Abstract
Purpose
Acute and late changes in magnetic resonance imaging of the pediatric brain have been described after radiotherapy (RT). We report the post-RT neuroimaging changes in the posterior fossa after intensity-modulated RT (IMRT) in children with medulloblastoma and contrast them with those of leptomeningeal disease.
Methods and Materials
We performed a retrospective review of 53 consecutive children with medulloblastoma who were treated with craniospinal RT followed by IMRT to the posterior fossa and chemotherapy between 1997 and 2006.
Results
After IMRT to the posterior fossa, 8 (15%) of 53 patients developed increased fluid-attenuated inversion-recovery signal changes in the brainstem or cerebellum and patchy, multifocal, nodular contrast enhancement at a median of 6 months. The enhancement superficially resembled leptomeningeal disease. However, the enhancement resolved without intervention at a median of 6 months later. The accompanying fluid-attenuated inversion-recovery signal changes occasionally preceded the enhancement, were often parenchymal in location, and resolved or persisted to a lesser degree. All 8 patients with transient magnetic resonance imaging changes in the posterior fossa were alive at last follow-up. In contrast, leptomeningeal disease occurred in 8 (15%) of our 53 patients at a median of 19.5 months after IMRT completion. Of these 8 patients, 7 demonstrated initial nodular enhancement outside the conformal field, and 7 patients died.
Conclusion
Magnetic resonance imaging changes can occur in the posterior fossa of children treated with IMRT for medulloblastoma. In our experience, these transient changes occur at a characteristic time and location after RT, allowing them to be distinguished from leptomeningeal disease.
Keywords: Intensity-modulated radiotherapy, Magnetic resonance imaging, Medulloblastoma, Pediatrics, Surveillance
INTRODUCTION
Medulloblastoma is the most common malignant brain tumor in children and accounts for about 20% of primary pediatric central nervous system tumors (1). The standard treatment in children ≥3 years is resection followed by craniospinal radiotherapy (RT) with a posterior fossa or tumor bed boost and then multiagent chemotherapy. Intensity-modulated RT (IMRT) is a highly conformal RT method and produces a heterogeneous radiation dose distribution within the target (2). Asymptomatic magnetic resonance imaging (MRI) changes have been described after RT in children treated with conventional RT for leukemia (3) and brain tumors (4–6). Fouladi et al. (6) reported diffuse white matter lesions after conformal RT. However, MRI changes primarily in the posterior fossa in association with IMRT have not been previously described.
In the treatment of medulloblastoma, IMRT provides the prescribed dose to the tumor bed or posterior fossa with relative sparing of the surrounding normal tissues. IMRT has been used to lessen the radiation dose to the temporal lobes and reduce radiation exposure to the cochlea by approximately 40%, resulting in decreased ototoxicity (7). In a previous report from our institutions, IMRT was shown to decrease ototoxicity despite the use of cisplatin chemotherapy compared with conventional RT (7). One potential disadvantage of IMRT is that the dose within the target can be heterogeneous, such that different regions within the posterior fossa will receive a gradient of doses. Potentially, this heterogeneous dose to the treated brain could produce a detectable effect on follow-up MRI. We report changes on MRI after treatment of childhood medulloblastoma with IMRT that can mimic tumor progression and describe how these changes can be distinguished from findings of leptomeningeal disease.
METHODS AND MATERIALS
Patient population
We performed a retrospective clinical and radiologic review of children with medulloblastoma who were treated at the Texas Children’s Hospital (Houston, TX) and received IMRT at the Methodist Hospital (Houston, TX) between August 1997 and November 2006. All medical records were reviewed for demographic, clinical information, and RT history. Disease was classified as average risk or high risk according to the postoperative tumor volume and the presence of metastatic disease (6). Average-risk disease was defined as a postoperative tumor volume of ≤1.5 cm2 residual disease on postoperative MRI, the absence of leptomeningeal disease, negative cerebrospinal fluid cytology, and no evidence of systemic disease. Patients with high-risk disease had at least one of the following: residual disease >1.5 cm2 on postoperative MRI, evidence of leptomeningeal disease, or positive cerebrospinal fluid cytology. All patients provided informed consent before treatment.
Radiation dosimetry
All patients were treated initially with craniospinal RT delivered using parallel opposed lateral fields to the cranium abutted to one or two spinal fields. Children with standard-risk medulloblastoma received an initial 23.4 Gy; those with high-risk disease received 36 Gy at 1.8 Gy/fraction to the craniospinal axis. In the earlier part of the study, patients with standard-risk disease received an additional 12.6 Gy to the entire posterior fossa, followed by a 19.8-Gy tumor bed boost (resection cavity ± residual tumor, with a 2-cm margin) using IMRT. In the latter part of the study, craniospinal RT was followed by an IMRT boost to the tumor bed, with a 1–2-cm margin at a prescribed dose of 28.8–32.4 Gy in 1.8-Gy fractions. Children with high-risk tumors either received an initial 36 Gy craniospinal dose followed by a tumor bed boost of 19.8 Gy with a 1–2-cm margin or they received an initial craniospinal dose of 39.6–45 Gy, followed by a tumor bed boost of 0–16.2 Gy. For IMRT, the dose constraints to the surrounding organs, including the cochlea, pituitary gland, hypothalamus, brainstem, and temporal lobes, were designated for each plan. In general, 100% of the planning target volume received 95% of the prescribed dose. In addition, no more than 10% of the planning target volume received >110% of the prescribed dose. Figure 1 shows an axial slice of an IMRT plan with sparing of the cochlea while still delivering the prescribed dose to the posterior fossa. The entire posterior fossa received varying radiation doses depending on the study period and the risk group category. Of the 53 patients, 11 (21%) received a prescribed dose of ≥50 Gy to the entire posterior fossa, 37 children (70%) received 36–45 Gy, and 5 patients (9%) received 23.4 Gy.
Fig. 1.
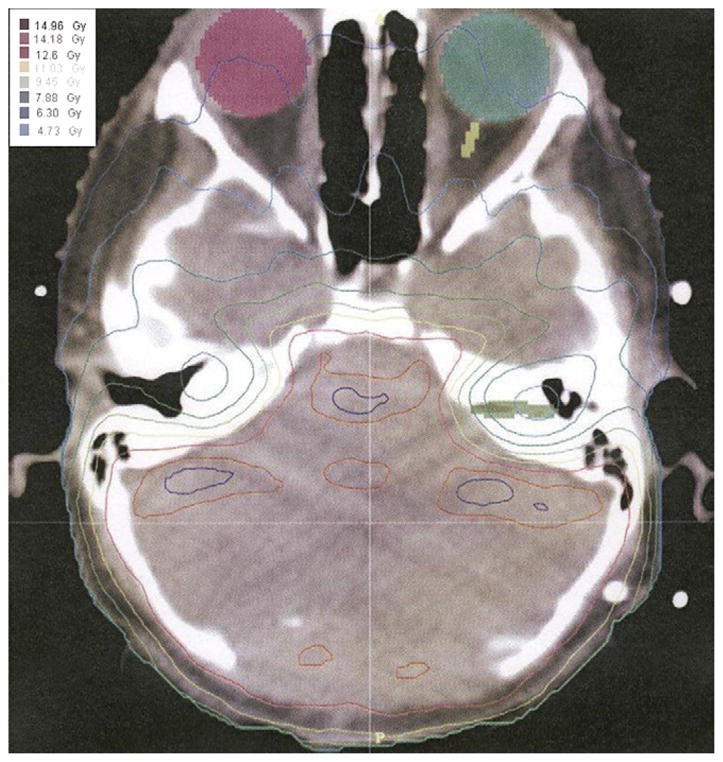
Intensity-modulated radiotherapy map for Patient 8. Example of dose heterogeneity and conformality of radiation dose to avoid normal structures.
Chemotherapy protocols
After RT, 51 of 53 patients received either conventional chemotherapy or high-dose chemotherapy with stem cell rescue. The conventional chemotherapy agents were vincristine, cisplatin, and lomustine or cyclophosphamide, similar to the treatment plan of a recently closed cooperative group study (8). High-dose chemotherapy was administered as part of a study and included high-dose cyclophosphamide, cisplatin, and vincristine or melphalan and cyclophosphamide (9, 10).
MRI examinations
All patients underwent postoperative and RT follow-up MRI performed on a 1.5T super-conducting magnet (Intera, Philips Medical Systems, Best, The Netherlands). The follow-up images were at 3-month or 6-month intervals, as determined by protocol. At a minimum, the pulse sequences consisted of precontrast T1-weighted sagittal and axial spin echo images (repetition time, 400–600 ms; excitation time, 10 ms; number of excitations, 2), fast spin-echo T2-weighted images (repetition time 4,000–5,000 ms; excitation time, 100 ms; number of excitations, 2), fluid-attenuated inversion-recovery (FLAIR) images (repetition time, 11,000 ms; excitation time, 140 ms; inversion time (TI) 2,600 ms; number of excitations, 2), and postcontrast T1-weighted spin echo images in all three planes. All sequences were acquired with a field of view of 20–24 cm and a section thickness of 5–6 mm. Gadolinium-based contrast material (Bayer Healthcare Pharmaceuticals, Wayne, NJ) was infused intravenously at a standard dose of 0.1 mmol/kg before the acquisition of the postcontrast images. After routine brain imaging, postcontrast T1-weighted sagittal and axial images were obtained through the spine. The images were analyzed on a dedicated picture archiving and communication system or Web-based viewing systems.
For all patients, the MRI scans of the brain were initially interpreted by a team of pediatric neuroradiologists. All images for each patient in this study were re-evaluated from the initial diagnosis through the subsequent examinations by a single pediatric neuroradiologist (J.Y.J.). Alterations in FLAIR signal and nodular enhancement were recorded, noting location (cerebrum, cerebellum, brainstem, upper spinal cord, or adjacent subarachnoid space), size, and appearance (patchy, nodular, linear), as well as any evolution and/or resolution of the changes. The sequential MRI spinal studies of all patients with positive brain findings were then reviewed to evaluate for abnormal intrathecal enhancement. Furthermore, the corresponding radiology reports were reviewed (J.A.M.), noting any possible positive findings with respect to the FLAIR signal or enhancement. The images of these patients were then re-inspected by the study neuroradiologist to ensure that no examinations with potential changes were overlooked.
RESULTS
Patient population
We identified 53 children with medulloblastoma who had undergone IMRT during the study period. Our patients included 40 males and 13 females. The median age at diagnosis was 8.1 years (range, 2.3–18.2 years; Table 1). The median patient follow-up period was 40 months (range, 2–104 months).
Table 1.
Patient characteristics (n = 53)
| Characteristic | Value |
|---|---|
| Gender (n) | |
| Male | 40 |
| Female | 13 |
| Age at medulloblastoma diagnosis (y) | |
| Median | 8.1 |
| Range | 2.3–18.2 |
| Chemotherapy (n) | |
| Conventional | 18 |
| High dose with stem cell rescue | 33 |
| Died before chemotherapy | 1 |
| Homeopathic | 1 |
| Prescribed radiation dose to entire posterior fossa (Gy) | |
| ≥50 | 11 |
| 36–45 | 37 |
| 23.4 | 5 |
Chemotherapy protocols
Chemotherapy was administered after RT (Table 1). Of the 53 patients, 18 received conventional chemotherapy and 33 high-dose chemotherapy with stem cell rescue (9, 11). One patient received homeopathic therapy, and 1 patient died before receiving chemotherapy.
MRI examinations
After completion of craniospinal RT, 8 (15%) of 53 patients developed local MRI signal abnormalities in the posterior fossa within the IMRT field. These signal abnormalities included patchy increased FLAIR signal in the cerebellum or brainstem at a median of 6 months (range, 4–9 months) and local patchy or nodular enhancement in the same locations at a median of 6 months (range, 4–11 months; Table 2). Figure 2 shows an example of FLAIR signal changes and nodular enhancement in Patient 8 (Table 2). One patient had additional involvement of the posterior temporal and occipital lobes, and another patient had involvement of the uppermost cervical cord. Although these changes resembled leptomeningeal disease (Fig. 3), the MRI changes seen after IMRT were often located in the parenchyma rather than the sulcal or cisternal spaces (Table 3). In 5 patients, FLAIR signal changes resolved within 11–15 months after IMRT, and in 3 patients, these FLAIR abnormalities persisted to a similar or lesser degree. Figure 4 shows an example of a patient who had changes on MRI after IMRT in whom the enhancement resolved and the FLAIR signal changes persisted. Abnormal enhancement had resolved 12 months (range, 9–25 months) after IMRT in all but 1 patient.
Table 2.
Clinical characteristics of 8 patients with MRI changes due to IMRT
| Pt. No. | Age at diagnosis (y) | Resection | Radiation dose (Gy) to posterior fossa* | Location of initial FLAIR signal change and enhancement | Associated new neurologic symptoms | Changes after IMRT (mo) |
Clinical outcome/follow-up (y) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Onset of FLAIR signal change | FLAIR signal change resolution | Onset of MRI enhancement | Enhancement resolution | |||||||
| 1 | 3.0 | GTR | 55.8 | Cerebellar hemispheres | None | 6 | Persistent | 6 | 13 | No recurrence/5 |
| 2 | 6.3 | GTR | 39.6 | Cerebellar hemispheres | None | 9 | 15 | 11 | 15 | No recurrence/4.5 |
| 3 | 5.1 | GTR | 23.4 | Medulla | None | 6 | 11 | 6 | 11 | No recurrence/1.2 |
| 4 | 9.8 | Near total | 36.0 | Pons and cerebellar hemispheres | None | 6 | Persistent | 7 | 25 | No recurrence/4 |
| 5 | 8.5 | GTR | 36.0 | Medulla | None | 7 | Persistent | 8 | Persistent | No recurrence/4 |
| 6 | 5.5 | GTR | 55.8 | Pons | Lower extremity weakness | 5 | 11 | 6 | 9 | No recurrence/8.8 |
| 7 | 3.9 | GTR | 54.0 | Cerebellar hemispheres | none | 4 | 8 | 7 | 9 | No recurrence/5.5 |
| 8 | 9.3 | GTR | 36.0 | Cerebellar hemispheres | none | 4 | 11 | 4 | 10 | Recurrence/4.5 |
Abbreviations: Pt. No. = patient number; GTR = gross total resection; IMRT = intensity-modulated radiotherapy; FLAIR = fluid-attenuated inversion-recovery; MRI = magnetic resonance imaging.
Tumor bed received 54–55.8 Gy.
Fig. 2.
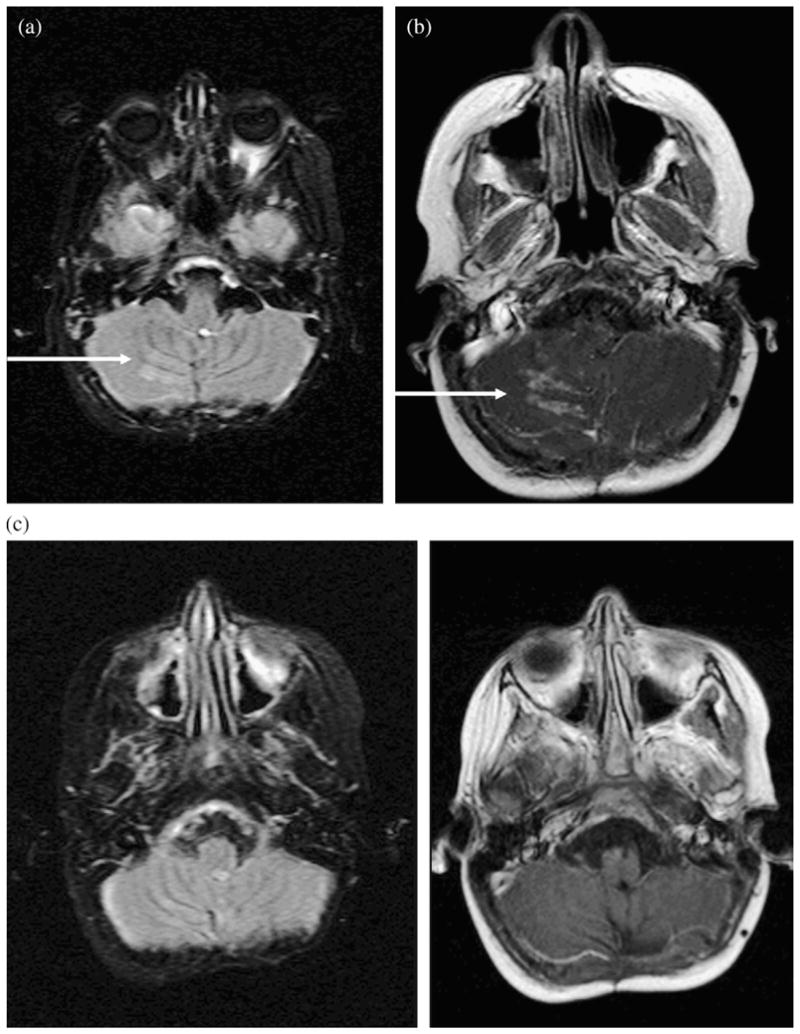
Magnetic resonance imaging scans of Patient 8. (a) Axial, fluid-attenuated inversion-recovery image demonstrating new, increased, nodular signal along periphery of right inferior cerebellar hemisphere at 4 months after intensity-modulated radiotherapy (IMRT). (b) Axial postcontrast T1-weighted image demonstrating avid nodular enhancement approximately 5.5 months after IMRT. (c) Abnormal fluid-attenuated inversion-recovery signal and enhancement had resolved at 12.5 months after IMRT.
Fig. 3.
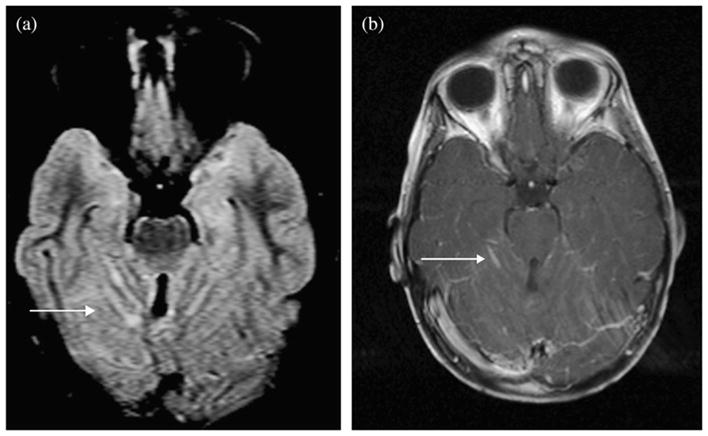
Magnetic resonance imaging scans of Patient 12 with leptomeningeal disease. (a) Axial fluid-attenuated inversion-recovery images demonstrating linear and nodular increased signal confined to superior cerebellar sulci. (b) Postcontrast T1-weighted images demonstrating linear enhancement in sulci.
Table 3.
Clinical characteristics of 8 patients with leptomeningeal disease
| Pt. No. | Age at diagnosis (y) | Resection | Radiation dose (Gy) to posterior fossa* | Location of initial FLAIR signal change and enhancement | Changes after IMRT (mo) |
Clinical outcome | |
|---|---|---|---|---|---|---|---|
| Onset of FLAIR signal change | Onset of MRI enhancement | ||||||
| 9 | 6.6 | GTR | 55.8 | Frontal lobe | NA | 16 | Died 46 mo after diagnosis |
| 10 | 6.5 | GTR | 55.8 | Caudate body and temporal lobe | 24 | 24 | Recurrent spinal disease 8.5 mo after IMRT; died 33 mo after diagnosis |
| 11 | 6.8 | GTR | 36.0 | Vermian fossa and frontal sulci | 5 | 5 | Died 10 mo after diagnosis |
| 12 | 8.0 | GTR | 36.0 | Cerebellar sulci | 16 | 16 | Died 25 mo after diagnosis |
| 13 | 12.3 | GTR | 36.0 | Cerebral and cerebellar sulci | 20 | 20 | Initial intrathecal recurrence at IMRT completion; died 47 mo after diagnosis |
| 14 | 14.1 | GTR | 36.0 | Lateral ventricle | 19 | 19 | Died 36 mo after diagnosis |
| 8 | 9.2 | GTR | 36.0 | Cerebellar sulcus | 43 | 43 | Initially had changes due to IMRT that resolved; alive 61 mo after diagnosis and 18 mo after recurrence |
| 15 | 5.8 | GTR | 36.0 | Insula | NA | 27 | Initial intrathecal recurrence 17 mo after IMRT; died 31 mo after diagnosis |
Abbreviations: NA = not applicable; other abbreviations as in Table 2.
Tumor bed received 54–55.8 Gy.
Fig. 4.
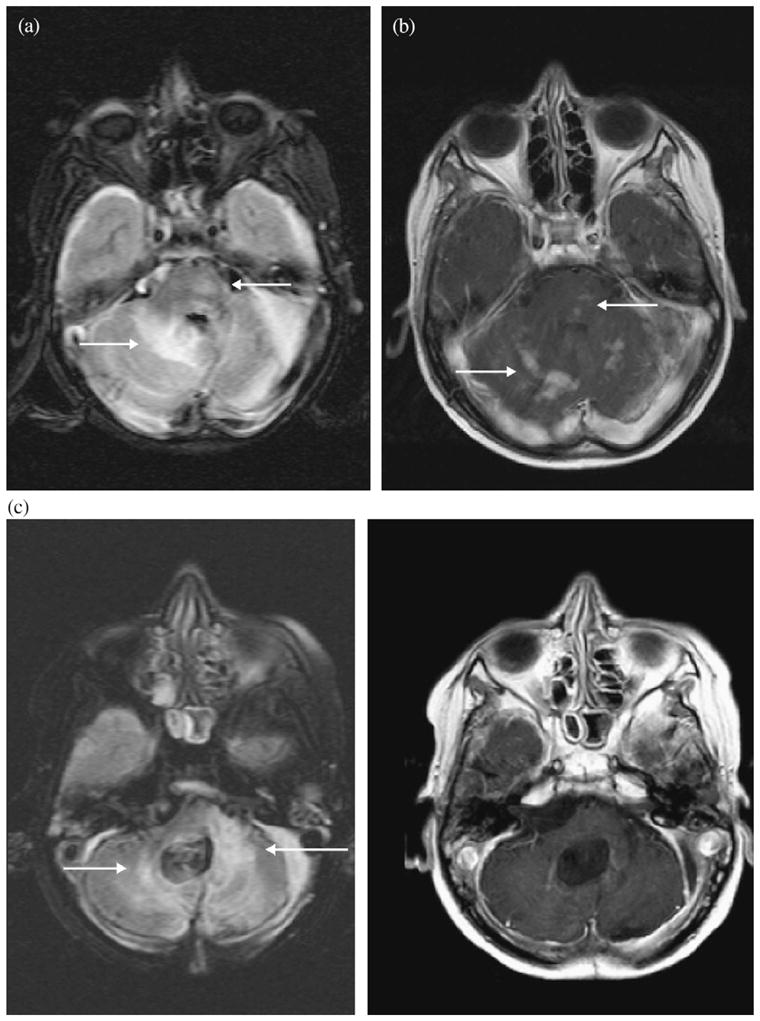
Magnetic resonance imaging scans of Patient 4. (a) Axial fluid-attenuated inversion-recovery images demonstrating patchy fluid-attenuated inversion-recovery signal in right cerebellar parenchyma and left pons at 5.5 months after intensity-modulated radiotherapy. (b) Axial T1-weighted images with avid patchy and nodular enhancement in same locations and at same time point. (c) Abnormal fluid-attenuated inversion-recovery signal changes have persisted and enhancement has resolved at 24.5 months after intensity-modulated radiotherapy.
In contrast, 15 (28%) of 53 patients had tumor recurrence at a median of 20 months (range, 1–53 months) from diagnosis. This subset of patients with recurrence included 8 patients (15%) with a combined imaging and clinical diagnosis of leptomeningeal recurrence at a median of 19.5 months (range, 5–43 months) from diagnosis (Table 3). Although none of the 8 patients had tissue verification of recurrent disease, 7 have died and 1 has demonstrated mild progression. Of these 8 patients with leptomeningeal disease, 7 presented with nodular enhancement outside the posterior fossa IMRT field: 4 had nodular enhancement localized to the thecal sac, 1 had nodular enhancement localized to the sulcal spaces supratentorially, 1 had nodular enhancement localized to the lateral ventricle, and 1 had enhancement in the sulcal spaces both supra- and infratentorially. Additionally, 1 patient had initially demonstrated IMRT changes and later presented with an enlarging nodule in the posterior fossa. An accompanying FLAIR signal was seen in association with these intracranial nodules primarily in the sulcal spaces. The 4 patients who initially had intrathecal nodules subsequently developed evidence of intracranial nodular enhancement.
Clinical characteristics and follow-up
Of the 8 patients we identified with changes in FLAIR signal and transient enhancement after IMRT, 4 (50%) had received conventional chemotherapy and 4 (50%) had received high-dose chemotherapy with stem cell rescue (Table 2). Of these 8 patients, 7 (88%) had had a primary tumor in the fourth ventricle or vermis and 1 had received IMRT for a primary tumor in the cerebellum that recurred locally (Table 2). Also, 2 (25%) of the 8 patients had had leptomeningeal disease at presentation and 6 (75%) had not. Seven of these patients (88%) had undergone gross total resection before receiving IMRT and one had undergone near total resection (Table 2). MRI changes after IMRT were observed in 3 (27%) of 11 children who had received ≥50 Gy to the entire posterior fossa, 4 (11%) of 37 who had received 36–45 Gy, and 1 (20%) of 5 who had received 23.4 Gy (Table 2).
All 8 patients with transient MRI changes after IMRT were alive at last follow-up (median, 4.3 years; range, 1.2–8.8 years), and 7 of the 8 patients remained disease free. At the initial MRI changes, 7 (88%) of the 8 patients had no new neurologic symptoms and 1 had lower extremity weakness that persisted. In general, these 8 patients experienced the long-term sequelae that are frequently seen in patients treated for brain tumors, including endocrine abnormalities, hearing and speech impairment, and gait abnormalities. Patient 5 developed radiation necrosis and quadriplegia 19 months after completing RT and had persistent FLAIR signal and enhancement abnormalities on MRI. The patient had received approximately 4,500–5,100 cGy to the area of radiation necrosis. This area was outside the clinical target volume and had not received a dose greater than that to the planning or clinical target volumes. Similar to children with transient MRI changes after IMRT, patients with leptomeningeal disease recurrence generally did not have new symptoms at the initial MRI change. However, unlike the excellent survival rate of patients with transient MRI changes after IMRT, 7 of 8 patients with leptomeningeal disease died (Table 3).
DISCUSSION
Generalized white matter changes on MRI after conventional RT have been previously described (5, 6). This is the first report of MRI changes after IMRT. Typically, changes in the posterior fossa with associated enhancement are suspicious for recurrent or progressive disease. The MRI findings after IMRT as described in our study (Fig. 2a,b) can be mistaken for leptomeningeal disease (Fig. 3a,b) or a combination of changes due to IMRT and late demyelination (Fig. 4). When leptomeningeal disease is suspected, biopsy is not uniformly performed. Also, patients might be removed from their original treatment protocols and enrolled in investigational studies.
White matter changes visible on MRI can be visualized after RT or chemotherapy (12–17). In a study of patients with acute lymphoid leukemia who were treated with chemotherapy with or without RT, the acute MRI changes noted included widening of the sulci or ventricles, hyper- or hypo-intense areas, and gray matter changes—findings believed to indicate cerebral atrophy, leukoencephalopathy, and brain necrosis (3). The MRI changes observed in our study differed from other treatment-related white matter changes in their combination of features: primarily posterior fossa location, subacute onset, transient nature, and lack of associated neurologic symptoms.
One proposed explanation for the radiographic changes we observed in some of our patients after IMRT is that these were white matter changes that might reflect areas in the posterior fossa that received the greater dose to the target. The greater target dose could result in a transient breakdown of the blood–brain barrier with or without concurrent transient demyelination and/or radiation-related small vessel damage. However, we observed a discordance between the prescribed radiation dose to the posterior fossa and the occurrence of white matter changes. In comparing the IMRT dose map and the MRI images, we would have expected the regions with the MRI changes to coincide with the greater radiation dose. However, we do not observe this association (Figs. 1 and 2). Although the white matter changes did not directly match the IMRT high-dose regions, we could not dismiss the radiation dose level as an influential factor associated with the transient MRI changes occurring after IMRT. The radiographic changes were seen in 27% of the patients who had received the larger radiation dose (>50 Gy) compared with 11% of patients who received the intermediate dose (36–45 Gy). The same patients who underwent IMRT had also undergone craniospinal RT, which can affect the homogeneity of the dose in the thinner portions of the head such as the posterior fossa region. The integral dose to the surrounding planning target volume could explain some of the white matter changes in the cerebellar hemispheres, brainstem, and temporal and occipital lobes, which received a greater dose than that delivered by craniospinal RT. In some patients, two IMRT plans were performed to cover the entire posterior fossa followed by a third IMRT plan for the tumor bed. Because of dose heterogeneity of the IMRT plans, the additive dose distribution just adjacent to the planning target volume or within the target volume of the plans could have affected the white matter changes seen. However, the number of patients receiving the greater vs. intermediate radiation dose (11 vs. 37) was too small to draw a definitive conclusion. Additionally, 1 of 5 patients who received the lowest dose (23.4 Gy) of IMRT to the posterior fossa and 3 of 11 patients who received the greatest dose (≥50 Gy) had transient MRI changes. The similar numbers in the two groups suggest that the MRI changes had no relation to the radiation dose received. However, the numbers were too small to draw a definitive conclusion. Therefore, we hypothesize that other undefined patient susceptibility factors contributed to the MRI changes we observed.
In our patients, the radiographic effects of IMRT consisted of a combination of changes on FLAIR images and parenchymal enhancement within the posterior fossa that occurred after a median of 6 months. In contrast, the MRI changes seen with leptomeningeal disease in our patient population occurred outside the conformal field of the posterior fossa, with enhancement and FLAIR changes occurring at a median of 19.5 months and 22 months, respectively, after IMRT completion. In the future, magnetic resonance spectroscopy performed when the MRI changes are noted might help to distinguish between radiation-related changes and recurrences early in the evolution of these changes. Although we do not have histologic proof that the areas of enhancement after IMRT were not tumor recurrence, the lack of progression of these abnormalities without therapeutic intervention strongly suggests that they did not represent neoplasm. Also, we did not observe an increase in local relapse in patients treated with IMRT.
CONCLUSION
Recurrent medulloblastoma has a dismal prognosis (18–24). Therefore, the distinction between radiographic changes related to normal tissue damage by chemoradiotherapy and true disease progression affects the patient’s subsequent treatment and prognosis. Patients who are believed to have disease recurrence might be enrolled in a Phase I or Phase II study. If the patient has radiographic changes resulting from RT, the “response to therapy” might be mistaken as therapeutic efficacy of the experimental agent. Our experience has demonstrated that patchy or nodular enhancement in the posterior fossa IMRT field, when detected at 6 months after IMRT and associated with patchy parenchymal FLAIR signal is most likely a post-RT effect rather than tumor recurrence. According to the findings in our patients, we recommend observing patients who have characteristic asymptomatic radiographic changes after IMRT with follow-up studies at frequent intervals to distinguish the effects of IMRT from true leptomeningeal disease and to avoid premature enrollment in investigational studies.
Acknowledgments
J. A. Muscal was supported by a grant from the National Heart, Lung, and Blood Institute (Grant T32 DK06440).
Footnotes
Presented as a poster at the American Society of Pediatric Hematology/Oncology, May 6, 2007, Toronto, ON, Canada.
Conflict of interest: none.
References
- 1.Blaney SM, Kun LE, Hunter J, et al. Tumors of the central nervous system. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 818–825. [Google Scholar]
- 2.Teh BS, Woo SY, Butler EB. Intensity modulated radiation therapy (IMRT): A new promising technology in radiation oncology. Oncologist. 1999;4:433–442. [PubMed] [Google Scholar]
- 3.Hertzberg H, Huk WJ, Ueberall MA, et al. for the German Late Effects Working Group. CNS late effects after ALL therapy in childhood: Part I. Neurological findings in long-term survivors of childhood ALL—An evaluation of the interferences between morphology and neuropsychological performance. Med Pediatr Oncol. 1997;28:387–400. doi: 10.1002/(sici)1096-911x(199706)28:6<387::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Bakardjiev AI, Barnes PD, Goumnerova LC, et al. Magnetic resonance imaging changes after stereotactic radiation therapy for childhood low grade astrocytoma. Cancer. 1996;78:864–873. doi: 10.1002/(SICI)1097-0142(19960815)78:4<864::AID-CNCR25>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Helton KJ, Edwards M, Steen G, et al. Neuroimaging-detected late transient treatment-induced lesions in pediatric patients with brain tumors. J Neurosurg. 2005;102(Suppl 2):179–186. doi: 10.3171/jns.2005.102.2.0179. [DOI] [PubMed] [Google Scholar]
- 6.Fouladi M, Chintagumpala M, Laningham FH, et al. White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol. 2004;22:4551–4560. doi: 10.1200/JCO.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 7.Huang E, Teh BS, Strother DR, et al. Intensity-modulated radiation therapy for pediatric medulloblastoma: Early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys. 2002;52:599–605. doi: 10.1016/s0360-3016(01)02641-4. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 9.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney DH, Jr, Strother D, Camitta B, et al. High-dose melphalan and cyclophosphamide with autologous bone marrow rescue for recurrent/progressive malignant brain tumors in children: A pilot Pediatric Oncology Group study. J Clin Oncol. 1996;14:382–388. doi: 10.1200/JCO.1996.14.2.382. [DOI] [PubMed] [Google Scholar]
- 11.Strother D, Ashley D, Kellie SJ, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem-cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiotherapy: Results of a collaborative study. J Clin Oncol. 2001;19:2696–2704. doi: 10.1200/JCO.2001.19.10.2696. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K, Takahashi S, Sato A, et al. Leukoencephalopathy in childhood hematopoietic neoplasm caused by moderate-dose methotrexate and prophylactic cranial radiotherapy—An MR analysis. Int J Radiat Oncol Biol Phys. 1995;32:913–918. doi: 10.1016/0360-3016(95)00565-g. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DA, Nitschke R, Bowman ME, et al. Transient white matter changes on MR images in children undergoing chemotherapy for acute lymphocytic leukemia. Radiology. 1991;180:205–209. doi: 10.1148/radiology.180.1.2052695. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney DH, Jr, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: An association with intermediate-dose intravenous methotrexate and intrathecal triple therapy—A Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 15.Pääkö E, Vainionpää L, Lanning M, et al. White matter changes in children treated for acute lymphoblastic leukemia. Cancer. 1992;70:2728–2733. doi: 10.1002/1097-0142(19921201)70:11<2728::aid-cncr2820701126>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Reddick WE, Russell JM, Glass JO, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich U, Wanke I, Mueller T, et al. White matter disease in children treated for malignant brain tumors. Childs Nerv Syst. 2002;17:731–738. doi: 10.1007/s00381-001-0526-3. [DOI] [PubMed] [Google Scholar]
- 18.Bouffet E, Doz F, Demaille MC, et al. Improving survival in recurrent medulloblastoma: Earlier detection, better treatment or still an impasse? Br J Cancer. 1998;77:1321–1326. doi: 10.1038/bjc.1998.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashley DM, Meier L, Kerby T, et al. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol. 1996;14:1922–1927. doi: 10.1200/JCO.1996.14.6.1922. [DOI] [PubMed] [Google Scholar]
- 20.Crafts DC, Levin VA, Edwards MS, et al. Chemotherapy of recurrent medulloblastoma with combined procarbazine, CCNU, and vincristine. J Neurosurg. 1978;49:589–592. doi: 10.3171/jns.1978.49.4.0589. [DOI] [PubMed] [Google Scholar]
- 21.Lefkowitz IB, Packer RJ, Siegel KR, et al. Results of the treatment of children with recurrent medulloblastoma/primitive neuroectodermal tumors with lomustine and vincristine. Cancer. 1990;65:412–417. doi: 10.1002/1097-0142(19900201)65:3<412::aid-cncr2820650306>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Wara WM, Le QT, Sneed PK, et al. Pattern of recurrence of medulloblastoma after low-dose craniospinal radiotherapy. Int J Radiat Oncol Biol Phys. 1994;30:551–556. doi: 10.1016/0360-3016(92)90940-j. [DOI] [PubMed] [Google Scholar]
- 23.Dunkel IJ, Boyett JM, Yates A, et al. for the Children’s Cancer Group. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. J Clin Oncol. 1998;16:222–228. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 24.Torres CF, Rebsamen S, Silber JH, et al. Surveillance scanning of children with medulloblastoma. N Engl J Med. 1994;330:892–895. doi: 10.1056/NEJM199403313301303. [DOI] [PubMed] [Google Scholar]


