Abstract
Sigma receptors once considered as a class of opioid receptors are now regarded as unique orphan receptors, distinguished by the ability to bind various pharmacological agents such as the progesterone (steroid), haloperidol (antipsychotic), and drugs of abuse such as cocaine and methamphetamine. The sigma-1 receptor is a 223 amino acid protein, proposed to have two transmembrane segments. We have developed a scheme for the purification of the guinea pig sigma-1 receptor following overexpression in Escherichia coli as a maltose binding protein (MBP) fusion and extraction with TritonX-100. Affinity chromatography using an amylose column and Ni2+ affinity column was used to purify the sigma-1 receptor. The sigma-1 receptor purified by this method is a 26 kDa polypeptide as assessed by SDS-PAGE, binds sigma ligands with high affinity and can be specifically photoaffinity labeled with the sigma-1 receptor photoprobe, [125I]-iodoazidococaine. Ligand binding using [3H]-(+)-pentazocine indicated that approximately half of the purified protein in Triton X-100 bound to radioligand. The MBP-sigma-1 receptor and the sigma-1 receptor in 0.5 % triton were maximally stable for approximately two weeks at −20°C in buffer containing 30 % glycerol.
INTRODUCTION
The sigma-1 receptor is a unique binding site occurring ubiquitously in many tissues. First thought to be an opioid receptor [1] the sigma-1 receptor was later reclassified based on binding to opioid antagonists naloxone and naltrexone, which it binds with extremely low affinity. The sigma-1 receptor is a 223 amino acid protein that has been cloned from different sources with all cloned sigma receptors sharing 90 % identity and 95 % similarity. The sigma-1 receptor protein shows no homology to any other mammalian proteins but has 30 % identity to a fungal sterol isomerase involved in cholesterol biosynthesis [2]. The sigma-1 receptor binds to a wide variety of different pharmacological agents including drugs of abuse such as cocaine and methamphetamine [3]. Based on hydropathy analysis there are three hydrophobic regions in the receptor of which the second has been proposed to be the single putative transmembrane segment [2]. However studies with green fluorescent protein (GFP) tagged sigma-1 receptors in Xenopus oocytes have suggested that the first and the second hydrophobic regions are both putative transmembrane segments [4], although this designation remains somewhat controversial.
Various functions have been proposed for the sigma-1 receptor including modulation of Ca2+ release [5], modulation of contractility, Ca2+ influx and beat rate in cultured cardiac myocytes [6], inhibition of proliferative response to mitogens [7], modulation of effects of cocaine [8] and inhibition of voltage gated K+ channels [9]. Even though these functions have been attributed to the sigma-1 receptor the exact signal transduction pathways regulated by the receptor are not clear. Although early evidence suggested that sigma-1 receptor might be coupled to G proteins, many recent reports indicate that this is not likely to be the case [9, 10]. The sigma-1 receptor has been shown to occur in a complex with voltage-gated K+ channels (Kv 1.4 and Kv 1.5), which has prompted the suggestion that it might serve as an auxiliary subunit to the K+ channels [4]. The sigma-1 receptor has also been shown to occur in a complex with the IP3 receptor on the endoplasmic reticulum [5]. Such evidence has led to the proposal that direct protein-protein interactions might play a role in sigma-1 receptor signal transduction. Studies with GFP tagged sigma-1 receptors demonstrated that sigma-1 receptors localized to endoplasmic reticulum lipid droplets containing caveolin 2 in NG108–15 neuroblastoma cells. N-terminal truncation of the sigma-1 receptor or treatment of the cells with the sigma-1 receptor ligand, (+)-pentazocine, resulted in translocation of sigma-1 receptor from the endoplasmic reticulum lipid microdomains to cytoplasmic lipid domains [11, 12]. Using rat primary hippocampal cultures Hayashi and Su also showed that sigma-1 receptors form galactoceramide enriched lipid domains at the endoplasmic reticulum in mature oligodendrocytes [13]. Although the physiological significance of these findings is unclear at present, the sigma-1 receptor does appear to regulate multiple cellular processes.
Sigma-1 receptor knockout mice have been reported which show no overt developmental phenotype but do lack the locomotor responses to the sigma ligand (+)-SKF100047 [14]. Furthermore, formalin induced non-acute pain is diminished in the sigma-1 receptor knockout mice [15]. It has been noted that perhaps there is redundancy in the biological role of sigma receptors such that other members of the family (eg. sigma-2 receptor) can substitute for a sigma-1 receptor deficiency. The sigma-2 receptor however, has not been cloned to date, but only characterized by ligand binding studies and photoaffinity labeling [16, 17]. Putative physiological roles, pathways and ligands associated with the sigma-1 and sigma-2 receptors have been recently reviewed [18–22].
Since the sigma-1 receptor is an integral membrane protein, the usual challenges for structural investigations such as crystallography and NMR spectroscopy are present. However its small size (223 amino acids) and relatively few transmembrane stretches is a potential advantage in this regard. Currently there is no report of large-scale functional heterologous overexpression and purification of the sigma-1 receptor. Here we present the first report on the expression and purification of the sigma-1 receptor from E. coli as a fusion to E. coli maltose binding protein (MBP), in a functional form (as defined by ligand binding) and in sufficient quantity for structural and biophysical work.
MATERIAL AND METHODS
Materials
The E. coli plasmids pMal-p2X and pMal-c2X, amylose resin and all restriction endonucleases were purchased from New England Biolabs, Beverly MA. Primers for PCR amplification were synthesized by Integrated DNA technologies, Coralville IA. Nova blues chemically competent E. coli and Factor Xa protease was purchased from Novagen, Madison WI. Anti-Maltose binding protein agarose was purchased from Vector laboratories, Burlingame CA. [H3]-(+)-Pentazocine was purchased from, Perkin Elmer Life Sciences, Wellesley, MA. Protease inhibitors and antibodies were purchased form Sigma-Aldrich, St. Louis, MO. IODO-GEN iodination tubes, supersignal chemiluminescent substrates and Superblock PBS blocking buffer were from PIERCE, Rockford, IL.
Preparation of the constructs
A cDNA encoding the guinea pig sigma-1 receptor was a kind gift from Harmut Glossmann (Instit fur Biochemische Pharmakologie, Universitat Innsbruck, Austria) [2]. 5′-Forward and 3′-Reverse oligonucleotides with the desired restriction sites (5′ BamH I, 3′ Hind III) were used to amplify the sigma-1 receptor cDNA by PCR and the restriction cleaved PCR product was then ligated into the vectors pMal p2X and pMal c2X previously digested with BamHI and HindIII. The 3′-reverse PCR primer incorporated a 6-histidine epitope at the C terminus of the protein. The protein encoded from the pMal p2X and c2X plasmids contained a factor Xa cleavage site between the MBP and sigma-1 receptor. For expression in COS-7 cells sigma-1 receptor PCR product was inserted between BamH1 and EcoR1 sites on the mammalian expression vector pcDNA3.1 (+). All plasmids were confirmed by DNA sequencing, which was carried out in Biotechnology Center at University of Wisconsin, Madison.
Expression and Purification of MBP-sigma-1R fusion protein
For protein expression the purified plasmid was transformed into the E. coli strain BL21 DE3 (Novagen). An overnight culture of cells in LB medium (1.0 % tryptone, 0.5 % yeast extract and 0.5 % NaCl) containing 0.2 % glucose and 100 μg/ml ampicillin was used to inoculate 2 L culture flasks containing 600–700 ml of the same medium. Cells were grown at 37°C with shaking at 250 rpm. At an OD600 of 0.7, isopropyl thio-β-D-galactoside (IPTG) was added to a final concentration of 0.7 mM and shaking was continued. Cells were harvested 4 h later by centrifuging the suspension at 4000 rpm for 25 min. The cell pellet weighting 2.5–4.0 g wet weight of cells, was stored at −20°C until further use.
Extractions from E.coli membranes
E. coli cell pellets from 14–18 L (40–60 g wet weight cells) culture were used in a single purification. The cells were thawed and resuspended in column buffer I (20 mM Tris, pH 8.0, 0.2 M NaCl, 1 mM 2-mercaptoethanol and 1 mM EDTA) supplemented with a protease inhibitor cocktail (Sigma) using 2–3 ml of buffer per gram of cells. The cell suspension was sonicated using a Branson sonifier 250 employing a 1 cm probe (output 50 %, 2 s bursts, 1 s lag) for 15 min on ice. The total cell lysate was centrifuged at 100000 G for 1 h to separate total particulate and soluble proteins. The pellet was resuspended in 80–100 ml of column buffer containing TritonX-100. We performed experiments using specifically photolabeled sigma-1 fusion protein to determine the ratio of total pellet protein to TritonX-100 (data not shown) that would achieve maximum extraction. The ratio of total pellet protein to TritonX-100 was optimized to be 1:4 (w/w). The thoroughly resuspended pellet was stirred at 4°C for 2 h for extraction of membrane proteins. The suspension was then centrifuged at 100000 g for 1 hr to obtain the solubilized fraction. This fraction was diluted with column buffer to a final TritonX-100 concentration of 1.0 % w/v.
Purification on an amylose column
10 ml of Amylose resin (New England Biolabs, E-802) was packed into a chromatographic column suitable for gravity flow applications. The resin was washed with two column volumes of distilled water and equilibrated with 2 column volumes of column buffer containing 1 % w/v TritonX-100 at a flow rate of 1–2 ml/min. The diluted extract (0.5–1 L) was loaded by gravity flow onto the amylose column at 4°C. After the extract was loaded, the amylose resin was washed with 10 column volumes of column buffer I and subsequently 3 column volumes of column buffer II (20 mM Tris, pH 8.0, 0.2 M NaCl, 1 mM 2-mercaptoethanol). The protein was eluted with elution buffer (20 mM Tris, pH 8.0, 0.2 M NaCl, 10 mM maltose). Five ml fractions were collected and aliquots analyzed by 12 % SDS PAGE. Protein estimation was carried out using the DC protein assay kit (Bio-Rad) and the Micro BCA protein assay kit (PIERCE).
Factor Xa cleavage of fusion protein
The purified fusion protein was incubated with Factor Xa protease (Novagen) at room temperature in a buffer containing 20 mM Tris, pH 8.0, 0.2 M NaCl and 5 mM CaCl2. One unit of Factor Xa was used for 75–100 μg of target protein. The reaction was allowed to proceed for 36–48 h for maximal cleavage (90–95 %) of the fusion protein.
Purification of the sigma-1 receptor on a Ni2+ column
HIS-Select HC Nickel affinity gel (Sigma P6611), in a packed bed volume of 1ml was washed with 5ml distilled water and equilibrated with 10ml of wash buffer (50 mM sodium phosphate pH 8.0, 0.3 M NaCl). This was performed in a batch format in a 15 ml falcon tube. The 15 ml tube was centrifuged at room temperature at 2400 rpm for 45 s to separate the Ni2+ resin from the supernatant. The factor Xa cleavage reaction was then incubated with the HIS-Select HC affinity gel and allowed to bind by to and fro shaking for 3 h at 4°C. The resin was then separated by centrifugation at 4000 rpm for 30 s and washed exhaustively with wash buffer. The protein was eluted with wash buffer containing 0.25 M imidazole. Eluted protein was analyzed by 12 % SDS-PAGE and stained with commassie blue.
Purification using Anti-Maltose binding protein agarose
The protein eluted from the Ni2+ column was incubated with 1ml of an agarose resin coupled with Anti-Maltose binding protein antibody (Vector laboratories, Burlingame CA) at 4°C for 18–24 h. The capacity of 1ml of packed resin was 0.4 mg for MBP. After incubation was complete, the mixture was centrifuged at 4000 rpm at room temperature and the resin separated.
Photoaffinity labeling
Photoaffinity labeling was performed using the sigma-1 receptor specific photolabel [I125]-Iodoazidococaine ([I125]-IACOC) as previously described [23]. E. coli homogenate (2 μg protein) was incubated in the presence and absence of 5 μM haloperidol in 60 mM Tris, pH 7.4 for 25 min on ice. [I125]-IACOC was then added to a concentration of 1nM and reaction was continued on ice for 15 min after which samples were irradiated for 6 s with a high pressure AH6-mercury lamp. Proteins were separated on a 12 % SDS PAGE and the gel was placed on a PhosphorImager (445 SI, Molecular Dynamics) exposure cassette for at least 8 h after which the cassette was scanned to develop the autoradiogram.
Ligand binding
Ligand binding was performed on E. coli membranes and purified receptor as described in the literature with modifications of incubation times and temperature [24, 25]. Curves were derived from five to eight different concentrations of [H3]-(+)-pentazocine (specific activity 36 Ci/mmol) ranging from 1nM to 300 nM. Haloperidol at 10 μM was used to determine non-specific binding. Binding was carried out in 50 mM Tris, pH 8.0 in a total volume of 100 μl. After incubation at 30°C for 45 min, the reaction was terminated by rapid filtration through glass fiber filters (Whatman GF/B), using a Brandel cell harvester (Brandel, Gaithersburg, MD). The glass fiber filters were previously soaked in 0.5 % polyethyleimine (PEI) for at least 1 h at room temperature. Filters were washed 4 times with 2 ml of ice-cold 50 mM Tris, pH 8.0. Radioactivity was quantified by liquid scintillation counting using a Packard model 1600CA scintillation counter. This method was suitable for both membranes and soluble sigma-1 receptor, the latter due to retention of soluble proteins on PEI coated filters due to charge interactions [26]. IODO-GEN (PIERCE) [27] radioiodinated pure MBP-sigma-1 receptor fusion protein was used to quantify the retention of the soluble fusion protein on PEI soaked filters. The amount of soluble protein that was retained on the filter was found to be dependent on the PEI concentration used on the filter (data not shown). We found a maximum of 50 % retention of soluble protein at 0.5 % PEI concentration. A correction factor in this regard was applied to all data from ligand binding experiments using the purified protein. Binding data were evaluated using GRAPHPAD PRISM (GRAPHPAD Software Inc., San Diego, CA, USA). For competition experiments Ki for ligands were calculated from the IC50 value using the Cheng Prussoff equation [28], Ki = IC50/(1 + [L]/Kd), where experimentally derived Kd value of [3H]-(+)-pentazocine for the purified sigma-1 receptor was 35 nM.
Preparation of polyclonal antibody against the sigma-1 receptor
Antibody was generated at Covance laboratories (Denver, Pennsylvania). New Zealand White (NZW) rabbits were immunized with 250 μg of pure MBP-sigma-1 receptor fusion protein with complete Freund’s adjuvant to initiate antibody production. For subsequent booster dose, five weeks after the initial dose, 125 μg of pure sigma-1 receptor with incomplete Freund’s adjuvant was used. 50 ml batch of serum from exsanguinated rabbits was precipitated with 50% ammonium sulfate and the antibody fraction dialyzed against phosphate buffered saline (PBS) pH 7.5 for 16 h at 4°C. To prepare the MBP-sigma-1 receptor or MBP affinity column, E. coli expressed and purified fusion protein or MBP was reacted with 5 ml of CNBr activated Sepharose 4B that was previously swelled and washed in 50 column volumes of 1 mM HCl and washed with each of 10 column volumes of distilled water and binding buffer (0.1 M NaHCO3 0.5 M NaCl pH 8.3). The reaction was allowed to continue overnight at 4°C. After eluting excess protein, unreacted groups on the column were blocked with 0.2 M glycine for 12 h at 4°C. For purification of all antibodies gainst the antigen, the dialyzed antibody fraction from serum was mixed with MBP-sigma-1 receptor affinity resin with gentle shaking at 4°C. For 1 ml of dialyzed serum 1ml of affinity resin was used. The column was washed with 5 column volumes of binding buffer and all fusion protein antibodies eluted with 5ml of antibody elution buffer (0.1 M glycine, pH 2.7). The pH was immediately adjusted to 8.0 with 3 M Tris, pH 8.8. For purification of sigma-1 receptor the eluate from the first MBP-sigma-1 receptor column was dialyzed against binding buffer for 16 h at 4°C and allowed to bind to the MBP affinity column for 12–16 h at 4°C. The flow through (4.5–5.0 ml) that contained the sigma-1 receptor antibody was collected at 4°C. Glycerol was added to a final concentration of 30 % v/v and the affinity purified antibody stored at a concentration of 150–250 μg/ml at −20°C.
Transient Expression in COS-7 cells
DNA purification, cell culture and transient transfection were performed as previously described [29]. COS-7 cells from a confluent 150 mm culture plate were harvested and transfected by electroporation with 15–50 μg of plasmid DNA using a Bio-Rad genepulser/capacitance extender unit. Cells were harvested with trypsin and homogenized by passage through a 32-gauge needle. The homogenate was centrifuged at 3000 g for 2 min to pellet cell debris and supernatant was used for western blotting.
Preparation of Guinea Pig liver membranes
Membranes were prepared as described previously [23]. Liver tissue was homogenized (10 ml buffer/g wet tissue) by 4 bursts of 10 s each using a brinkman polytron on setting 6 in ice cold sodium phosphate buffer (10 mM pH 7.4) containing 0.32 M sucrose and a cocktail of protease inhibitors (20 μg/ml leupeptin, 5 μg/ml soybean trypsin inhibitor, 100 μM Phenylmethylsulfhonyl fluoride (PMSF), 100 μM benzamidine and 1 mM EDTA). The membrane suspension after homogenization was centrifuged for 10 min at 17000 g and the supernatant was recentrifuged for 60 min at 105000 g. The pellet was resuspended in homogenization buffer, snap frozen and stored at −80°C.
Western Blotting
After analyzing the protein samples by 12 % SDS PAGE the proteins were transferred onto polyvinyldifluoride (PVDF) membrane (Millipore, 0.45 μm) in CAPS buffer (10 mM 3-(Cyclohexylamino)-1-propanesulfonic acid (CAPS), pH 10.5, 0.5 % w/v DTT and 15 % v/v methanol) at 65 V for 1 h. The PVDF membrane was blocked with Superblock PBS (Pierce) containing 0.05 % Tween-20 at room temperature for 1 h or overnight at 4°C. The blot was then washed 3 times for 10 min each in buffer PBST (PBS containing 0.05 % Tween 20) before incubating with primary antibody (5000 X dilution) in PBST for 1 h at room temperature. The membrane was then washed thoroughly with PBST and incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG, (Sigma-Aldrich, St. Louis, MO) at 100000 X dilution in PBST for 1 h at room temperature. HRP was detected with enhanced chemiluminescence (ECL) reagents from PIERCE.
RESULTS
Expression and Purification of the sigma-1 receptor as a MBP fusion protein
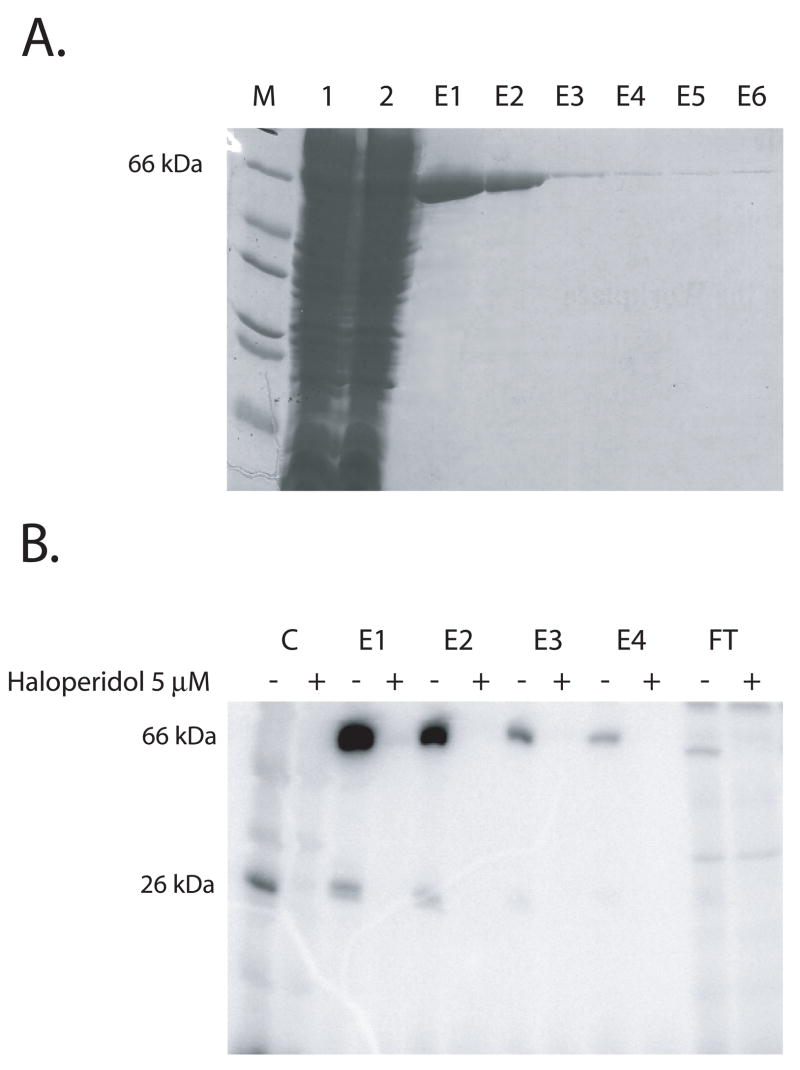
When expressed by itself the sigma-1 receptor localizes to inclusion bodies in E. coli (data not shown). Thus we explored the ability of N-terminal fusion partners for the sigma-1 receptor to enhance its solubility during E. coli expression. Glutathione-S-transferase (GST) and intein constructs were found not to influence the solubility of the sigma-1 receptor in E. coli since both sigma-1 fusion proteins still resulted in localization to inclusion bodies in a form that did not bind [3H]-(+)-pentazocine or radioiodinated sigma-1 receptor photoprobes (eg. [125I]Iodoazidococaine [23]). To generate the MBP-sigma-1 receptor fusion protein, primers were used to amplify the sigma-1 receptor ORF and the product was ligated into the New England Biolabs vector pMal p2X and pMal c2X [30] such that the MBP sequence is placed on the N terminus of the sigma-1 receptor. The PCR primers were designed to place a 6-histidine epitope on the C-terminus of the sigma-1 receptor. Upon transformation in E. coli BL21 (DE3), it was found that the vector pMal p2X resulted in the expression of 66 kDa MBP-sigma-1 receptor fusion protein as evidenced by SDS/Polyacrylamide gel electrophoresis (PAGE) analysis. Use the E. coli strains C41 (DE3) and C43 (DE3) that have been applied for overexpression of transmembrane proteins [31] did not result in a higher yields of expression (data not shown). The concentration of IPTG at 0.7 mM was found to be optimal for maximal expression. The fusion protein was found to be present in the E. coli particulate fraction after a 100000 G, 1 hr centrifugation. We explored the ability of a series of detergents 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate) (CHAPS), lauryl dimethyl amine oxide (LDAO), TritonX-100 and digitonin to effectively extract the fusion protein from E. coli membranes (data not shown). Purification of the fusion protein was carried out by extracting the E.coli particulate fraction with TritonX-100 at a detergent to protein ratio of 4:1 (w/w) and centrifugation at 100000 g for one hour to obtain the solubilized fraction. This MBP-sigma-1 receptor fusion protein was chromatographed over an amylose column, which resulted in efficient binding of the MBP-sigma-1 fusion protein to the amylose (Fig 1A). After washing the column with 10–15 column volumes of buffer to remove nonspecifically adsorbed protein, the MBP-sigma-1 fusion was eluted in buffer containing 10 mM maltose (Fig. 1A lanes E1–E6). The fusion protein migrated at the position of the 66 kDa molecular weight marker on 12 % SDS/PAGE (Fig. 1A). The amylose column afforded a greater than 90 % purification in a single step. The eluted fractions containing the MBP-sigma-1 fusion at 66 kDa were specifically photoaffinity labeled with [I125]-IACOC [23] (Fig. 1B lanes E1– E4).
Figure 1. Amylose column purification of the MBP-sigma-1 receptor and photoaffinity labeling with [I125] IACOC.
(A) Commassie blue stained 12 % SDS/polyacrylamide gel showing samples from amylose column purification of the MBP-sigma-1 receptor fusion protein. Lanes 1 and 2 are Triton solubilized column load and flow through respectively. Lanes E1 to E6 are elution fractions from the amylose column eluted with elution buffer containing 10 mM maltose. (B) Autoradiogram from photoaffinity labeling of protein fractions shown in (A) using the sigma-1 receptor photoprobe [I125]IACOC in the absence (−) and presence (+) of 5μM haloperidol as a protector. Lanes C (−/+) are Cos-7 cells transfected with sigma-1 receptor as a standard. Lanes E1 to E4 are elutions 1 through 4 and FT is column flow through. The specifically labeled lower molecular weight band is the sigma-1 receptor occurring due to non- specific cleavage of the fusion protein.
Factor Xa cleavage and purification of sigma-1 receptor
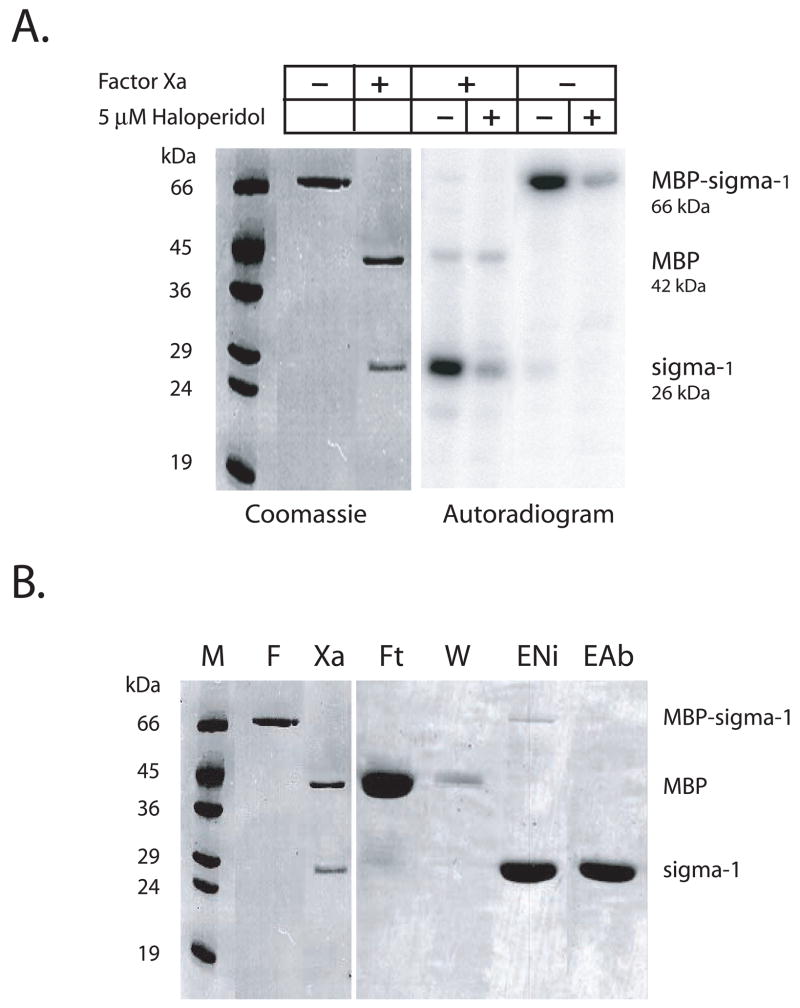
The purified fusion protein was cleaved with protease factor Xa to separate the MBP from the sigma-1 receptor. The proteolysis reaction occurred optimally at room temperature for 48 h. Approximately 5–10 % of the fusion protein remained undigested. The cleavage conditions did not affect the activity of the protein as assayed by ligand binding and photoaffinity labeling (Fig. 2A).
Figure 2. Factor Xa cleavage and Ni2+ affinity purification of the sigma-1 receptor.
(A) Commassie blue stained 12 % SDS/polyacrylamide gel showing Factor Xa cleavage of the MBP- sigma-1 receptor fusion protein (left). On the right photoaffinity labeling of the factor Xa reaction shows that the label is incorporated into the sigma-1 receptor. There is no specific labeling of the MBP. Haloperidol at 5μM is used to show specificity of the photolabeling. (B) The Factor Xa (Xa) reaction is purified on the Ni2+ affinity resin. The MBP flows through (Ft) and can be completely removed in the wash (W); Elution of the Ni column with buffer containing 0.25 M imidazole elutes the sigma-1 receptor and uncleaved fusion protein (ENi). Treatment of the Ni2+ eluate with anti-MBP linked Sepharose removed the fusion protein (EAb).
The factor Xa reaction was passed over Ni2+ affinity matrix whereby the MBP lacking the 6-histidine epitope was removed in the flow through while the sigma-1 receptor and undigested fusion protein bound to the column (Fig. 2B). Following extensive washing of the column to remove bound MBP the sigma-1 receptor and the undigested fusion protein were collected by elution buffer containing 0.25 M imidazole. At this stage of purification the sigma-1 receptor was greater than 90 % pure but contained some fusion protein as an impurity (Fig. 2B lane ENi). To remove the contaminant fusion protein the imidazole eluate was treated with Sepharose beads linked to anti-MBP antibody. The pure sigma-1 receptor was collected in the flow through (Fig. 2B lane EAb).
Ligand binding assays on the sigma-1 receptor
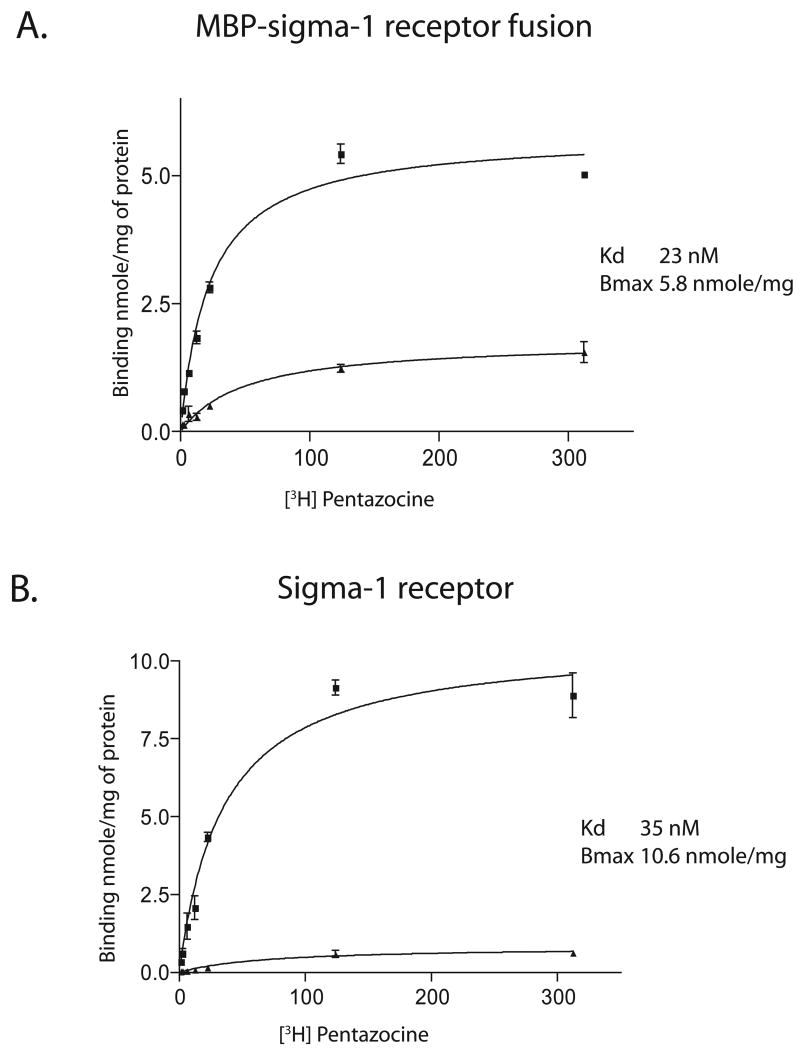
We performed [3H]-(+)-Pentazocine binding assays on the purified sigma-1 receptor. We used filter binding assays for both E. coli membranes as well as the purified fusion protein and sigma-1 receptor. It has been shown that charge interactions lead to retention of soluble proteins on polyethyleneimine coated filters [26]. The method has been used previously for soluble extracts containing the sigma receptor [25]. To test if all, or only a fraction of the soluble protein is retained on the filter we radio-iodinated purified fusion protein and sigma-1 receptor with IODO-GEN, Iodination reagent (PIERCE) and Na125I [27]. Efficient separation of free [125I] from protein bound radioactivity was achieved by the use of a G50 spin column and the homogeneity of the iodinated protein determined by SDS-PAGE and autoradiography (data not shown). When the radio-iodinated protein was passed through the PEI coated glass fiber filters we found that 50 % of the fusion protein and the sigma-1 receptor was retained on the filters. This factor was therefore applied in analysis of all binding data on the soluble receptor. Ligand binding was performed at 30°C for 45 min. Representative [3H]-(+)- pentazocine binding curves for the fusion protein and sigma-1 receptor are shown in Fig. 3. Table 1 documents a representative purification starting with 5 L of E. coli culture, which represents a wet cell mass of 12–15 g. The purification process achieved a 700-fold purification starting from E. coli particulate fraction overexpressing the MBP-sigma-1 receptor fusion protein. Slight variability was seen in Kd values between different preparations and on different times (days) following purification. The Kd of [3H]-(+)-pentazocine for the MBP-sigma-1 receptor fusion protein was between 10–25 nM and for the pure sigma-1 receptor was 15–35 nM. From the Bmax values obtained in the binding assays and the total protein used in the binding assay it was seen that approximately 40–60 % of the purified protein could bind to [3H]-(+)-pentazocine in the presence of 0.5 % Triton X-100. Reducing the triton concentration to 0.05 % did not result in an increased Bmax indicating that reduced ligand binding was not dependent on detergent concentration. These results suggest, conservatively, that the purification protocol resulted in approximately a 50 % yield in terms of native sigma-1 receptor. Other possibilities exist that could also account for the binding data, for example that Triton X-100 interfered with ligand binding (although the TritonX-100 concentration during ligand binding was 0.05 %) or that the stochiometry of [3H]-(+)-pentazocine binding to the sigma-1 receptor is not 1:1.
Figure 3. Representative [3H]-(+)-Pentazocine binding curves.
(A) MBP-sigma-1 receptor fusion protein and (B) Purified sigma-1 receptor. (■) Total binding, (▲) Non-specific binding measured in the presence of 5 μM haloperidol. The Kd of [3H]-(+)-pentazocine (35 nM) was applied to the Cheng-Prusoff equation for the data in Fig. 4 as described in the experimental section.
Table 1. Purification summary table.
Purification was performed as described in the text. Total receptor was determined in each of the different fractions by saturation binding to [3H]-(+)-pentazocine (concentrations from 1–100 nM). Total protein was estimated by the BCA protein assay. A total yield of 1.2 mg of sigma-1 receptor protein was obtained from 5 L of E. coli cell suspension (12–16 g wet weight cells). This protein yield is proportional to 20 L purification as described in the text. Saturating [3H]-(+)-pentazocine binding indicated 700-fold purification for the sigma-1 receptor starting with the crude E. coli particulate fraction expressing the MBP-sigma-1 receptor fusion protein.
| Total receptor (nmoles) | Relative yield (%) | Protein Concentration (mg/ml) | Total Protein (mg) | Specific Binding (pmoles/mg) | Fold purification | |
|---|---|---|---|---|---|---|
| Total E. coli pellet | 26.8 | 100 | 15 | 1200 | 22.3 | 1 |
| Total extract | 23.4 | 87 | 4.5 | 360 | 65.1 | 2.9 |
| Amylose column elution | 19.1 | 71 | 0.2 | 3.2 | 5980 | 267 |
| Nickel column elution | 20.2 | 75 | 0.15 | 1.2 | 16800 | 753 |
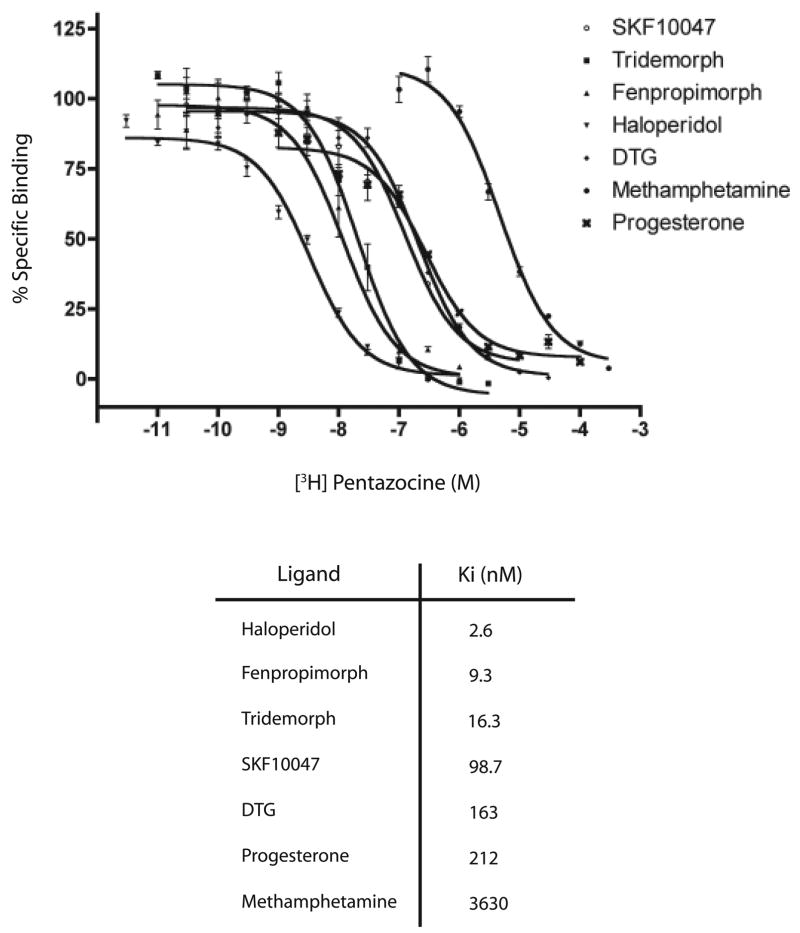
We used competition experiments with [3H]-(+)-pentazocine to determine the Kd values for different sigma ligands on the purified sigma-1 receptor preparation. Figure 4 shows the representative displacement curves for inhibition of [3H]-(+)-pentazocine binding. The rank order of potency for the various ligands used were: haloperidol > fenpropimorph > tridemorph > SKF10047 > DTG > progesterone > methamphetamine. The Kd values for the ligands on the pure receptor were similar to that reported for sigma-1 receptor on brain and liver membranes [2, 3, 32]. Progesterone, which has been suggested to be an endogenous modulator of the sigma-1 receptor [33], exhibited a Kd of 212 nM on the purified sigma-1 receptor preparation.
Figure 4. [3H]-(+)-Pentazocine displacement curves on the purified sigma-1 receptor.
Binding experiments were performed as described in the Experimental section. Incubation was at 30°C for 1 hr in 50 mM Tris, pH 8.0. Ki was calculated by the Cheng-Prussoff equation, Ki = EC50/(1+ [L]/Kd) using the software GRAPHPAD PRISM. The [3H]-(+)-pentazocine concentration ([L]) was 10 nM. The Kd of [3H]-(+)-pentazocine was taken to be 35 nM. Non-specific binding was determined in the presence of 5μM haloperidol.
Polyclonal antibody against the sigma-1 receptor
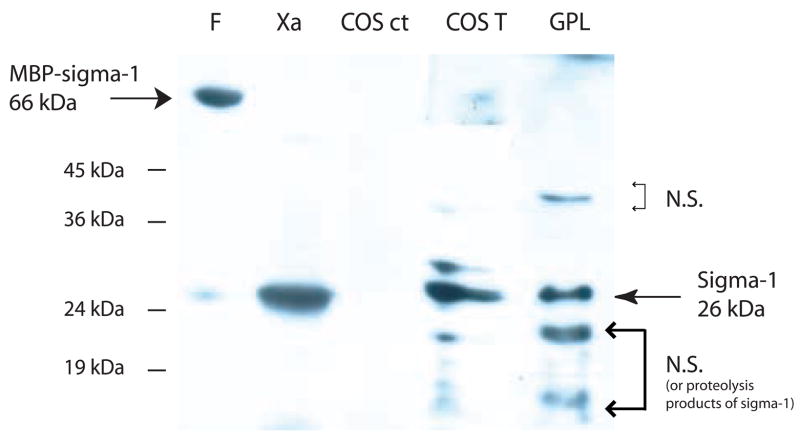
We used the MBP-sigma-1 receptor fusion protein to generate antibody against the sigma receptor. The fusion protein was used as antigen to initiate the antibody production and pure sigma-1 receptor was used for the booster doses. This work was performed at Covance laboratories (Denver, Pennsylvania). For purification of the sigma-1 receptor antibodies from serum, a two-step process was optimized. Following 50% ammonium sulfate precipitation, the total antibody fraction was chromatographed on an affinity column immobilized with MBP-sigma-1 receptor fusion protein. Anti-MBP and anti-sigma-1 receptor antibodies retained on the column were eluted with low pH buffer. This eluate was then bound to an affinity matrix immobilized with MBP. The flow through from the second column was found to be non-reactive to MBP and recognized only the sigma-1 receptor as assessed by western blotting. Fig. 5 lanes F (fusion) and Xa (Xa cleavage consisting of MBP and sigma-1 receptor), demonstrate that the antibody only recognized the sigma-1 receptor. This affinity purified polyclonal antibody was very effective for detection of the sigma-1 receptor from guinea pig liver membrane and transfected sigma-1 receptor from COS-7 cells while it did not detect the sigma-1 receptor in untransfected COS-7 cells where sigma-1 receptor levels are very low (Fig. 5 GPL, COS T and COS Ct).
Figure 5. Western blot using the affinity purified sigma-1 receptor polyclonal antibody.
Lanes, F, Fusion protein. Xa, Factor Xa cleavage of fusion protein. COS ct, total homogenate from COS-7 cells. COS T, total homogenate from COS-7 cells overexpressing sigma-1 receptor. GPL, Guinea pig liver membrane fraction. Lower molecular weight bands (lanes COS T and GPL) may be nonspecific (NS) labeling or lower molecular weight proteolytic fragments of the sigma-1 receptor
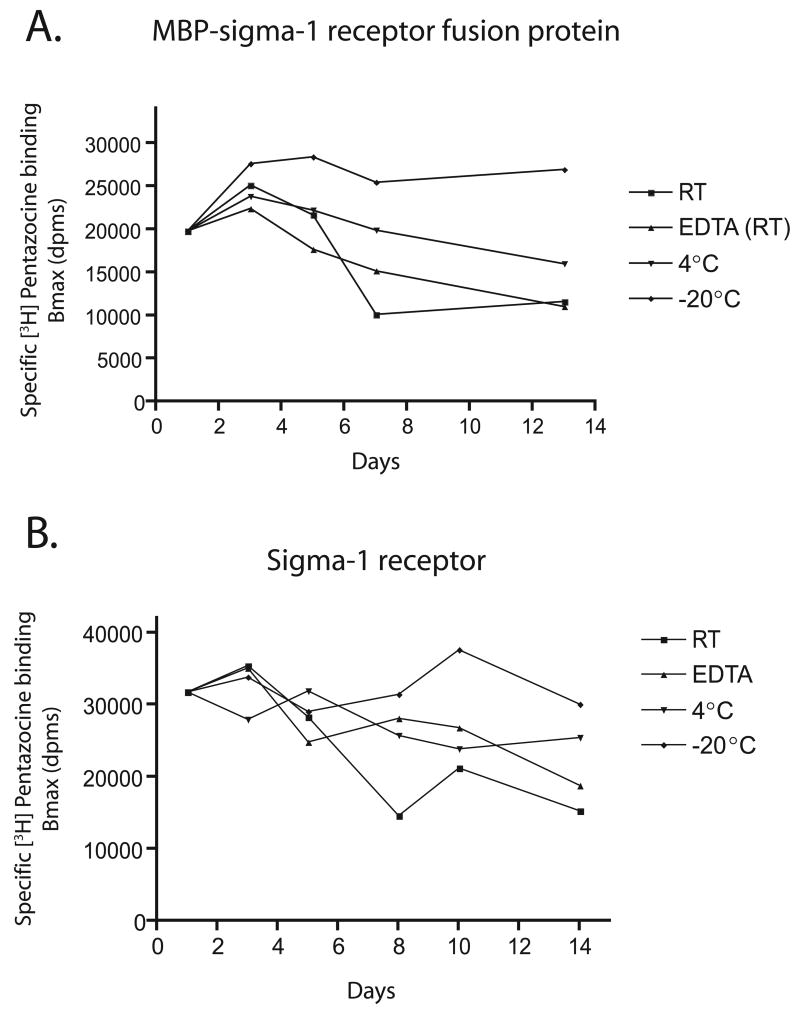
Stability of the MBP-sigma-1 receptor fusion protein and the purified sigma-1 receptor
Fig. 6A summarizes data illustrating the stability of the purified MBP-sigma-1 receptor fusion protein as well as the finally purified sigma-1 receptor. The MBP-sigma-1 receptor fusion was stable for at least 13 days at −20°C in the presence of 30 % glycerol, 50 mM Na phosphate pH 8.0 and 0.3 M NaCl (storage buffer). Other methods of storage at room temperature or at 4°C in the absence of glycerol resulted in as much as a 50 % loss of Bmax for [3H]-(+)-pentazocine activity. The purified sigma-1 receptor was seen to lose 30 % ligand binding activity at room temperature and 15 % binding activity at 4°C over a period of 14 days (Fig. 6B). Addition of 5 mM EDTA did not affect stability at room temperature. The pure sigma-1 receptor was relatively stable at −20°C in storage buffer containing 30 % glycerol.
Figure 6. Stability of the MBP-sigma-1 receptor fusion protein. (A) and pure sigma-1 receptor (B) under various conditions.
The stability of the fusion protein and pure sigma-1 receptor was assayed by saturation [3H]-(+)-pentazocine (1– 50 nM) binding on various days following purification. The protein (20–25 μg) was in buffer containing 20 mM Tris, pH 7.6, 0.2 M NaCl and 0.5 % TritonX-100. Binding assays were performed as described in the Experimental section. Non-specific binding was determined in the presence of 5 μM haloperidol. All samples were analyzed in triplicate and data reported as mean ± st. error. RT, room temperature, EDTA (RT), samples at room temperature containing 5 mM EDTA, 4°C, samples at 4°C, −20°C, samples in freezer at −20°C.
DISCUSSION
At present there is no report of overexpression and purification of the mammalian sigma-1 receptor in a form that binds ligand with high affinity. We have achieved overexpression in E. coli and purification of the guinea pig sigma-1 receptor. Since the sigma-1 receptor is a mammalian membrane protein our attempts at expression of the protein by itself in E.coli resulted in localization to inclusion bodies and denatured protein (data not shown). To obtain functional expression of sigma-1 receptor from E. coli we explored various fusion protein partners, namely Glutathione S transferase (GST), intein and MBP for the sigma-1 receptor. Maltose binding protein (MBP) has been shown to be an effective fusion partner to enhance the solubility of insoluble proteins [34]. We found the MBP-sigma-1 receptor fusion protein to express at reasonable levels in the E.coli particulate fraction. Interestingly only the periplasmic (pMal p2X) but not the cytoplasmic (pMal c2X) MBP fusion with the sigma-1 receptor could be expressed and extracted from E.coli membranes (data not shown). We hypothesize that when the periplasmic MBP-sigma-1 receptor is transported to the periplasmic space in the E. coli, the sigma-1 receptor is embedded in the E. coli membrane which may be important for proper folding of the protein.
When octyl glucoside, CHAPS, LDAO and TritonX-100 were tested for their ability to extract the MBP-sigma-1 receptor fusion from E. coli membranes, TritonX100 at a detergent to protein ratio of 4:1 (w/w) was found to be most effective. The amylose column that binds to MBP afforded a greater than 95 % purification of the MBP-sigma-1 receptor from the E. coli extract in the first step (Fig. 1). The factor Xa site between the MBP and the sigma-1 receptor was utilized to cleave the MBP affinity tag from the receptor. Removal of the MBP did not deleteriously alter the ability of the sigma-1 receptor to bind the radioiodinated sigma-1 receptor photoprobe, [I125] IACOC, or the sigma-1 receptor ligand [3H]-(+)-pentazocine. The 6-histidine tag on the C terminus of the sigma-1 allowed capture of the factor Xa cleaved sigma-1 receptor on a Ni2+ affinity matrix. The Ni2+ affinity column purification of the sigma-1 receptor after factor Xa cleavage also provides a convenient means of exchanging TritonX-100 with any detergent of choice, as needed for structural studies.
The MBP-sigma-1 receptor and pure sigma-1 receptor were characterized by ligand binding studies. The Kd value of [3H]-(+)-pentazocine for the MBP-sigma-1 receptor was between 10–25 nM and for the purified sigma-1 receptor was between 15–34 nM. This is in reasonable agreement with the Kd value of [3H]-(+)-pentazocine for the sigma-1 receptor in guinea pig brain membranes [35]. From [3H]-(+)-pentazocine binding assays it was determined that approximately 50 % of the purified protein showed binding activity, assuming 1:1 stoichometry of [3H] pentazocine binding to the sigma-1 receptor, unless the half of sites binding is due to dimerization of the sigma-1 receptor. Various sigma ligands were tested for their binding affinity on the purified sigma-1 receptor. The sigma ligands haloperidol, SKF10047 and DTG, the yeast sterol isomerase inhibitors fenpropimorph and tridemorph as well as the ligands progesterone and methamphetamine inhibited [3H] pentazocine binding on the pure sigma-1 receptor. Similar Kd values for sigma-1 receptor in membranes and purified sigma-1 receptor suggest that the receptor purified as a MBP-sigma-1 fusion protein retains the main structural features of the sigma-1 receptor ligand binding site.
In summary, we have overexpressed the recombinant guinea pig sigma-1 receptor in E.coli as an MBP-sigma-1 receptor fusion protein. The sigma-1 receptor can be cleaved from the MBP and affinity purified in a form that retains specific binding to sigma-1 receptor ligand [3H]-(+)-pentazocine. Ligand binding studies indicate that approximately 50 % of the receptor is competent to bind ligand. The MBP-sigma-1 fusion protein and the purified sigma-1 receptor maintained a stable Bmax and Kd as measured by [3H]-(+)-pentazocine binding, for approximately two weeks at −20°C in 30 % glycerol in phosphate buffer containing 0.5 % TritonX-100. The E. coli expression and purification of the sigma-1 receptor can be reasonably applied to produce biochemical amounts of sigma-1 receptor for further biochemical characterization.
Acknowledgments
We thank Dr. Michael K. Sievert for providing valuable assistance in molecular biology and protein purification techniques and helpful discussions. We thank Dr. Marty Arbabian for preparing [125I]-iodoazidococaine. This work was supported by NIH grant R01 MH065503 to A. E. R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 2.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J Pharmacol Exp Ther. 1994;269:1300–1309. [PubMed] [Google Scholar]
- 7.Spruce BA, Campbell LA, McTavish N, Cooper MA, Appleyard MV, O’Neill M, Howie J, Samson J, Watt S, Murray K, McLean D, Leslie NR, Safrany ST, Ferguson MJ, Peters JA, Prescott AR, Box G, Hayes A, Nutley B, Raynaud F, Downes CP, Lambert JJ, Thompson AM, Eccles S. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004;64:4875–4886. doi: 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- 8.Romieu P, Martin-Fardon R, Bowen WD, Maurice T. Sigma 1 receptor-related neuroactive steroids modulate cocaine-induced reward. J Neurosci. 2003;23:3572–3576. doi: 10.1523/JNEUROSCI.23-09-03572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000;526(Pt 3):527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong W, Werling LL. Evidence that the sigma(1) receptor is not directly coupled to G proteins. Eur J Pharmacol. 2000;408:117–125. doi: 10.1016/s0014-2999(00)00774-3. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 2003;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2004;101:14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 15.Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 17.Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]
- 18.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 19.Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- 20.Aydar E, Palmer CP, Djamgoz MB. Sigma receptors and cancer: possible involvement of ion channels. Cancer Res. 2004;64:5029–5035. doi: 10.1158/0008-5472.CAN-03-2329. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 22.Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berl) 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- 23.Kahoun JR, Ruoho AE. (125I)iodoazidococaine, a photoaffinity label for the haloperidol-sensitive sigma receptor. Proc Natl Acad Sci U S A. 1992;89:1393–1397. doi: 10.1073/pnas.89.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganapathy ME, Prasad PD, Huang W, Seth P, Leibach FH, Ganapathy V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J Pharmacol Exp Ther. 1999;289:251–260. [PubMed] [Google Scholar]
- 25.Torrence-Campbell C, Bowen WD. Differential solubilization of rat liver sigma 1 and sigma 2 receptors: retention of sigma 2 sites in particulate fractions. Eur J Pharmacol. 1996;304:201–210. doi: 10.1016/0014-2999(96)00109-4. [DOI] [PubMed] [Google Scholar]
- 26.Bruns RF, Lawson-Wendling K, Pugsley TA. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- 27.Salacinski PR, McLean C, Sykes JE, Clement-Jones VV, Lowry PJ. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen) Anal Biochem. 1981;117:136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 29.Thiriot DS, Sievert MK, Ruoho AE. Identification of human vesicle monoamine transporter (VMAT2) lumenal cysteines that form an intramolecular disulfide bond. Biochemistry. 2002;41:6346–6353. doi: 10.1021/bi015779j. [DOI] [PubMed] [Google Scholar]
- 30.Maina CV, Riggs PD, Grandea AG, 3rd, Slatko BE, Moran LS, Tagliamonte JA, McReynolds LA, Guan CD. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene. 1988;74:365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- 31.Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 32.Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8–C7 isomerase. Br J Pharmacol. 1997;121:1–6. doi: 10.1038/sj.bjp.0701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramamoorthy JD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Cocaine-sensitive sigma-receptor and its interaction with steroid hormones in the human placental syncytiotrophoblast and in choriocarcinoma cells. Endocrinology. 1995;136:924–932. doi: 10.1210/endo.136.3.7867601. [DOI] [PubMed] [Google Scholar]
- 34.Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8:1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Costa BR, Bowen WD, Hellewell SB, Walker JM, Thurkauf A, Jacobson AE, Rice KC. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989;251:53–58. doi: 10.1016/0014-5793(89)81427-9. [DOI] [PubMed] [Google Scholar]