTRODUCTION
It has been recognized since the early 1960s, concomitant with the development of GH assays with sufficient specificity and sensitivity to detect changes in concentrations, that starvation results in increased GH secretion1 and that obesity is associated with a decrease in serum GH levels2. Visceral adiposity3, 4, increased insulin secretion5 and elevated free fatty acids6-8 have been demonstrated to be strong determinants of GH secretion in obesity. In chronically undernourished patients, low IGF-1 levels appears to stimulate GH production, through a feedback mechanism9.
The determinants of IGF-1 levels across the weight spectrum are less well understood. Although GH secretion is known to be an important determinant of IGF-1 production, chronic starvation is associated with GH resistance and relatively low IGF-1 levels, and obesity with decreased GH secretion and a decrease in IGF-1 production, but not to the degree that might be expected based on the degree of reduced GH secretion. As GH stimulates lipolysis independent of IGF-1 effects, which are largely anabolic, increased GH secretion in starvation serves the function of mobilizing fat stores in the setting of reduced energy availability. However, the mechanisms underlying the development of GH resistance in states of chronic undernutrition or the increased GH sensitivity in obesity are not as well established. Recently and importantly, GH-induced activation of the Janus kinase (JAK)/STAT signaling pathway has been shown to be reduced in muscle and fat from human subjects during fasting10. However, the factors contributing to GH resistance under these conditions are not well understood. The low levels of IGF-1 in anorexia nervosa and relatively preserved levels in obesity cannot be attributed to abnormalities in binding globulin levels, cleavage or action, as IGF-1 bioactivity is reduced in anorexia nervosa11 and preserved in obesity12. In contrast, insulin plays an important role by modulating growth hormone binding in the liver, as has been shown by Leung et al. in a hepatoma cell culture model13. In this experimental model, increasing concentrations of insulin increased hepatic GH receptor protein and GH binding. Gonadal steroids also may be determinants of GH and hepatic IGF-1 production. Estrogens stimulate GH release in some, but not all, experimental models and may therefore drive IGF-1 levels through this mechanism14. However, oral estrogen decreases hepatic production of IGF-115 by suppressing GH-induced JAK2 phosphorylation16.
Testosterone exerts direct independent stimulatory effects on GH and IGF-1 release in men17. Likewise, we have recently shown that testosterone levels independently predict IGF-1 levels in obese women women with normal reproductive function, who have androgen levels at the higher end of the normal range4, but whether androgens are important determinants in women with low androgen levels is unknown. Our findings in obese eumenorrheic women are consistent with data demonstrating that women with polycystic ovary syndrome have higher IGF-1 levels than women without the syndrome of comparable weight18. In addition, healthy women of reproductive age receiving oral contraceptives containing progestins with higher androgenecity and postmenopausal women receiving hormone replacement therapy with greater androgenecity have higher IGF-1 levels than those receiving preparations of lower androgenecity19. Although we have shown in a large group of women with anorexia nervosa that androgen levels are reduced20, it is not known whether this is a significant determinant of GH resistance in women with anorexia nervosa.
Our aim was to investigate the determinants of GH and IGF-1 levels in women across the weight spectrum, from severe undernutrition to marked obesity. We hypothesized that in very low weight women, as in obesity, trunk fat mass would be a significant determinant of endogenous GH concentrations and androgen levels would be important determinants of IGF-1 levels. We therefore studied women with anorexia nervosa, obesity and normal-weight controls with overnight sampling for GH, IGF-1, testosterone, SHBG, estradiol, insulin and free fatty acids.
SUBJECTS and METHODS
Subjects
We studied thirty-two female ambulatory, community-dwelling subjects who were recruited through advertising. Inclusion criteria for all groups included ages 18-45, and for normal-weight and obese women, eumenorrhea. Women with anorexia nervosa fulfilled all DSM-IV diagnostic criteria for anorexia nervosa, including weight <85% of ideal body weight and amenorrhea for at least three months. Exclusion criteria for all groups included hypothalamic or pituitary disorders, hypothyroidism, hyperthyroidism, estrogen or glucocorticoid use, and diabetes mellitus.
Materials and Methods
The study was approved by the Partners Healthcare Inc. Institutional Review Board, and written informed consent was obtained from each study participant. Each obese and normal-weight participant was admitted during the follicular phase of the menstrual cycle to the Clinical Research Center at the Massachusetts General Hospital. As all women with anorexia nervosa were amenorrheic, their study visits were not timed with respect to menstrual periods or other events. Serum was collected every hour for 12h starting at 2000h under standardized conditions. Subjects were served dinner between 1800h and 1900h and were not offered breakfast until after the 8 a.m. blood draw. Vigorous activity, including exercise, was prohibited during this admission. Equal aliquots of serum from each hour sample were combined into one 12h-pooled sample. Clinical characteristics, but none of the endocrine data reported here, on a subset of the subjects studied were reported in previous publications4, 12, 21, 22.
Biochemical Analyses
Serum samples were collected and stored at -80° C. Serum total IGF-I levels were measured using a solid-phase enzyme-labeled chemiluminescent immunometric assay on the Immulite 2000 automated immunoanalyzer (Siemens Medical Solutions Diagnostics, Los Angeles, CA) with an inter-assay coefficient of variation (cv) of approximately 4%. Serum total GH levels were measured using an immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX) with a sensitivity of 0.01 ng/ml, with an inter-assay cv of 3-5%. Estradiol, insulin, and SHBG were measured by automated immunoassay (Architect; Abbott Diagnostics, Chicago, IL). The lower limit of detection of estradiol was 10 pg/ml, with an intra-assay cv of 1-6%. The lower limit of detection of insulin was 1.0 μU/ml, with an intra-assay cv of 2-5% and with an inter-assay cv of 2-10%. The lower limit of detection of SHBG was 0.01 nmol/l, with an intra-assay cv of 6-10%. Serum testosterone levels were measured by a solid-phase radioimmunoassay Coat-A-Count RIA kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA) with a minimum detection limit of 2.2 pg/ml and an intra-assay cv of 1-2%. Free testosterone levels were calculated from total testosterone and SHBG using the laws of mass action23. Nonesterified fatty acids were measured on an automated immunoanalyzer (Architect; Abbott Diagnostics, Chicago, IL) using a commercially available kit (Wako Chemicals USA, Richmond, VA) with a sensitivity of 0.0014 mEq/L, and with an intra-assay cv of <1% .
Body Composition Measures
Body composition was measured by dual-energy x-ray-absorptiometry (DXA) using a Hologic QDR 4500 scanner (Hologic Inc., Waltham, MA), with an accuracy error for body fat mass of 1.7% and for fat-free mass of 2.4%24.
Statistical Analysis
JMP Statistical Discoveries software (version 4.0.2, SAS Institute, Inc., Cary, NC) was used for statistical analyses. Variables were log-transformed to approximate a normal distribution and means compared with an overall analysis of variance (ANOVA). Corrections for multiple comparisons were performed using the Tukey-Kramer method. Univariate regression models were constructed after log transformation of variables, and Pearson's coefficients are reported, except for correlations with IGF-1, for which quadratic fits were used. As a secondary analysis, dummy variables for anorexia nervosa and obesity were entered into least squares regression models examining the relationships between GH and both BMI and trunk fat and between IGF-1 and androgen levels. Stepwise regression models were constructed to investigate independent predictors of mean GH and IGF-I levels. The following log-transformed variables were entered into the stepwise regression models: trunk fat mass, insulin, free fatty acid, estradiol and free testosterone levels. Statistical significance was defined as p value < 0.05. Results are expressed as mean ± SEM.
RESULTS
Clinical Characteristics of Study Subjects and Endocrine Data
Clinical characteristics and endocrine data are presented in Table 1. We studied 11 AN (BMI ≤ 18.0 kg/m2), 11 normal-weight (19.0 < BMI < 25 kg/m2) and 10 obese (BMI ≥ 30 kg/m2) women of comparable age. Mean BMI differed between the groups, as designed, with women with AN and obesity having lower and higher BMIs, respectively, than the controls.
Table 1.
Clinical Characteristics
| Anorexia Nervosa BMI ≤ 18.0 kg/m2 (n = 11) | Lean 19.0 < BMI < 25 kg/m2 (n = 11) | Obese BMI ≥ 30 kg/m2 (n = 10) | |
|---|---|---|---|
| Age (yr) | 31.7 ± 2.2 | 30.5 ± 2.3 | 33.3 ± 1.9 |
| BMI (kg/m2) | 16.6 ± 0.5* | 22.3 ± 0.6 | 35.2 ± 0.9* |
| Weight (kg) | 45.3 ± 1.7* | 62.7 ± 2.0 | 95.6 ± 5.1* |
| Trunk Fat (kg) | 2.9 ± 0.3* | 7.4 ± 0.7 | 19.6 ± 1.5* |
| TSH (uU/ml) | 1.7 ± 0.4 | 2.1 ± 0.5 | 1.2 ± 0.3 |
| Estradiol (pg/ml) | 23 ± 4* | 75 ± 14 | 77 ± 16 |
| Testosterone (ng/dl) | 8.8 ± 3.2 | 9.3 ± 2.2 | 26.3 ± 5.4* |
| Free Testosterone (pg/ml) | 0.8 ± 0.4 | 1.5 ± 0.3 | 5.1 ± 1.1* |
| SHBG (nmol/l) | 123 ± 17* | 56 ± 7 | 33 ± 4 |
| Insulin (uIU/ml) | 5.3 ± 0.8 | 6.1 ± 0.7 | 11.6 ± 1.7* |
| Free Fatty Acids (meq/l) | 0.46 ± 0.05 | 0.41 ± 0.03 | 0.43 ± 0.04 |
p < 0.02 compared to lean subjects
Endocrine Data
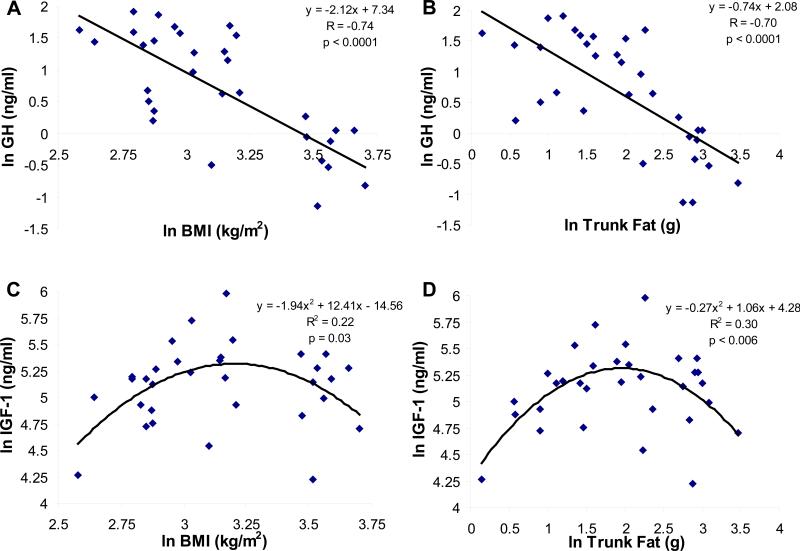
Mean pooled overnight GH levels were strongly inversely associated with BMI (R= -0.74, p < 0.0001) (Figure 1, Panel A) and trunk fat (R= -0.70, p < 0.0001) for the group as a whole (Figure 1, Panel B). When dummy variables for the obese group and the group with anorexia nervosa were entered into the models, BMI (p=0.009) and trunk fat (p=0.003) remained significant predictors of GH, but the dummy variables were not, except for obesity, which was a predictor of trunk fat (p=0.009). Mean pooled overnight GH levels were lower in the obese than the normal-weight women and the group with anorexia nervosa (obese: 0.76 ± 0.1 ng/ml, normal-weight: 3.4 ± 0.5 ng/ml, anorexia nervosa: 3.8 ± 0.6 ng/ml, p < 0.0001). Pooled IGF-1 levels were quadratically related to both BMI (R2= 0.22, p= 0.03) (Figure 1, Panel C) and trunk fat (R2= 0.30, p < 0.006) (Figure 1, Panel D), with lower mean levels in the women with anorexia nervosa and those with obesity compared with the normal-weight group (obese: 164 ± 16 ng/ml, normal-weight: 222 ± 25 ng/ml, anorexia nervosa: 147 ± 11 ng/ml, p= 0.016). Mean GH levels were positively associated with mean IGF-1 levels in the group as a whole (R=0.42, p=0.02).
Figure 1.
Panel A: A linear inverse relationship was observed between GH and BMI (R= -0.74, p< 0.0001). Panel B: A linear inverse relationship was observed between GH and trunk fat (R= -0.70, p< 0.0001). Panel C: A quadratic relationship was observed between IGF-1 levels and BMI (R2= 0.22, p= 0.03). Panel D: A quadratic relationship was observed between IGF-1 levels and trunk fat (R2= 0.30, p< 0.006). Log-transformed data are shown.
Mean pooled free testosterone levels were higher in obese women and lower in women with anorexia nervosa than in normal-weight women (obese: 5.1 ± 1.1 pg/ml, normal-weight: 1.5 ± 0.3 pg/ml, anorexia nervosa: 0.8 ± 0.4 pg/ml, p < 0.0003). Mean pooled total testosterone levels were higher in the obese than normal-weight women or women with anorexia nervosa (obese: 26.3 ± 5.4 ng/dl, normal-weight: 9.3 ± 2.2 ng/dl, anorexia nervosa: 8.8 ± 3.2 ng/dl, p= 0.0034) (Table 1). Pooled estradiol levels were lower in the women with anorexia nervosa than in obese or normal-weight women (obese: 77 ± 16 pg/ml, normal-weight: 75 ± 14 pg/ml, anorexia nervosa 23 ± 4 pg/ml, p= 0.01). Mean pooled insulin levels were higher in the obese group of women than in normal-weight women or women with anorexia nervosa (obese: 11.6 ± 1.7 uIU/ml, normal-weight: 6.1 ± 0.7 uIU/ml, anorexia nervosa: 5.3 ± 0.8 uIU/ml, p= 0.001. There was no difference in mean pooled free fatty acid levels between the groups (obese: 0.43 ± 0.04 meq/l, normal-weight: 0.41 ± 0.03 meq/l, anorexia nervosa: 0.46 ± 0.05 meq/l, p= 0.64). All of the significant differences between groups delineated above remained significant after correcting for multiple comparisons.
Mean pooled GH levels were inversely associated with mean pooled insulin levels (R= -0.56, p= 0.001), and there was a trend toward an inverse association with free testosterone levels (R= -0.34, p=0.060). GH levels did not correlate significantly with estradiol, total testosterone or free fatty acid levels.
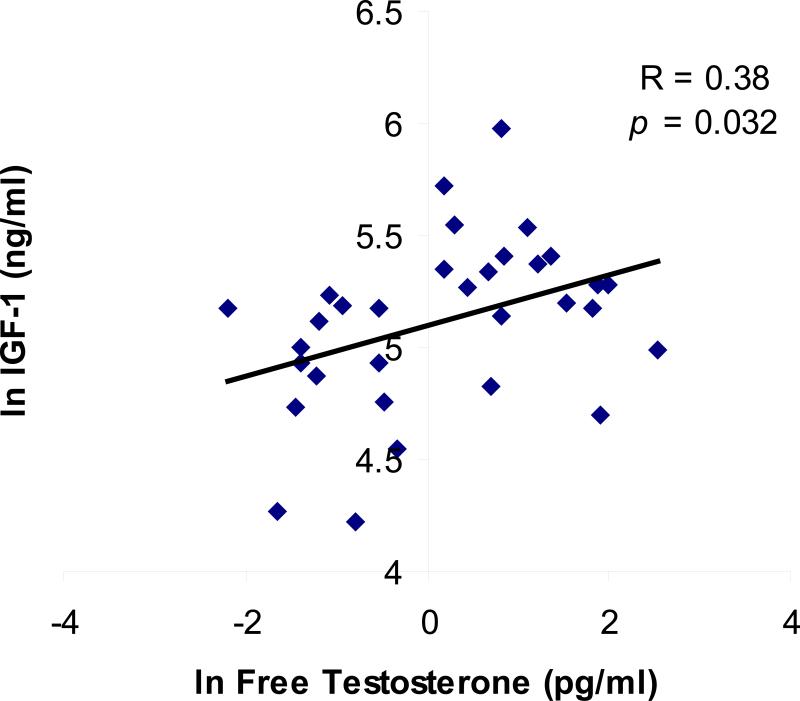
Mean pooled IGF-1 levels were positively associated with total testosterone (R= 0.35, p=0.048) and free testosterone levels (R= 0.38, p=0.032) (Figure 2), and there was a trend toward an association with mean pooled estradiol levels (R= 0.33, p=0.067). IGF-1 levels did not correlate with insulin or free fatty acid levels. Dummy variables for anorexia nervosa and obesity were then entered into the models. For the relationship between IGF-1 and total testosterone, both anorexia nervosa (p=0.015) and obesity (p=0.002) were significant predictors. For the association between IGF-1 and free testosterone, obesity (p=0.003), but not anorexia nervosa (p=0.18) were significant predictors in the model.
Figure 2.
Pooled overnight IGF-1 levels were associated with pooled free testosterone levels across the body composition spectrum (R= 0.38, p= 0.032) (log-transformed data shown).
Stepwise regression models were constructed to investigate the determinants of the variability of pooled GH and IGF-1 levels. In the group as a whole, trunk fat was a negative predictor and the strongest determinant of mean GH levels, accounting for 49% of the variability in levels (R2= 0.49, p<0.0001), with an additional 7% of the variability attributable to estradiol (cumulative R2= 0.56, p=0.042). There was a trend toward a negative effect of insulin p=0.084). When weight was entered into the model instead of trunk fat, weight was the strongest, and a negative, predictor of GH (R2= 0.44, p<0.0001), with an additional 9% of the variability attributable to free testosterone (cumulative R2= 0.53, p=0.031). In a stepwise regression model, free testosterone was a positive, and the only independent predictor of IGF-1 levels, accounting for 14% of the variability of IGF-1 levels (R2= 0.14, p=0.032); this was unchanged when weight was entered into the model instead of trunk fat.
When the obese study subjects were excluded, i.e. stepwise regression models were constructed for the group of women with anorexia nervosa and normal-weight controls only, the model identified no significant determinants of GH levels. Free testosterone was a positive and the only significant independent predictor of IGF-1 levels, accounting for 36% of the variability (R2=0.36, p=0.003). There was a trend toward a positive effect of estradiol. (p= 0.053).
DISCUSSION
In this study, we investigated determinants of IGF-1 and GH levels throughout the weight spectrum, from obesity to anorexia nervosa. We measured potential determinants using overnight sampling for integrated measurements of these factors. We demonstrate that androgen levels are significant determinants of IGF-1 levels throughout the weight spectrum, including at the low end of the weight range, in which relative hypoandrogenemia is present. We could not definitively establish that the relationships between IGF-1 and androgens were comparable within the anorexia nervosa and obese groups, and further studies would be necessary in a larger group of women to investigate this. Although the reduction in IGF-1 levels may be adaptive in chronic starvation by reducing anabolism, it may also have deleterious effects, contributing to bone and muscle loss in women with anorexia nervosa. In addition, although the most significant determinant of GH levels in obese women was truncal adipose tissue mass, this did not significantly contribute to mean overnight GH levels in women with anorexia nervosa. These data support the hypothesis that GH secretion is differentially regulated at the low and high end of the weight spectrum in women.
Testosterone is an important factor in the regulation of GH release and has been shown in men to stimulate IGF-1 levels independently of GH. Some, but not all studies have demonstrated that testosterone stimulates or positively predicts IGF-1 levels in men. Gibney et al. administered GH with and without testosterone to men with hypopituitarism and demonstrated a greater increase in IGF-1 levels in those men administered the combination than those administered GH alone, suggesting that testosterone augments the effects of GH on hepatic IGF-1 production17. In contrast, in a similar study, Fisker et al. did not detect any change in total or free IGF-1 levels when testosterone administration was added to GH replacement in hypopituitary men25. Published data in women demonstrate that women with polycystic ovary syndrome have higher IGF-1 levels than women of comparable weights but without hyperandrogemia18. Moreover, women receiving progestins with higher androgenecity19 have higher IGF-1 levels than those receiving progestins with lower androgenecity. In addition, large cross-sectional studies demonstrate an association between IGF-1 levels and androgens in women26. In normal-weight eumenorrheic young women, both GH secretion and serum IGF-I have been shown to increase in the periovulatory period in concert with increases in estradiol levels27. Although this may reflect a causative relationship between estradiol and IGF-1, androgens increase at midcycle as well23, and it is not clear to which gonadal steroids to attribute this relationship. We have recently shown that free testosterone levels are an independent predictor of IGF-1 levels in obese eumenorrheic women4. However, the current paper is the first to examine whether androgen levels are associated with IGF-1 concentrations at the low end of the female weight spectrum. Because the relationship between IGF-1 and most other known factors that affect the regulation of the hypothalamic-pituitary-IGF-1 axis, including GH, BMI and adiposity, are altered in a state of chronic starvation, it was not clear a priori whether the relationship observed in obese and normal-weight women of reproductive age would hold for women with anorexia nervosa, i.e. that low androgen levels are a factor contributing to low IGF-1 levels in this population. Our data suggest that the relationship does hold. However, it should be noted that as causality cannot be established with a cross-sectional study, it is possible that IGF-1 is stimulating androgen production or that a third factor, associated with both IGF-1 and testosterone levels, is the cause of the association observed. Further studies are needed to clarify this point.
Nearly 50% of the variability of mean overnight GH levels was explained by truncal adipose tissue mass, as has been previously described by our group and others in obese women3, 4. Of note, this relationship was driven by the obese group of women, as the multivariate regression model did not identify truncal fat mass as a determinant of GH levels when obese women were excluded from the analysis. This is consistent with the hypothesis that GH secretion in states of undernutrition is driven by decreased circulating IGF-1, through a feedback mechanism, rather than body composition parameters or other circulating factors, e.g. free fatty acid or insulin levels. The lack of a statistically significant relationship in our study may also reflect the much smaller range in truncal adiposity among undernourished and normal-weight subjects when compared with obese women. However, it should be noted that our BMI range at the lower end was extremely low (13.2 kg/m2). We therefore should have been able to detect such an association if it were present. As trunk and abdominal fat have been shown to be important determinants of GH secretion in non-obese study subjects28, 29, it is also possible that we were unable to detect an existing association between truncal fat and GH due to our sample size. It should also be noted that trunk fat as determined by DXA measures subcutaneous and visceral adipose tissue; in addition, when we entered weight into the multivariate models instead of truncal fat, we obtained similar results for weight as we had for truncal fat, suggesting that our study was not able to definitively identify the specific element of body composition responsible for effects on GH secretion. Another important point is that GH was inversely associated with nocturnal insulin levels. Although it is certainly possible that insulin is merely a surrogate for obesity, it has also been shown in a mouse pituitary cell culture experiment5 that insulin downregulates pituitary growth hormone, GHRH-receptor and ghrelin receptor mRNA, suggesting a potential direct role of insulin in regulating GH.
Limitations of this study include its cross-sectional design and small size. In addition, we were able to sample peripheral blood only, and portal circulation of insulin and other hormones may differ from peripheral concentrations. Another limitation is that we pooled hourly serum samples for mean overnight GH levels. As the pulsatility of GH secretion in itself may be an important determinant of IGF-I9, 30, 31, we might have been able to learn more about the regulation of the GH-IGF-1 axis had we had been able to characterize GH pulsatility over a 24-hour period. Finally, although IGF-1 is thought to be secreted into the circulation predominantly by hepatocytes, IGF-1 derived from other sources, for example adipocytes, has not been ruled out as a possible source of circulating IGF-1 in obese women and men.
In summary, we demonstrate that free testosterone is a significant determinant of IGF-1 levels in women of reproductive age over a wide weight range. Further studies are warranted to determine whether the relationship between IGF-1 and androgens is comparable within the anorexia nervosa to that within women of normal weight and with obesity. It would also be important to determine whether androgen deficiency contributes to bone and skeletal muscle loss indirectly through its effect on IGF-1 levels in states of chronic undernutrition.
Acknowledgements
We thank the nurses and bionutritionists of the Massachusetts General Hospital Clinical Research Center and the subjects who patients who participated in the study.
Funding: This work was supported in part by the following NIH grants: R01 HL077674, R01 DK52525, MO1 RR01066 and UL1 RR025758
Footnotes
Declaration of interest: The authors have no conflicts of interest to report.
References
- 1.Roth J, Glick SM, Yalow RS, Bersonsa Hypoglycemia: a potent stimulus to secretion of growth hormone. Science. 1963;140:987–988. doi: 10.1126/science.140.3570.987. [DOI] [PubMed] [Google Scholar]
- 2.Beck P, Koumans JH, Winterling CA, Stein MF, Daughaday WH, Kipnis DM. Studies of Insulin and Growth Hormone Secretion in Human Obesity. J Lab Clin Med. 1964;64:654–667. [PubMed] [Google Scholar]
- 3.Pijl H, Langendonk JG, Burggraaf J, Frolich M, Cohen AF, Veldhuis JD, Meinders AE. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86:5509–5515. doi: 10.1210/jcem.86.11.8061. [DOI] [PubMed] [Google Scholar]
- 4.Utz AL, Yamamoto A, Sluss P, Breu J, Miller KK. Androgens may mediate a relative preservation of IGF-I levels in overweight and obese women despite reduced growth hormone secretion. J Clin Endocrinol Metab. 2008;93:4033–4040. doi: 10.1210/jc.2008-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147:2754–2763. doi: 10.1210/en.2005-1549. [DOI] [PubMed] [Google Scholar]
- 6.Cordido F, Peino R, Penalva A, Alvarez CV, Casanueva FF, Dieguez C. Impaired growth hormone secretion in obese subjects is partially reversed by acipimox-mediated plasma free fatty acid depression. J Clin Endocrinol Metab. 1996;81:914–918. doi: 10.1210/jcem.81.3.8772550. [DOI] [PubMed] [Google Scholar]
- 7.Kok P, Buijs MM, Kok SW, Van Ierssel IH, Frolich M, Roelfsema F, Voshol PJ, Meinders AE, Pijl H. Acipimox enhances spontaneous growth hormone secretion in obese women. Am J Physiol Regul Integr Comp Physiol. 2004;286:R693–698. doi: 10.1152/ajpregu.00595.2003. [DOI] [PubMed] [Google Scholar]
- 8.Moller N, Gormsen LC, Schmitz O, Lund S, Jorgensen JO, Jessen N. Free fatty acids inhibit growth hormone/signal transducer and activator of transcription-5 signaling in human muscle: a potential feedback mechanism. J Clin Endocrinol Metab. 2009;94:2204–2207. doi: 10.1210/jc.2008-2624. [DOI] [PubMed] [Google Scholar]
- 9.Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab. 1999;84:2056–2063. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- 10.Moller L, Dalman L, Norrelund H, Billestrup N, Frystyk J, Moller N, Jorgensen JO. Impact of fasting on growth hormone signaling and action in muscle and fat. J Clin Endocrinol Metab. 2009;94:965–972. doi: 10.1210/jc.2008-1385. [DOI] [PubMed] [Google Scholar]
- 11.Stoving RK, Chen JW, Glintborg D, Brixen K, Flyvbjerg A, Horder K, Frystyk J. Bioactive insulin-like growth factor (IGF) I and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2323–2329. doi: 10.1210/jc.2006-1926. [DOI] [PubMed] [Google Scholar]
- 12.Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK. Bioactive insulin-like growth factor-I in obesity. J Clin Endocrinol Metab. 2009;94:3093–3097. doi: 10.1210/jc.2009-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85:4712–4720. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 14.Meinhardt UJ, Ho KK. Regulation of growth hormone action by gonadal steroids. Endocrinol Metab Clin North Am. 2007;36:57–73. doi: 10.1016/j.ecl.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72:374–381. doi: 10.1210/jcem-72-2-374. [DOI] [PubMed] [Google Scholar]
- 16.Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CK, Low TH, Leong GM, Ross RJ, Ho KK. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci U S A. 2003;100:1016–1021. doi: 10.1073/pnas.0337600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibney J, Wolthers T, Johannsson G, Umpleby AM, Ho KK. Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am J Physiol Endocrinol Metab. 2005;289:E266–271. doi: 10.1152/ajpendo.00483.2004. [DOI] [PubMed] [Google Scholar]
- 18.Van Dam EW, Roelfsema F, Helmerhorst FH, Frolich M, Meinders AE, Veldhuis JD, Pijl H. Low amplitude and disorderly spontaneous growth hormone release in obese women with or without polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:4225–4230. doi: 10.1210/jc.2002-012006. [DOI] [PubMed] [Google Scholar]
- 19.Campagnoli C, Abba C, Ambroggio S, Peris C. Differential effects of progestins on the circulating IGF-I system. Maturitas. 2003;46(Suppl 1):S39–44. doi: 10.1016/j.maturitas.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, Herzog DB, Klibanski A. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92:1334–1339. doi: 10.1210/jc.2006-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, Klibanski A, Miller KK. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43:135–139. doi: 10.1016/j.bone.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab. 2008;93:2507–2514. doi: 10.1210/jc.2008-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- 24.Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S. Growth Hormone-Releasing Hormone in HIV-Infected Men With Lipodystrophy. JAMA. 2004;292:210–218. doi: 10.1001/jama.292.2.210. [DOI] [PubMed] [Google Scholar]
- 25.Fisker S, Norrelund H, Juul A, Skakkebaek NE, Christiansen JS, Jorgensen JO. The growth hormone (GH)-insulin-like growth factor axis during testosterone replacement therapy in GH-treated hypopituitary males. Growth Horm IGF Res. 2001;11:104–109. doi: 10.1054/ghir.2001.0195. [DOI] [PubMed] [Google Scholar]
- 26.Maccario M, Ramunni J, Oleandri SE, Procopio M, Grottoli S, Rossetto R, Savio P, Aimaretti G, Camanni F, Ghigo E. Relationships between IGF-I and age, gender, body mass, fat distribution, metabolic and hormonal variables in obese patients. Int J Obes Relat Metab Disord. 1999;23:612–618. doi: 10.1038/sj.ijo.0800889. [DOI] [PubMed] [Google Scholar]
- 27.Ovesen P, Vahl N, Fisker S, Veldhuis JD, Christiansen JS, Jorgensen JO. Increased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the periovulatory interval in normal women. J Clin Endocrinol Metab. 1998;83:1662–1667. doi: 10.1210/jcem.83.5.4761. [DOI] [PubMed] [Google Scholar]
- 28.Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90:768–774. doi: 10.1210/jc.2004-0894. [DOI] [PubMed] [Google Scholar]
- 29.Vahl N, Jorgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol. 1997;272:E1108–1116. doi: 10.1152/ajpendo.1997.272.6.E1108. [DOI] [PubMed] [Google Scholar]
- 30.Chowen JA, Frago LM, Argente J. The regulation of GH secretion by sex steroids. Eur J Endocrinol. 2004;151(Suppl 3):U95–100. doi: 10.1530/eje.0.151u095. [DOI] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Bowers CY. Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest. 2003;26:799–813. doi: 10.1007/BF03345229. [DOI] [PubMed] [Google Scholar]