Abstract
Purpose
The aim of this study was to investigate the impact of a distal subtotal gastrectomy on the quality of life (QoL).
Materials and Methods
The QoL data of 126 patients were obtained on their 5th annual follow-up visit after a curative distal subtotal gastrectomy for gastric cancer (Group A). The QoL data of 130 age- and gender-adjusted healthy population were obtained from the individuals who visited the health screening center for a medical check-up (Group B). There were 42 women and 84 men in the study group and their mean age was 56.0±11.1 years. QoL was assessed using the Korean versions of the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire Core 30 (QLQ-C30) and QLQ-STO22.
Results
The EORTC QLQ-C30 global health status and QoL scores of Group A and Group B were 63.9±22.7 and 61.3±22.1, respectively (p=0.361). Group A revealed a better score for emotional functioning (84.1±16.1 and 75.2±21.4, respectively; p<0.001), cognitive functioning (82.0±16.4 and 75.0±21.4, respectively; p=0.004) and fatigue (27.7±20.8 and 33.8±23.2, respectively; p=0.028). However, Group A revealed a worse score for nausea and vomiting (14.8±20.0 and 10.2±16.0, respectively; p=0.042), financial difficulties (14.8±22.9 and 7.1±16.1, respectively; p=0.002), reflux (16.7±17.7 and 10.1±17.0, respectively; p=0.003), eating restrictions (13.6±15.2 and 6.6±10.2, respectively; p<0.001) and body image (23.3±25.4 and 16.2±24.6, respectively; p=0.023).
Conclusion
The QoL of long-term survivors after a distal subtotal gastrectomy is still influenced by the surgery itself even though they are considered to be free of disease.
Keywords: Stomach neoplasms, Gastrectomy, Quality of life
Introduction
The number of long-term survivors after surgical resection for gastric cancer has been increasing as a result of early detection and the improved surgical techniques. Although survivors may be rendered free of disease by surgery, they may suffer from post-operative symptoms and functional losses. Thus, it is imperative to give more attention to the post-surgical quality of life (QoL) of the patients with gastric cancer (1-3).
There have been many attempts to evaluate the QoL of patients with breast cancer (4), lung cancer (5) or rectal cancer (6). The instrument developed by Spitzer et al. (7), which measures the QoL of cancer patients in general, and the instrument developed by Troidl et al. (8), which measures the QoL of patients following gastric resection, have often been used to evaluate the QoL of patients with gastric cancer. Korenaga et al. (9) have developed their own sets of questions to evaluate the QoL of post-gastrectomy patients. Generic QoL instruments designed in the form of questionnaires have recently been developed, and these include the Functional Assessment of Cancer Therapy-General (FACT-G) by the Functional Assessment of Chronic Illness Therapy QoL group and the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) by the EORTC QoL group (10,11). The instruments from these groups are significant because they have additional site-specific modules in addition to the core questionnaires. For example, the EORTC QoL group has developed a gastric cancer specific module, the EORTC QLQ-STO22 (12,13). The EORTC QLQ-C30 has been translated into Korean and its validity has been demonstrated (14). In addition, the EORTC QLQ-STO22 has also been translated into Korean and some attempts have been made to assess the QoL of gastric cancer patients in Korea (15,16).
There have been attempts to evaluate the post-surgical QoL of long-term survivors from gastric cancer. However, most of them compared the QoL of gastric cancer patients by the surgical approach only. For example, they compared QoL by different levels of lymph node dissections or by different techniques (17-19). Few studies have examined the QoL of long-term survivors from gastric cancer using the EORTC QLQ-C30 and QLQ-STO22.
The aim of this study was to investigate the impact of a distal subtotal gastrectomy on the QoL of patients 5 years after surgery when they were generally considered to be free of disease, and to compare the outcomes with that of an age- and gender-adjusted healthy population using the Korean versions of the EORTC QLQ-C30 and QLQ-STO22.
Materials and Methods
The QoL data were obtained from patients, who agreed to participate in the study, on their 5th annual follow-up visit between January 2008 and March 2009 after they had previously undergone a curative distal subtotal gastrectomy for gastric adenocarcinoma at Kyungpook National University Hospital. Patients were excluded if they had any significant past medical histories or any signs of recurrence, and a patient with renal cell carcinoma who underwent a right nephrectomy, and a patient with ovarian cancer who underwent a total hysterectomy and bilateral salpingo-oophorectomy were excluded. A total of 126 patients were eligible for inclusion in the study group. The QoL data of 130 healthy population were obtained from individuals who visited our health screening center for an annual medical check-up. Those individuals with significant past medical histories or abnormal test results which required further medical treatment were excluded.
There were 42 women and 84 men in the long-term survivor group and their mean age was 56.0±11.1 years. The staging definition was in accordance with the sixth edition of the International Union Against Cancer (UICC) classification. One hundred eleven patients (88.1%) had stage I disease, 10 patients (7.9%) had stage II disease and 5 patients (4.0%) had stage III disease. Continuity of the digestive tract was reconstructed by performing stapled Billroth I gastroduodenostomy. D2 or more extended lymphadenectomy was performed. There were 44 women and 86 men in the healthy population with a mean age of 55.9±10.6 years. There was no significant statistical difference between the long-term survivors and the healthy population for gender (p=0.931; chi-square test) and age (p=0.950; Student's t-test).
The QoL was assessed using the Korean versions of the EORTC QLQ-C30 and QLQ-STO22 provided by the EORTC QoL group. The EORTC QLQ-C30 consists of 30 items and the EORTC QLQ-STO22 consists of 22 items. The QoL assessment was based on the answers to all 52 items, as completed by the responders themselves.
The EORTC QLQ-C30 is composed of 15 scales, including the global health status and QoL, five functional scales (physical, role, cognitive, emotional and social) and nine symptom scales and single items (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties). The EORTC QLQ-STO22 is composed of nine scales, including dysphagia, pain, reflux, eating restrictions, anxiety, dry mouth, taste, body image and hair loss. The raw scores of each scale were linearly transformed into scores from 0 to 100 in accordance with the EORTC QLQ-C30 Scoring Manual and the Scoring procedure for the EORTC-STO22. From the EORTC QLQ-C30, a high score for the global health status and QoL represents high QoL, and a high score for a functional scale represents high QoL. A high score for a symptom scale or a single item represents poor QoL. A high score for a scale from the EORTC QLQ-STO22 represents poor QoL.
The QoL of the long-term survivors after a distal subtotal gastrectomy for gastric cancer was compared to that of the age- and gender-adjusted healthy population. Student's t-test was used to compare between the groups. A p-value of less than 0.05 was considered statistically significant.
Results
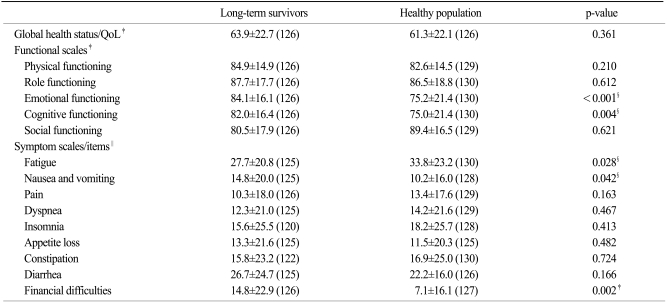
For the EORTC QLQ-C30, the global health status and QoL scores of the long-term survivors and healthy population were 63.9±22.7 and 61.3±22.1, respectively, but the difference did not reach the statistical significance (p=0.361). Among the functional scales, the long-term survivors had better scores for emotional (p<0.001) and cognitive functioning (p=0.004). Among the symptom scales and single items, the long-term survivors had a better score for fatigue (p=0.028) and worse scores for nausea and vomiting (p=0.042) and financial difficulties (p=0.002) than did the healthy population (Table 1).
Table 1.
Comparison of the QoL* between the long-term survivors and the healthy population, as measured by the Korean version of the EORTC QLQ-C30†
Values in parentheses are numbers of responders. *quality of life, †European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, ‡a higher score represents a better outcome, §statistically significant, ∥a higher score represents a worse outcome.
For the EORTC QLQ-STO22, the long-term survivors had worse scores for reflux (p=0.003), eating restrictions (p<0.001) and body image (p=0.023) than the healthy population. The long-term survivors showed a trend to have a worse score for dysphagia, but the difference did not reach statistical significance (p=0.098). The long-term survivors had a better score than the healthy population for hair loss (p=0.001) (Table 2).
Table 2.
Comparison of the QoL* between the long-term survivors and the healthy population, as measured by the Korean version of the EORTC QLQ-STO22†, ‡
Values in parentheses are the numbers of responders. *quality of life, †European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, ‡a higher score represents a worse outcome, §statistically significant.
Discussion
Cancer patients may have poor QoL due to the disease itself before surgery. After surgery, they may still suffer from poor QoL due to oncologic problems and the consequences of surgery. Our main concern was whether these patients would have poor QoL after their oncological problems were solved.
There have been several studies that have investigated the post-surgical QoL of patients with gastric cancer. However, many of these studies investigated the QoL of patients with unresolved oncological problems (20-22). The QoL of long-term survivors has also been investigated, but most of these studies examined the impact of procedural differences on the QoL of patients who underwent surgery for gastric cancer (18-20).
Korenaga et al. (9) assessed the QoL of long-term survivors after surgery for gastric cancer. Their instrument examined such parameters as the consistency of food, volume of food, appetite and the changes in the body weight and performance status of patients 1 to 3 years, 3 to 6 years, 6 to 10 years and more than 10 years after surgery. The majority of patients had a diet of normal consistency, but the study revealed a decreased food volume intake and appetite loss in more than half the patients. In addition, the majority of patients experienced weight loss, but their performance status showed gradual improvement.
Tyrväinen et al. (23) studied the QoL of long-term survivors of gastric cancer between 6 and 19 years (median: 9 years) after a total gastrectomy, and they compared their QoL with that of a healthy population. The short form (SF) 36 and the 15 dimensional (D) health surveys were used to assess QoL. The SF36 did not show QoL differences between the two groups. While the 15D did not show QoL differences between the two groups in general, the post-gastrectomy patients had poorer QoL for sleeping, distress and bladder and bowel function. It was concluded that patients following a total gastrectomy did just as well as the healthy population, except in the above-mentioned sub-dimensions.
We utilized the EORTC QLQ-C30 and QLQ-STO22 to measure the QoL of long-term survivors of gastric cancer 5 years after a distal subtotal gastrectomy, and we compared their QoL with that of a healthy population. Even though there was no statistically significant difference in the global health status and QoL between the two groups, the study did indicate QoL deterioration as related to upper gastrointestinal symptoms such as nausea and vomiting, reflux and eating restrictions in the long-term survivors. Other than the upper gastrointestinal symptoms, financial difficulties and a poor body image had an impact on the QoL of the long-term survivors, although they were considered to be free of disease at the time of follow-up.
Other than the extent of resection, this study differs from the study of Tyrväinen et al. for the length of the post-surgical period. The time that elapsed after surgery was much shorter in our study.
Another difference is the instruments used to assess QoL. The SF 36 consists of 8 dimensions: physical functioning, role limitation due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The 15D consists of 16 categories, 15 dimensions (mobility, vision, hearing, breathing, sleeping, eating, speech, eliminating, usual activities, mental function, discomfort and symptoms, depression, distress, vitality and sexual activity) and a total score. The SF 36 and the 15D are similar to the generic QoL instrument, the EORTC QLQ-C30. When QoL is assessed with specifically using the site-specific module, that is, the EORTC QLQ-STO22, it revealed deterioration of the QoL in certain sub-dimensions that remained undetected by the generic QoL instruments.
The QoL of the healthy population was worse than that of the long-term survivors for emotional functioning, cognitive functioning and fatigue. We hypothesized that these outcomes had been confounded by the anxieties experienced by the healthy population as they awaited the outcomes of their annual medical check-up.
The healthy population had better QoL for financial difficulties. This could be related to the fact that the QoL data of the healthy population were assessed from people who underwent health screening on their own expense. It is unlikely that people with financial difficulties would visit the health screening center.
The scale for hair loss is often used to assess the QoL as related to chemotherapy or radiotherapy for various types of cancer (24,25). The scale for hair loss from the EORTC QLQ-STO22 sheds light on how a history of gastric cancer affects the survivors long after surgery although they were considered to be free of disease. We compared the scale for hair loss between the long-term survivors and the healthy population, and this sub-dimension revealed poorer QoL in the healthy population than in the cancer survivors. The numbers of responders to this item from the long-term survivors and the healthy population were relatively small at 50 and 56, respectively, because the number of eligible participants who were experiencing hair loss was small. However, the number was large enough to allow statistical analysis, and it appears that hair loss did not have as much of an impact on the QoL of long-term survivors as it did on the healthy population. The former cancer patients appeared to pay less attention to their hair as long as they remain free of disease following cancer surgery. It seems that the QoL of long-term survivors is less influenced by personal matters compared with that of the healthy population.
Conclusion
Disparity in the QoL between long-term survivors after curative surgery for gastric cancer and healthy population does exist 5 years after surgery, especially in relation to upper gastrointestinal symptoms such as nausea and vomiting, reflux, and eating restrictions.
The QoL of long-term survivors of gastric cancer after a distal subtotal gastrectomy is still influenced by the surgery itself even though they are considered to be free of disease.
References
- 1.Calman KC. Quality of life in cancer patients: an hypothesis. J Med Ethics. 1984;10:124–127. doi: 10.1136/jme.10.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blazeby JM. The role of quality of life assessment in gastric cancer. Jpn J Clin Oncol. 2000;30:246. doi: 10.1093/jjco/30.5.246. [DOI] [PubMed] [Google Scholar]
- 3.Kaptein AA, Morita S, Sakamoto J. Quality of life in gastric cancer. World J Gastroenterol. 2005;11:3189–3196. doi: 10.3748/wjg.v11.i21.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiebert GM, de Haes JC, van de Velde CJ. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients: a review. J Clin Oncol. 1991;9:1059–1070. doi: 10.1200/JCO.1991.9.6.1059. [DOI] [PubMed] [Google Scholar]
- 5.Erridge SC, Gaze MN, Price A, Kelly CG, Kerr GR, Cull A, et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005;17:61–67. doi: 10.1016/j.clon.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Hölzel D. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg. 2003;238:203–213. doi: 10.1097/01.sla.0000080823.38569.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34:585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 8.Troidl H, Kusche J, Vestweber KH, Eypasch E, Maul U. Pouch versus esophagojejunostomy after total gastrectomy: a randomized clinical trial. World J Surg. 1987;11:699–712. doi: 10.1007/BF01656592. [DOI] [PubMed] [Google Scholar]
- 9.Korenaga D, Orita H, Okuyama T, Moriguchi S, Maehara Y, Sugimachi K. Quality of life after gastrectomy in patients with carcinoma of the stomach. Br J Surg. 1992;79:248–250. doi: 10.1002/bjs.1800790321. [DOI] [PubMed] [Google Scholar]
- 10.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 12.Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 13.Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–2268. doi: 10.1016/j.ejca.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 15.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 16.Bae JM, Kim S, Kim YW, Ryu KW, Lee JH, Noh JH, et al. Health-related quality of life among disease-free stomach cancer survivors in Korea. Qual Life Res. 2006;15:1587–1596. doi: 10.1007/s11136-006-9000-8. [DOI] [PubMed] [Google Scholar]
- 17.Wu CW, Chiou JM, Ko FS, Lo SS, Chen JH, Lui WY, et al. Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. Br J Cancer. 2008;98:54–59. doi: 10.1038/sj.bjc.6604097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda K, Shiraishi N, Etoh T, Shiromizu A, Inomata M, Kitano S. Long-term quality of life after laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2007;21:2150–2153. doi: 10.1007/s00464-007-9322-9. [DOI] [PubMed] [Google Scholar]
- 19.Tomita R, Tanjoh K, Fujisaki S. Novel operative technique for vagal nerve- and pyloric sphincter-preserving distal gastrectomy reconstructed by interposition of a 5 cm jejunal J pouch with a 3 cm jejunal conduit for early gastric cancer and postoperative quality of life 5 years after operation. World J Surg. 2004;28:766–774. doi: 10.1007/s00268-004-6987-2. [DOI] [PubMed] [Google Scholar]
- 20.Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, et al. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048–1055. doi: 10.1007/s002689900515. [DOI] [PubMed] [Google Scholar]
- 21.Svedlund J, Sullivan M, Liedman B, Lundell L, Sjödin I. Quality of life after gastrectomy for gastric carcinoma: controlled study of reconstructive procedures. World J Surg. 1997;21:422–433. doi: 10.1007/pl00012265. [DOI] [PubMed] [Google Scholar]
- 22.Huang CC, Lien HH, Wang PC, Yang JC, Cheng CY, Huang CS. Quality of life in disease-free gastric adenocarcinoma survivors: impacts of clinical stages and reconstructive surgical procedures. Dig Surg. 2007;24:59–65. doi: 10.1159/000100920. [DOI] [PubMed] [Google Scholar]
- 23.Tyrväinen T, Sand J, Sintonen H, Nordback I. Quality of life in the long-term survivors after total gastrectomy for gastric carcinoma. J Surg Oncol. 2008;97:121–124. doi: 10.1002/jso.20925. [DOI] [PubMed] [Google Scholar]
- 24.Paccagnella A, Favaretto A, Oniga F, Barbieri F, Ceresoli G, Torri W, et al. Cisplatin vs. carboplatin in combination with mitomycin and vinblastine in advanced non small cell lung cancer. A multicenter, randomized phase III trial. Lung Cancer. 2004;43:83–91. doi: 10.1016/s0169-5002(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 25.Slotman BJ, Mauer ME, Bottomley A, Faivre-Finn C, Kramer GW, Rankin EM, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol. 2009;27:78–84. doi: 10.1200/JCO.2008.17.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]