Abstract
Purpose
The purpose of this study was to evaluate the clinicopathological features and immunohistochemical features of gastrointestinal stromal tumor (GIST), and specifically the expressions of platelet derived growth factor receptor A (PDGFRA), protein kinase C theta (PKC theta), discovered on GIST-1 (DOG-1), p16 and p27.
Materials and Methods
Total 118 patients who underwent surgical resection for GIST at our institution between Jan 1997 and Dec 2007 were retrospectively studied. Immunohistochemical staining for c-kit, PDGFRA, PKC-theta, DOG-1, p16 and p27 was performed on a tissue microarray of the 118 GIST. The clinicopathologic parameters, the disease-free survival (DFS) and the overall survival rate were analyzed along with immunohistochemistry.
Results
The immunohistochemical stains for c-kit, CD34, PKC-theta, PDGFRA, DOG-1, p16 and p27 were positive in 89.8%, 72.0%, 56.8%, 94.9%, 90.7%, 69.5% and 44.1% of the tumor samples, respectively. The immunohistochemical expression of c-kit was strongly correlated with PKC-theta (p=0.000), DOG-1 (p=0.000) and CD34 (p=0.002). The DFS rate was significantly decreased for the patients with peritoneal GIST, high risk GIST, ≥10 cm-sized GIST, ≥10 mitoses/50 high power fields (HPFs) and p16 positivity (p=0.001, p=0.004, p=0.001, p=0.003 and p=0.028). GISTs ≥10 cm, epithelioid tumor cell type, and c-kit, and DOG-1 negativity were significantly associated with shorter period of overall survival (p=0.048, p=0.006, p=0.000 and p=0.000).
Conclusion
The expression of p16 and no expression of c-kit and DOG-1 in GISTs, as well as peritoneal tumor site, high risk group, large tumor size, epithelioid tumor cell type and numerous mitoses, may be potentially prognostic factors for predicting worse outcome for patients who suffer from GIST.
Keywords: Gastrointestinal stromal tumors, Immunohistochemistry, Prognosis
Introduction
Gastrointestinal stromal tumor (GIST) is one of the mesenchymal tumors arising from the gastrointestinal (GI) tract, and they are mostly diagnosed in the stomach, small intestine and colorectum (1,2). GIST is histologically defined as KIT (CD117, stem cell factor receptor)-positive mesenchymal spindle or epithelioid cell tumors from the GI tract (3). The relationship between GIST and the activating mutations in KIT was first reported by Hirota et al. (4) These KIT mutations contribute to the development of GIST, and the origin of GIST may be the interstitial cell of Cajal (ICC) (4).
A few new immunohistochemical (IHC) markers are also useful for identifying KIT negative GIST. Platelet derived growth factor receptor A (PDGFRA) and protein kinase C theta (PKC theta) are the examples of these IHC markers (5,6). PDGFRA germ line mutations are also associated with c-kit negative GISTs (7). These have been recently discovered on GIST-1 (DOG-1) and this may help make the diagnosis of GISTs, including the PDGFRA mutants that fail to express KIT antigen (8).
The aim of this study is to clarify the difference between the immunohistochemical markers for GISTs and to assess the clinicopathological and immunohistochemical characteristics of GISTs.
Materials and Methods
1. The patients and tumor samples
One hundred eighteen cases of GIST were selected from among all the GIST cases that underwent resection at Dongsan Medical Center from 1997 to 2007. The hematoxylin and eosin stained tumor slides were reviewed and classified by the National Institutes of Health (NIH) criteria (2). The clinical and follow-up data (gender, age, medical history, recurrence and survival) were obtained from the medical records.
2. Tissue microarray
The 118 patients were chosen for the analysis based on the availability of tumor samples to construct tissue microarray block. Three 5 mm diameter-cores of the representative areas were selected from each tumor sample, and then these were manually embedded in new paraffin blocks using skin biopsy needle.
3. IHC staining
The IHC staining was performed on the 5 µm thick sections that were cut from the tissue microarray blocks, which were composed of formalin-fixed, paraffin-embedded surgical specimens from all 118 patients. These sections were placed on slides and the sections on the slides were dewaxed and rehydrated in the graded series of alcohol solutions. IHC staining was performed using an automated system (Autostainer 360, Lab Vision, Fremont, CA). The primary antibodies used in this investigation were KIT (CD117) (1 : 400, Dako, Denmark), CD34 (1 : 400, Neomarkers, Fremount, CA), PDGFRA (1 : 300, Santa Cruz Biotechnology, Santa Cruz, CA), PKC theta (1 : 50, Biosciences, Franklin Lakes, NJ), DOG-1 (1 : 300, Novocastra, UK), p16 (1 : 100, Santa Cruz Biotechnology) and p27 (1 : 300, Santa Cruz Biotechnology). The expression was scored as positive if >5% of the tumor cells were reactive with any intensity.
4. Statistical analysis
Statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL). The correlation analysis of the expression of c-kit and the other antibodies with the clinicopathologic variables were done using chi-squared tests, and the disease-free survival (DFS) rate and overall survival (OS) rate were calculated and compared using the Kaplan-Meier method and log-rank tests. A multivariate analysis was done by Cox's regression analysis to identify the prognostic factors for DFS and OS. p-value less than 0.05 were considered statistically significant.
Results
1. Patient and tumor characteristics
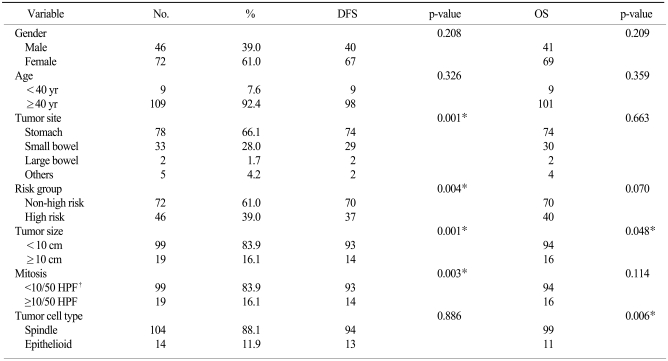
Of the 118 patients, 46 (39.0%) were male and 72 (61.0%) were female, and their ages ranged from 32 to 83 (mean age, 57.7) years. The origin of the GISTs was stomach in 78 patients (66.1%), small intestine for 33 (28.0%), peritoneum for 5 (4.2%) and large intestine for 2 (1.7%). The tumor sizes were <2 cm in diameter in 13 cases (11.0%), ≥2 cm and <5 cm in 52 (44.1%) and ≥5 cm in 53 (44.9%).
Microscopically, the spindle cell type was the most common and this was found in 104 cases (88.2%), followed by epithelioid cell type in 11 (9.3%) and mixed type in 3 (2.5%). Mitoses were observed in <5 among 50 high power fields (HPFs) in 61 cases (51.7%), in ≥5 and <10 HPFs in 38 cases (32.2%) and in ≥10 HPFs in 19 cases (16.1%). According to the NIH criteria (2), 11 cases (9.3%) were graded as very low risk, 39 (33.1%) were graded as low risk, 22 (18.6%) were graded as intermediate risk and 46 (39.0%) were graded as high risk.
For statistical analysis, the patients' ages were divided as <40 years (9 cases, 7.6%) and ≥40 years (109 cases, 92.4%). The sizes of the tumors were divided as <10 cm (98 cases, 83.1%) and ≥10 cm (20 cases, 16.9%). Mitoses was also divided as <10/50 HPFs (99 cases, 83.9%) and ≥10/50 HPFs (19 cases, 16.1%). The very low risk, low risk and intermediate risk groups were reclassified as non-high risk group (72 cases, 61.0%), as compared to high risk group (46 cases, 39.0%). Three cases of mixed cell type GIST were added to epithelioid type due to the presence of epithelioid cells. The patient and tumor characteristics are shown in Table 1.
Table 1.
Disease free survival (DFS) and overall survival (OS) according to the clinical characteristics
*statistical significant, †high power fields.
2. Immunohistochemistry (IHC)
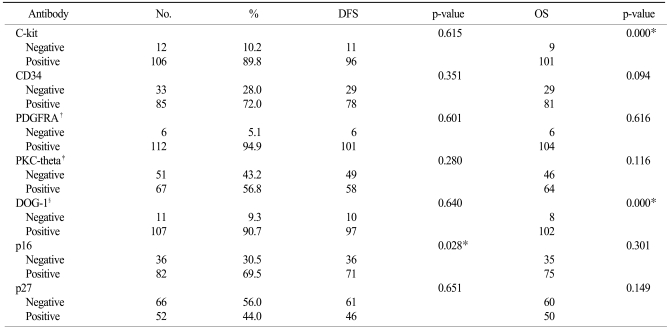
Of the 118 GISTs, c-kit was positive in 106 cases (89.8%), CD34 was positive in 85 (72.0%), PKC-theta was positive in 67 (56.8%), PDGFRA was positive in 112 (94.9%), DOG-1 was positive in 107 cases (90.7%), p16 was positive in 82 (69.5%) and p27 was positive in 52 (44.1%) (Table 2).
Table 2.
Disease free survival (DFS) and overall survival (OS) according to the clinical characteristics
*statistical significant, †platelet derived growth factor receptor A, ‡protein kinase C theta, §discovered on gastrointestinal stromal tumor-1.
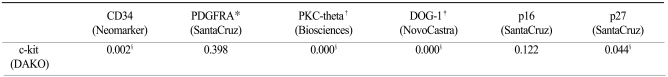
The IHC expression for c-kit was strongly correlated with expression of PKC-theta (p=0.000), DOG-1 (p=0.000), CD34 (p=0.002) and p27 (p=0.044). But PDGFRA (p=0.398) and p16 (p=0.122) expressions were not correlated with c-kit expression (Table 3).
Table 3.
Correlations between c-kit and other immunohistochemical antidodies in gastrointestinal stromal tumor (GIST)
*platelet derived growth factor receptor A, †protein kinase C theta, ‡discovered on gastrointestinal stromal tumor-1, §statistical significant.
3. Statistical analysis
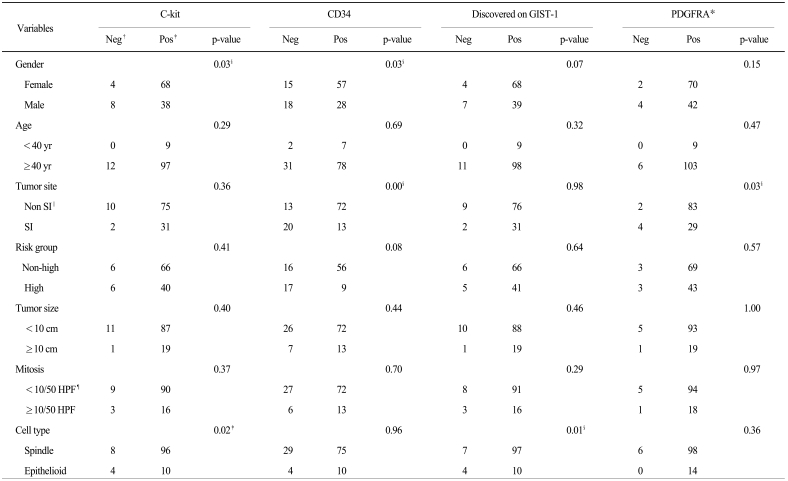
As a result of the correlation analysis of the clinicopathological and IHC characteristics, c-kit (p=0.02) and DOG-1 (p=0.01) positivity are correlated with spindle cell type, positivity for c-kit (p=0.03) and CD34 (p=0.03) were correlated with female gender, positivity for CD34 (p=0.00) and PDGFRA (p=0.03) were correlated with non-small intestine origin, positivity for p16 was correlated with patient age ≥40 years old (p=0.01) and mitoses ≥10/50 HPFs (p=0.01), and positivity for p27 was correlated with tumor ≥10 cm (p=0.01) (Table 4).
Table 4.
Clinicopathologic characteristics related to immunohistochemical stains of gastrointestinal stromal tumor (GIST)
*platelet derived growth factor receptor A, †negative stain, ‡positive stain, §statistical significant, ∥small intestine, ¶high power field.
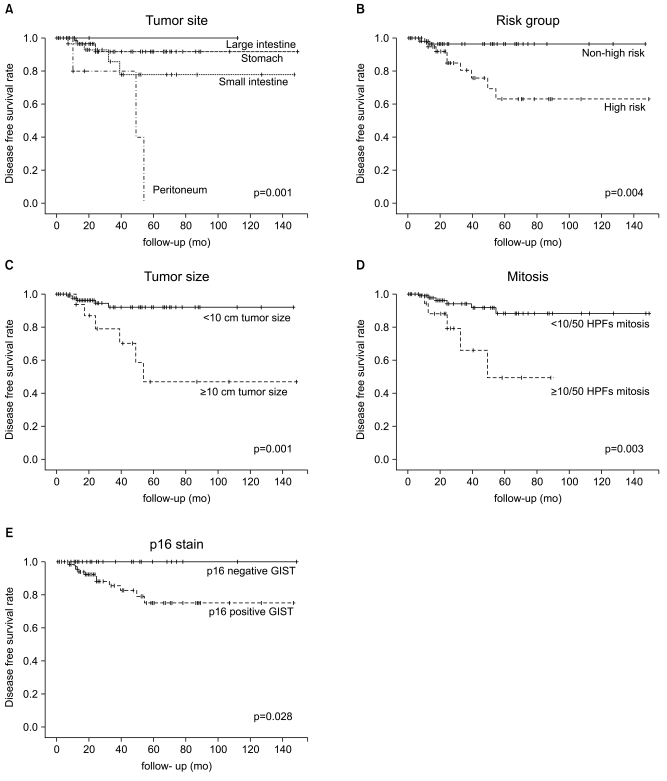
The disease free survival (DFS) rate of all the patients was poor for peritoneal GIST (p=0.001), high risk GIST (p=0.004), tumor size ≥10 cm (p=0.001), mitoses ≥10/50 HPFs (p=0.003) and p16 positivity (p=0.028) (Fig. 1).
Fig. 1.
Disease free survival (DFS) rate of all patients was poor in peritoneal gastrointestinal stromal tumor (GIST) (A), high risk GIST (B), tumor size ≥10 cm (C), mitoses ≥10/50 HPFs (D), and p16 positivity (E). HPF, high power field.
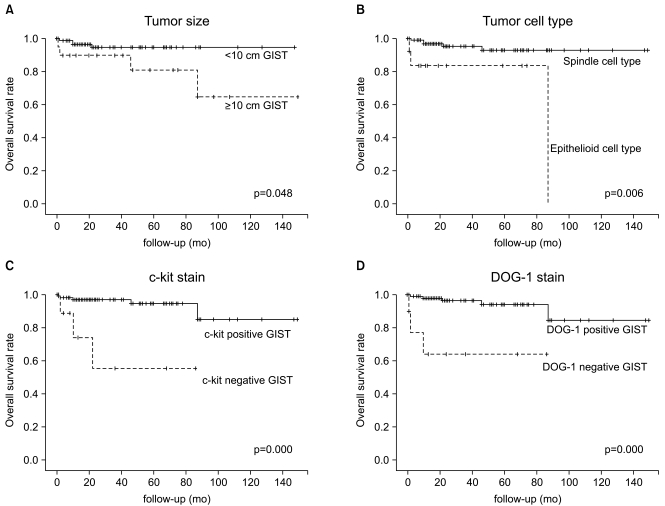
The OS rate was also poor for tumor size ≥10 cm (p=0.048), epithelioid tumor cell type (p=0.006), negative c-kit expression (p=0.000) and negative DOG-1 expression (p=0.000) (Fig. 2, Tables 1 and 2).
Fig. 2.
Overall survival (OS) rate was also poor in tumor size ≥10 cm (A), epithelioid cell type (B), negative c-kit (C), and negative DOG-1 (D). GIST, gastrointestinal stromal tumor; DOG-1, discovered on GIST-1.
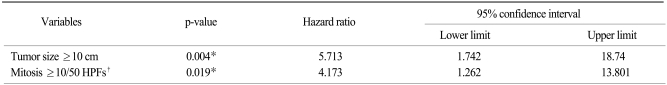
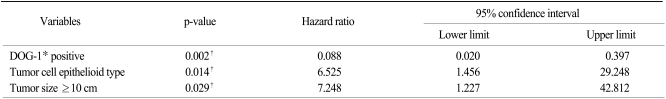
On the multivariate analysis, tumor size ≥10 cm (p=0.004) and mitotic figures ≥10/50 HPFs were independent prognostic factors for poor DFS (Table 5), where DOG-1 negativity (p=0.002), an epithelioid tumor cell type (p=0.014) and tumor size ≥10 cm (p=0.029) were independent prognostic factors for poor OS (Table 6).
Table 5.
Cox's multivariate model for disease-free survival
*statistical significant, †high power field.
Table 6.
Cox's multivariate model for disease-free survival
*discovered on gastrointestinal stromal tumor-1, †statistical significant.
Discussion
GIST is the most common mesenchymal tumor (1), which accounts for about 0.1% to 3% of all GI tumors (9). These tumors were previously classified as leiomyomas, leimyosarcomas or schwannomas, according to their histologic features. The KIT protein expression and mutations of the kit gene were first found in GIST in 1998 (4), and the authors of that study demonstrated that GIST might originate from the pacemaker cells of the GI tract, that is, ICC (10). GIST has since been comfirmed by IHC stains for c-kit and CD34 to arise from Cajal (pacemaker) cells.
The sites where GISTs are usually involved are stomach (50% to 60%), small intestine (20% to 30%) and colorectum (10%) (2,11). Rabin reviewed 93 GISTs, and in that study, there were 40 females and 53 males with a ratio of 1 : 1.3, and the ages ranged between 26 and 89 years (mean age, 62 years) (11). In our study, 46 patients (39.0%) were male and 72 patients (61.0%) were female, and their ages ranged from 32 to 83 years (mean age, 57.4 years). The most common tumor sites were stomach (78 cases, 66.1%) and small intestine (33 cases, 28.0%). Other less common sites of GISTs were peritoneum (5 cases, 4.2%) and large intestine (2 cases, 1.7%). GISTs usually occur in older adults, and rarely in children and young adults.
IHC stains such as CD34, smooth muscle actin (SMA) and S100 desmin, as well as c-kit (CD117), are necessary for making an accurate diagnosis of GIST and for making the differential diagnosis between GIST and other mesenchymal tumors. Liu et al. (12) revealed that CD117 (c-kit) and CD34 showed diffuse strong positive expressions in GISTs, and the positive rates were 98.1% and 92.3% of the GISTS in that study. Rabin et al. (11) reported 40% to 70% of GIST's were positive for CD34, 20% to 30% were positive for SMA, 10% were positive for S100 protein and <5% were positive for desmin. Although the diagnosis of GIST may be made by light microscopy, pathologists commonly employ a panel of IHC markers, including anti-CD34, smooth-muscle actin, desmin, S100 and c-kit, to confirm the diagnosis. However, making the diagnosis can be difficult for the c-kit negative cases that exhibit the same morphological, cytogenetic and molecular features as those of the c-kit positive GISTs This is of great importance since the use of imatinib mesylate has led to a dramatic improvement in the survival rates of GIST patients, in addition to improving their quality of life (6). While intragenic PDGFRA activating mutations are present in some of the c-kit negative GISTs, the oncogenic events underlying the pathogenesis of the other GISTs remain unknown. The existence of c-kit-negative GIST points to the need for additional markers, including PDGFRA (6), PKC-theta (5,13) and DOG-1 (8). Of the 118 GIST's in our study, c-kit was positive in 106 cases (89.8%), CD34 was positive in 85 (72.0%), PKC-theta was positive in 67 (56.8%), PDGFRA was positive in 112 (94.9%) and DOG-1 was positive in 107 (90.7%). An IHC expression for c-kit was strongly correlated with PKC-theta, DOG-1 and CD34, so a combination of these IHC stains would be helpful to confirm the diagnosis of GIST. Although the PDGFRA positivity showed a high frequency in contrast to the positivity for c-kit, there was reciprocal positivity between c-kit and PDGFRA. So, antibodies such as DOG-1, PKC-theta and CD34 together with c-kit can be used as an important tool for diagnosing GISTs with high specificity and sensitivity.
A diagnosis of GIST is not always possible using IHC techniques for detecting c-kit (14). Hirota et al. (4) first reported that sequencing of the c-kit complementary DNA and encoding a proto-oncogenic receptor tyrosine kinase (KIT) from GISTs revealed mutations in the region between the transmembrane and tyrosine kinase domains (4). Most GISTs (more than 80%) contain oncogenic kit or PDGFRA receptor tyrosine kinase mutations. Kit mutations are in exon 11, exon 9, exon 13 and exon 17 (15,16) and PDGFRA mutations are in exon 18, exon 12 and exon 14 (15,17), in increasing order of frequency. These mutations cause functional changes in the KIT and PDGFRA proteins (15). Most kit-mutant proteins are sensitive to imatinib (15); however, the GISTs with exon 17 kit-mutation are primarily resistant to imatinib, and exon 9 kit-GIST mutants are less sensitive to imatinib than the exon 11 mutants (18). GIST patients with exon 9 and 11-mutant tumors have better outcome than those with no detectable mutations (the wild-type genotype). PDGFRA mutations show a strong predilection for gastric GISTs with an epithelioid morphology, and the reported incidence of kit mutation-negative GISTs was 35% (17). In our study, 12 cases among the 118 GISTs showed the absence of c-kit expression by IHC, although the clinicopathological features were typical of GISTs. Those 12 cases also showed positivity for PDGFRA (12 cases, 100%), p16 (6 cases, 50%), CD34 (4 cases, 33.3%), DOG-1 (4 cases, 33.3%) and PKC-theta (1 case, 8.3%). So, a mutation study for kit or PDGFRA, as well as an antibody staining panel for these proteins, is needed to confirm the diagnosis of GIST.
STI 571 (Gleevec®, Novartis, Switzerland) is an inhibitor of tyrosine kinase, and it was recently developed and has been effectively used for treating GISTs (19). Therefore, the interest in the pathological parameters has been increased for predicting the prognosis of patients who have GISTs and who are treated with Gleevec. The biological behavior of GIST is difficult to predict because some GISTs metastasize to other sites, whereas others remain asymptomatic for years (20), although various clinicopathologic criteria for making the prognosis have been suggested. There is not enough information about the correlation between the GIST-associated proteins (such as c-kit, CD34, PDGFRA, PKC-theta and DOG-1) and the clinicopathological characteristics. The currently accepted high risk group, which has large tumor size, high mitotic activity, necrosis, infiltration and metastasis and hypercellularity, has tended to have poor prognosis (21,22) Although these variables have independent value for predicting the prognosis of patients with GISTs, better methods are still required to accurately predict the clinical course.
Nakamura et al. (23) found that the cyclin-cdk complex was significantly correlated with tumor grade, tumor size and mitotic activity. This cyclin-cdk complex may be correlated with these factors because alteration in cyclin-cdk complex, which are cell cycle regulator proteins, causes dysregulation of proliferation. In contrast to a study that reported worse prognosis with loss of p16 expression (INK4A) (21), Schmieder et al. (24) reported that the expression of p16 (INK4A) in GIST was useful in predicting poor outcome. In our study, p16 (INK4A) positivity was correlated with an older age (≥40 years old) and many mitotic figures (≥10/50 HPFs), and p27 (KIP1) positivity was correlated with large size of tumor (≥10 cm). The DFS rate was significantly decreased for the patients with peritoneal GIST, high risk GIST, tumor size ≥10 cm, mitoses ≥10/50 HPFs and p16 positivity. GISTs ≥10 cm, epithelioid cell type and c-kit and DOG-1 negativity were significantly associated with shorter period of OS. Especially, tumor size ≥10 cm and mitotic figures ≥10/50 HPFs were the significant independent prognostic factors for poor DFS, where DOG-1 negativity, epithelioid tumor cell type and tumor size ≥10 cm were the significant independent prognostic factors for poor OS. In conclusion, the expression of p16 (INK4A) and no expression of c-kit and DOG-1, in addition the clinicopathologic characteristics such as peritoneal tumor site, high risk group, large tumor size ≥10 cm, an epithelioid cell type and numerous mitoses ≥10/50 HPFs, may be the potential prognostic factors for predicting worse outcomes. Research needs to be done to find treatment for these high risk patients.
Conclusion
A tumor size ≥10 cm and mitotic figures ≥10/50 HPFs were the significant independent prognostic factors for poor DFS, where DOG-1 negativity, epithelioid tumor cell type and tumor size ≥10 cm were the significant independent prognostic factors for poor OS. The expression of p16 (INK4A) was correlated with an older age (≥40 years old) and numerous mitotic figures (≥10/50 HPFs), and the DFS rate was also significantly decreased for the patients with p16 (INK4A) positive GISTs. Therefore, p16 (INK4A) positivity may be potential prognostic factor for predicting worse outcome, as well as such clinicopathologic characteristics as peritoneal site of tumor, high risk group, large tumor size ≥10 cm, epithelioid tumor cell type and numerous mitoses ≥10/50 HPFs.
Footnotes
The present research has been funded a Bisa Research Grant of Keimyung University in 2002.
References
- 1.Connolly E, Gaffney E, Reynolds J. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178–1186. doi: 10.1002/bjs.4352. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher C, Berman J, Corless C, Gorstein F, Lasota J, Longley B, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10:81. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Lasota J. Gastrointestinal stromal tumors-definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 5.Motegi A, Sakurai S, Nakayama H, Sano T, Oyama T, Nakajima T. PKC theta, a novel immunohistochemical marker for gastrointestinal stromal tumors (GIST), especially useful for identifying KIT-negative tumors. Pathol Int. 2005;55:106–112. doi: 10.1111/j.1440-1827.2005.01806.x. [DOI] [PubMed] [Google Scholar]
- 6.Sevinc A, Camci C, Yilmaz M, Buyukhatipoglu H. The diagnosis of C-kit negative GIST by PDGFRA staining: clinical, pathological, and nuclear medicine perspective. Onkologie. 2007;30:645–648. doi: 10.1159/000109978. [DOI] [PubMed] [Google Scholar]
- 7.Chompret A, Kannengiesser C, Barrois M, Terrier P, Dahan P, Tursz T, et al. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 8.West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705–712. doi: 10.1007/s10434-000-0705-6. [DOI] [PubMed] [Google Scholar]
- 10.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–389. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rabin I, Chikman B, Lavy R, Sandbank J, Maklakovsky M, Gold-Deutch R, et al. Gastrointestinal stromal tumors: a 19 year experience. Isr Med Assoc J. 2009;11:98–102. [PubMed] [Google Scholar]
- 12.Liu FY, Qi JP, Xu FL, Wu AP. Clinicopathological and immunohistochemical analysis of gastrointestinal stromal tumor. World J Gastroenterol. 2006;12:4161–4165. doi: 10.3748/wjg.v12.i26.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, et al. PKCtheta expression in gastrointestinal stromal tumor. Mod Pathol. 2006;19:1480–1486. doi: 10.1038/modpathol.3800673. [DOI] [PubMed] [Google Scholar]
- 14.Isaac JC, Willmore C, Holden JA, Layfield LJ. A c-kit-negative gastrointestinal stromal tumor with a platelet-derived growth factor receptor alpha mutation. Appl Immunohistochem Mol Morphol. 2006;14:52–56. doi: 10.1097/01.pai.0000156866.84350.ec. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher JA, Rubin BP. KIT mutations in GIST. Curr Opin Genet Dev. 2007;17:3–7. doi: 10.1016/j.gde.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 18.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H. Treatment of inoperable gastrointestinal stromal tumor (GIST) with Imatinib (Glivec, Gleevec) Med Klin (Munich) 2002;97(Suppl 1):28–30. [PubMed] [Google Scholar]
- 20.Kim T, Lee H, Kang Y, Choe M, Ryu M, Chang H, et al. Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clin Cancer Res. 2004;10:3076. doi: 10.1158/1078-0432.ccr-03-0581. [DOI] [PubMed] [Google Scholar]
- 21.Huang HY, Huang WW, Lin CN, Eng HL, Li SH, Li CF, et al. Immunohistochemical expression of p16INK4A, Ki-67, and Mcm2 proteins in gastrointestinal stromal tumors: prognostic implications and correlations with risk stratification of NIH consensus criteria. Ann Surg Oncol. 2006;13:1633–1644. doi: 10.1245/s10434-006-9188-4. [DOI] [PubMed] [Google Scholar]
- 22.Nemoto Y, Mikami T, Hana K, Kikuchi S, Kobayashi N, Watanabe M, et al. Correlation of enhanced cell turnover with prognosis of gastrointestinal stromal tumors of the stomach: relevance of cellularity and p27kip1. Pathol Int. 2006;56:724–731. doi: 10.1111/j.1440-1827.2006.02038.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, et al. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828–837. doi: 10.1016/j.humpath.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Schmieder M, Wolf S, Danner B, Stoehr S, Juchems MS, Wuerl P, et al. p16 expression differentiates high-risk gastrointestinal stromal tumor and predicts poor outcome. Neoplasia. 2008;10:1154–1162. doi: 10.1593/neo.08646. [DOI] [PMC free article] [PubMed] [Google Scholar]