Abstract
Call rate suppression is a common short-term solution for avoiding acoustic interference in animals. It has been widely documented between and within frog species, but the effects of non-anuran calling on frog vocalizations is less well known. Heterospecific acoustic interference on the calling of Oophaga pumilio (Bauer, 1994) (formerly Dendrobates pumilio) males was studied in a lowland, wet tropical forest in SE Nicaragua. Acoustic playback experiments were conducted to characterize the responses of O. pumilio males to interfering calls of cicadas, two species of crickets and a sympatric dendrobatid frog, Phyllobates lugubris. Call rate, call bout duration, percent of time calling, dominant frequency and latency to first-call were analyzed. Significant call rate suppression was observed during all stimulus playbacks, yet no significant differences were found in spontaneous call rates during pre- and post-playback trials. Dominant frequency significantly decreased after P. lugubris playback and first-call latency significantly decreased in response to both cicada and tree cricket playbacks. These results provide robust evidence that O. pumilio males can dynamically modify their calling pattern in unique ways, depending on the source of the heterospecific acoustic interference.
Keywords: amphibian, vocalizations, playback experiments, call rate suppression, dendrobatidae
In anurans, advertisement calling plays a vital role not only in territorial maintenance but also in female attraction (Bunnell 1973, Wells 1977, Narins & Capranica 1978, Wells 2007). Female mate choice is often based largely or exclusively on advertisement call characteristics (Ryan & Rand 1990, Lopez & Narins 1991, Gerhardt 1994). For example, increased levels of calling in males of Epipedobates trivittatus result in higher mating success (Roithmair 1994). However, calling is energetically very expensive (Bucher et al. 1982, Taigen & Wells 1986, Gerhardt & Huber 2002). In fact, in a study of Hyla microcephala, calling is identified as the most energetically demanding activity performed by ectothermic vertebrates (Taigen & Wells 1989).
Moreover, call detection has been shown to be impaired when there is a high level of interfering noise (Narins 1982, Narins & Zelick 1988, Wollerman 1999), therefore individuals should be selective with regard to their call timing in the face of acoustic interference (Zelick & Narins 1982, 1983, Taigen & Wells 1986, Gerhardt & Schwartz 1995). Acoustic communication interference can manifest itself when two species have such similar call characteristics that the calling of one inhibits the calling of another (Páez et al. 1993).
Oophaga pumilio (formerly Dendrobates pumilio, see Grant et al. 2006) is a moderate-sized (19–24 mm) diurnal, aposematically colored dendrobatid frog found in lowland to subtropical evergreen forest habitats from Panama to Nicaragua (Savage 1968). Dendrobatid frogs are territorial and exhibit strong site fidelity (McVey et al. 1981, Pröhl 2005). They are also remarkably responsive to speaker playbacks and exhibit positive phonotaxis to conspecific calls (Bunnell 1973).
In competitive conspecific male interactions, frogs may exhibit increases or decreases in call duration and/or call frequency (Taigen & Wells 1989, Wagner 1989, Bee et al. 1999). Although these interactions have been proposed to be the most significant source of acoustic interference (Wollerman 1999), heterospecific calling interference has also been found to be significant (Littlejohn & Martin 1969, Drewry 1970, Zelick & Narins 1983, Páez et al. 1993). In many lowland wet tropical forests, there is a high level of background noise, much of it attributable to orthopterans (Narins & Zelick 1988, Narins 1995, Gerhardt & Huber 2002). In addition, cicada calls (Homoptera, Cicadidae) have been shown to significantly inhibit O. pumilio calling behavior (Páez et al. 1993). Although cicadas are the loudest known insects (Bennet-Clark 1999), orthopterans also may use abdominal resonators to generate prodigiously high sound levels (Van Staaden & Römer 1997). Furthermore, other sympatric species, such as the dendrobatid frog Phyllobates lugubris, produce calls with dominant frequencies similar to those of O. pumilio but have not been tested for inhibitory effects. In this study, we measured the vocal responses of O. pumilio males to playbacks of conspecific calls and to interfering calls of the aforementioned species. The responses to these stimuli form the basis for testing the hypothesis that males of O. pumilio significantly change their calling pattern in unique ways, depending on the source of the heterospecific acoustic interference.
METHODS
Study area
The study was conducted at Refúgio Bartola, located adjacent to the Indio Maíz Reserve (latitude 10° 97′ N, longitude 83° 16′ W; ca 30 m a.s.l., in southeastern Nicaragua) from 2–16 May 2007. Established in 1990, the Indio Maíz biological reserve covers 2640 km2 of pristine lowland, wet tropical forest (IRENA 1992, Nygren 2004). The park is located on the Rio San Juan, near the border between Nicaragua and Costa Rica. The region receives ca. 4 m of rain annually with a mean annual temperature of 26°C. A short dry season typically falls between February and April; the remainder of the year is rainy. Historically, the mean rainfall recorded in San Juan del Norte, Nicaragua (located within 75 km of our study site) for the month of May is 517 mm, midway between the annual minimum in March of 165 mm and the summer maximum in July of 874 mm (Portig 1965). The reserve is strictly protected in order to preserve biodiversity. No commercial deforestation or extensive cattle farming is allowed.
Recordings and playbacks
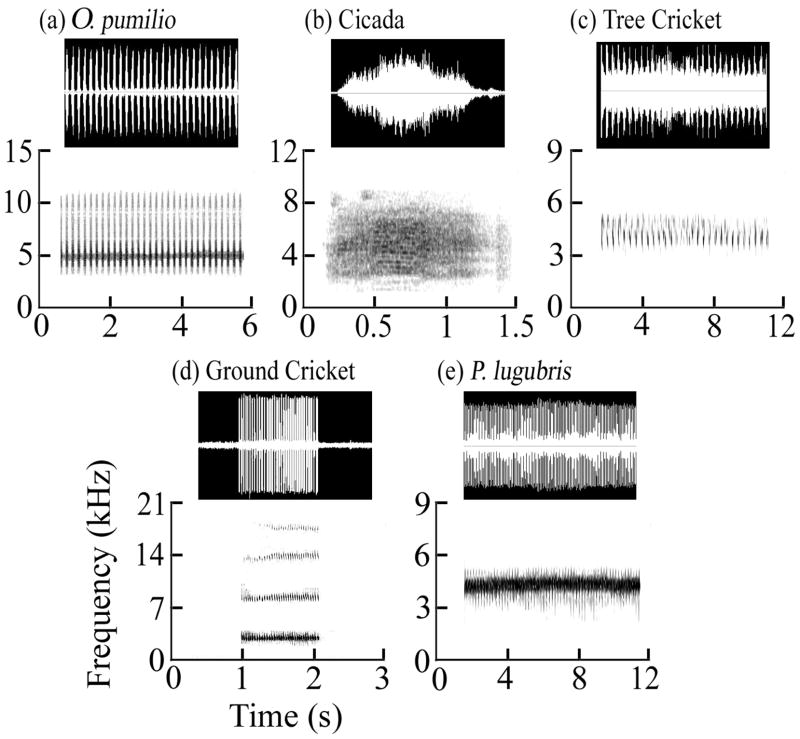
In order to determine the effect of interfering calls on the vocalizations of males of O. pumilio, responses to a control playback stimulus (a representative call of a male O. pumilio) were compared with responses to four experimental stimuli. We determined the optimal playback call length and dominant frequency for our control stimulus from spontaneous vocalizations of 30 individual males of O. pumilio recorded using a digital recorder (Marantz PMD 671) with a unidirectional microphone (Optimus #33-3017). In this paper, we define a "call note" as an individual utterance, a “call bout” as a collection of call notes where successive call bouts are separated by a relatively long period of silence, and “call rate” as the number of call notes produced per second during a bout. For this sample, the average call bout duration for O. pumilio was 46.4 s. To study call interference, we edited our stimulus recordings so that the ratio of stimulus-on time to stimulus-off time (duty cycle) was 50%, and the total length of the cycle was equal to the average call bout duration found in the frogs (46.4 s). We then selected an individual whose spontaneous call characteristics most closely matched those of the population mean in (a) desired call bout duration (23.2 s) and (b) dominant frequency (4.91 kHz). Our experimental stimuli [cicada (family Cicadidae), tree cricket (subfamily Oecanthiinae), ground cricket (subfamily Gryllinae) and Phyllobates lugubris] were edited to be of the same duration (23.2 s). These species were chosen because they all occur within the same microhabitat as O. pumilio, were observed to call at the same time of day and contain dominant frequencies within 0.6 octave of the average O. pumilio dominant frequency [Fig. 1: (1) O. pumilio: 4.77 kHz, (2) cicada: 4.56 kHz, (3) tree cricket: 4.48 kHz, (4) ground cricket: 3.27 kHz, (5) P. lugubris: 4.65 kHz]. The control and experimental playback levels were all equalized at 75 dB SPL at 1 m. Our tree cricket recording corresponds to Sonotype 2 in Brandes et al. (2006). (T.S. Brandes, pers. comm.). Each of the heterospecific experimental stimuli was made from a recording of the spontaneous calling of a representative individual from the local population. For each heterospecific stimulus, the representative call was chosen by applying several fixed criteria: (1) the calling individual had to be acoustically isolated, that is, all calling individuals in the background were at least 20 dB below the recording level of the focal individual, and (2) the microphone had to be located within 1 m of the caller resulting in a signal-to-noise ratio of the recording of at least 10 dB. These recordings were then low-pass filtered using Raven Pro (1.3β version, Cornell Laboratory of Ornithology) to remove high-frequency background noise and band-reject filtered to eliminate inter-harmonic noise. The Oophaga, cicada, tree cricket, ground cricket and Phyllobates stimuli were low-pass filtered at 11 kHz, 10 kHz, 5 kHz, 18 kHz and 5 kHz, respectively. Care was taken not to remove any harmonics or other high-frequency call components below the highest visible harmonic in the sound spectrogram. With this procedure, we could be reasonably certain that the frogs would be responding exclusively to our stimuli, and not to any spurious sounds in the background.
FIGURE 1.
Waveforms and spectrograms for the vocalizations of (a) Oophaga pumilio, (b) Cicada, (c) Tree Cricket, (d) Ground Cricket, and (e) Phyllobates lugubris. Note the similarity in the dominant frequencies in the spectrograms across calls.
To ensure that no individual was tested multiple times we sampled along a different trail each day and the distance between sampled frogs was never less than 5 m (Fig. 2). Furthermore, because there was a noted difference in response to our recordings when cicadas were calling nearby vs. when they were not, we carried out our study only in the absence of their calling. After the identity of a vocalizing male O. pumilio was visually confirmed, a powered loudspeaker (PAL Tivoli) was placed 1 m away from the frog, facing toward it. The playback experiment was divided into two parts. The first part (call bout) was repeated five times and consisted of a 23.2 s control stimulus (O. pumilio call) followed by an equal period of silence. Following a two-minute silent period, the second call bout (repeated five times) consisted of a 23.2 s period during which we presented one of the four experimental stimuli followed by an equal period of silence. All responses were recorded with the digital recorder and unidirectional microphone.
FIGURE 2.
Study site map indicating distribution of Oophaga pumilio males sampled. Each dot represents a recording/playback site. Each day a different trail was traversed to avoid multiple sampling of individual frogs.
Following the playbacks, the individual was captured and its snout-vent length was measured to the nearest 0.5 mm. Temperature (Kestrel 3000 digital thermometer) at the focal male’s calling site was also measured to the nearest 0.5°C. For each playback stimulus except one (cricket 2, N=11), 15 individuals were sampled for a total of 56 individuals.
Analyses
Call rate (call notes/s), call bout durations (s), percent time calling (%), dominant frequencies (kHz) and first-call latencies (s) were determined using Raven Pro (1.3β version, Cornell Laboratory of Ornithology). The data were entered into a spreadsheet and analyzed using Microsoft Excel 2000 Premium. For each individual tested, the parameters of the responses recorded for the five call bouts of the control and experimental stimuli were averaged to find a single value for each. The averages were analyzed to determine whether the experimental stimuli had significantly changed O. pumilio calling (SPSS 14.0 for Windows).
For individuals for which we were able to collect a complete data set in response to the interfering call, i.e., pre-playback calling, during playback and post-playback calling, we used a repeated measures ANOVA with a post-hoc pairwise comparisons test with a Sidak correction. The covariates of temperature and snout-vent length were included in the ANOVA model. For dominant frequency and first-call latency, we were unable to take measurements of responses during the playback, therefore only pre- and post-playback averages were compared in a paired samples t-test.
RESULTS
Cicada
Broadcast of cicada calls significantly decreased call rates of O. pumilio males (repeated measures ANOVA: F=170.52, df=1, 14, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.001, post-playback vs. during playback P<0.01). There was no significant difference between pre-playback and post-playback call rates (P>0.05). Call bout duration significantly decreased during cicada calling when compared to pre-playback only, although it seems to be approaching significance for during vs. post-playback (repeated measures ANOVA: F=8.258, df=1, 9, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.05, post-playback vs. during playback P>0.05). There was no significant difference between pre-playback and post-playback (P>0.05). Percent time calling also showed significant decreases during cicada broadcasting when compared to pre-playback only (repeated measures ANOVA: F=8.806, df=1, 9, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.05, post-playback vs. during playback P>0.05). Again, there was no significant difference between pre-playback and post-playback (P>0.05). Dominant frequencies (Paired samples t-test: t= −0.436, df=13, P>0.05, N=14) and first-call latencies (t=1.83, df=13, P>0.05) were not significantly affected by the interfering cicada stimulus, since differences between the values obtained during playback and those from either the pre- or post-playback periods were non-significant. Of the covariates, snout-vent length was found to affect call rate, whereas temperature did not (Sidak post-hoc comparisons test: Snout-Vent Length F=12.189, df=1, P<0.01; Temperature F=2.335, df=1, P>0.05).
Tree cricket
Tree cricket calls also evoked a significant decrease in O. pumilio call rates during stimulus playback with respect to pre- and post-playback values (repeated measures ANOVA: F=170.52, df=1, 14, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.001, post-playback vs. during playback P<0.01), but during the post-playback period, the frog returned to pre-playback call rates (P>0.05). Call bout duration and percent time calling significantly decreased while tree cricket calls were broadcast with respect to pre- and post-playback values. (Call bout duration: repeated measures ANOVA: F=1.859, df=1, 10, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.001, post-playback vs. during playback P<0.05; percent time calling: repeated measures ANOVA: F=1.859, df=1, 10, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.001, post-playback vs. during playback P<0.05). For both call bout duration and percent time calling, there was no significant change between pre- and post-playback (P >0.05). Mean first-call latency significantly decreased between pre- and post-playback call bouts (Paired samples t-test: t=4.547, df=14, P<0.001, N=15), but mean dominant frequency did not (t=0.569, df=13, P>0.05, N=14). Neither of the covariates was found to have a significant effect (Sidak post-hoc comparisons test: Snout-Vent Length F=2.201, df=1, P>0.05; Temperature F=0.635, df=1, P>0.05).
Ground cricket
We observed a significantly reduced call rate during stimulus playback (repeated measures ANOVA: F=198.46, df=1, 6, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P<0.01, post-playback vs. during playback P<0.05). Pre- and post-playback call rates were not significantly different (P>0.05). Call bout duration and percent time calling were not significantly affected by ground cricket calling (call bout duration: repeated measures ANOVA: F=0.006, df=1, 5, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P>0.05, post-playback vs. during playback P>0.05, pre-playback vs. post-playback P >0.05; percent time calling: repeated measures ANOVA: F=0.006, df=1, 5, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P>0.05, post-playback vs. during playback P>0.05, pre-playback vs. post-playback P>0.05). Mean dominant frequency (Paired samples t-test: t= −0.417, df=7, P>0.05, N=8) did not change significantly between pre- and post-playback periods, nor did mean first-call latency (t=2.102, df=8, P>0.05, N=9), although the lack of significance in these cases may be attributed to a small sample size. Neither of the covariates was found to have a significant effect (Sidak post-hoc comparisons test: Snout-Vent Length F=4.806, df=1, P>0.05; Temperature F=0.026, df=1, P>0.05).
Phyllobates lugubris
Playbacks of the call of P. lugubris resulted in a significant change in call rate between during playback period and post-playback period only, although call rate appears to be approaching significance for pre-playback vs. during playback (repeated measures ANOVA: F=397.25, df=1, 14, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P>0.05, post-playback vs. during playback P<0.001). Call rate differences between pre- and post-playback periods were not significant (P>0.05). Call bout duration and percent time calling differences were not found to be significant in response to P. lugubris calling (call bout duration: repeated measures ANOVA: F=0.827, df=1, 11, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P>0.05, post-playback vs. during playback P>0.05, pre-playback vs. post-playback P>0.05; percent time calling: repeated measures ANOVA: F=0.827, df=1, 11, P<0.001; Sidak post-hoc comparisons: pre-playback vs. during playback P>0.05, post-playback vs. during playback P>0.05, pre-playback vs. post-playback P>0.05). Mean dominant frequency significantly changed between pre- and post-playback periods (Paired samples t-test: t=2.821, df=14, P<0.05, N=15) as did the mean first-call latency (t=4.115, df=14, P<0.01). Neither of the covariates was found to significantly affect call rate (Sidak post-hoc comparisons test: Snout-Vent Length F=0.269, df=1, P>0.05; Temperature F=4.232, df=1, P>0.05).
DISCUSSION
Since calling is such an energetically expensive activity, many anurans adjust their calling pattern to avoid acoustic interference (Awbrey 1978, Bucher et al. 1982, Zelick & Narins 1982, 1985, Taigen & Wells 1989). In addition to altering call timing, they may change other call parameters, since significant correlations exist between aerobic metabolic costs and signaling rate or duration (Taigen et al. 1985, Lopez et al., 1988, Gerhardt & Huber 2002). To choose to call during periods of high levels of acoustic activity would be maladaptive given the higher probability that the call will be masked, thus resulting in wasted energetic effort. Call suppression is a common response used by anurans to minimize acoustic interference and to mediate the energetic cost of advertisement calls (Zelick & Narins 1983; Penna & Hamilton-West 2007).
In all experimental trials (n=57), O. pumilio males responded by decreasing their calling rates when an acoustically interfering stimulus was broadcast (Table 1). Females of many anuran species prefer males producing high call rates (Lopez & Narins 1991, Pröhl 2003), however calling at high rates that are energetically costly during periods when there is a significant chance of signal masking is clearly non-optimal. Nevertheless, since males with high calling persistence have greater mating success (Wells 1977, Roithmair 1994), it would be maladaptive to cease calling altogether. Therefore, in the face of acoustic interference, O. pumilio males instead choose to decrease their call rates, a compromise that allows them to maintain advertisement call emission while reducing energy expenditure. A similar response has been observed in two sympatric species, Dendropsophus ebraccatus and D. microcephalus, in which males decrease their calling rates in order to reduce acoustic interference with each other (Schwartz & Wells 1983). Although on average no significant change was found between pre- and post-playback call rates in O. pumilio, it should be noted that the post-playback call rates did not change consistently. Some individuals increased their post-playback calling rate (n=36), and others (n=21) decreased it. Thus, over the population sampled, these opposing changes effectively cancelled each other out. This differential response to the experimental stimulus still remains to be thoroughly investigated.
TABLE I.
Mean values ± standard deviations of the call parameters of O. pumilio in response to playback of experimental stimuli.
| Parameter | Stimulus | Cicada | Tree Cricket | Ground Cricket | P. lugubris |
|---|---|---|---|---|---|
| Call rate (call notes/s) | Pre-playback | 4.01 ± 0.88*** | 4.64 ± 0.93*** | 4.12 ± 1.08** | 3.97 ± 0.86 |
| During playback | 0.70 ± 1.29 | 1.82 ± 1.73 | 2.77 ± 0.73 | 3.20 ± 1.08 | |
| Post-playback | 3.75 ± 1.72** | 4.61± 1.64** | 4.47 ± 1.08* | 4.34 ± 0.79*** | |
| Call bout duration (s) | Pre-playback | 14.01 ± 3.63* | 17.23 ± 3.14*** | 13.01 ± 2.35 | 15.04 ± 3.87 |
| During playback | 7.53 ± 5.79 | 5.96 ± 4.46 | 10.39 ± 3.13 | 12.53 ± 5.24 | |
| Post-playback | 12.18 ± 3.94 | 13.08 ± 5.37* | 10.07 ± 4.83 | 12.60 ± 4.12 | |
| Percent Time calling | Pre-playback | 60.37 ± 15.64* | 74.26 ± 13.54*** | 56.07 ± 10.14 | 64.82 ± 16.69 |
| During playback | 32.46 ± 24.94 | 25.71 ± 19.24 | 44.79 ± 13.51 | 54.01 ± 22.61 | |
| Post-playback | 52.52 ± 16.93 | 56.41 ± 23.16* | 43.40 ± 20.80 | 54.33 ± 17.76 | |
| Dominant frequency (kHz) | Pre-playback | 4.74 ± 0.19 | 4.80 ± 0.15 | 4.64 ± 0.76 | 4.90 ± 0.18 |
| Post-playback | 4.75 ± 0.19 | 4.69 ± 0.22 | 4.72 ± 0.31 | 4.84 ± 0.17* | |
| First-call latency (s) | Pre-playback | 26.82 ± 2.32 | 27.44 ± 3.73 | 26.40 ± 8.97 | 27.32 ± 3.93 |
| Post-playback | 22.89 ± 7.38 | 19.64 ± 7.04*** | 17.31 ± 9.83 | 19.76 ± 5.95** |
For call rate, call bout duration and percent time calling, measurements were taken for pre-stimulus playback, during stimulus playback and post-stimulus playback periods. We were unable to measure dominant frequency and first-call latency during stimulus playbacks, therefore only pre- and post-playback values were analyzed. For call rate, call bout duration and percent time calling, asterisks indicate the level of significance difference between pre-/post-playback periods and during playback periods, and for dominant frequency and first call latency, asterisks indicate the level of significance difference between pre- and post-playback periods
P < 0.05;
P < 0.01;
P < 0.001
In addition to modifying their call rate, O. pumilio males are able to alter their call bout duration and percent time calling in the presence of an interfering stimulus. Furthermore, they are able to do so selectively. Significant decreases in call bout duration and percent time calling were found in response to tree cricket and cicada calling, but not for ground cricket and P. lugubris calling. O. pumilio significantly decreased call bout duration and percent time calling during tree cricket broadcasts and post-playback, increased both parameters significantly. However in response to cicada broadcasts, O. pumilio calling did not recover as quickly, and therefore during and post-playback values did not significantly differ. This showed that in the post-playback silence, the frog was still affected by the stimulus. Cicadas are the loudest known insects (Bennet-Clark 1999), therefore it is reasonable that they would generate a longer lasting response.
Dominant frequency significantly changed only in response to playbacks of Phyllobates lugubris calls. Following stimulus playback, males of O. pumilio lowered their dominant frequency on average (Table 1). The dominant frequency (4.65 kHz) of the P. lugubris call is the closest of all stimuli tested to the average O. pumilio calling frequency (4.77 kHz). Anurans often use frequency to assess other males (Davies & Halliday 1978, Wagner 1989). For example, males of Acris crepitans blanchardi will often lower the dominant frequency of their calls to advertise their (exaggerated) fighting prowess and to repel intruding males (Wagner 1989). Moreover, males of Leptodactylus albilabris will shift their dominant calling frequency to match the frequency of the stimulus as a precursor to aggressive behavior (Lopez et al. 1988). Similarly, the isolated calls of P. lugubris closely resemble those of O. pumilio and could therefore be considered a potent acoustic masker for O. pumilio calls. However, since interspecific aggressive interactions have not been reported in dendrobatid frogs, males of O. pumilio may be engaging in deliberate masking of P. lugubris’s calls, a behavior similar to that reported from males of the Central American hylid, Smilisca sila (Ryan 1986).
First-call latency was found to significantly decrease after playbacks of P. lugubris and tree cricket calls (Table 1), two species sympatric with O. pumilio. Since P. lugubris, tree crickets and O. pumilio all share the same microhabitat, it is likely that males of O. pumilio have habituated to P. lugubris and tree cricket calls and therefore will more readily vocalize (shorter first call latency) than they would in response to an unfamiliar call. In contrast, the ground cricket recording was taken from the edge of the rainforest where no O. pumilio were observed calling. Therefore first-call latency might be expected to be longer for ground cricket should O. pumilio males require increased processing time to assess this “novel” acoustic stimulus.
A second possible explanation for the observed decrease in first-call latency is purely mechanistic. The ground cricket calls at a dominant frequency of 3.27 kHz, which is markedly lower than that of O. pumilio at 4.77 kHz and thus would be expected to be a relatively ineffective masker. The anuran auditory system is often tuned to certain species-specific bands (Capranica & Moffat 1983). Therefore, not all acoustic frequencies will have the same masking effect (Wollerman 1999). Neurophysiologically, signals falling at the edge of the receiver passband exhibit longer response latencies than those near the center of the passband (Hau et al. 2004).
Temperature did not significantly affect call rate in response to any of the stimuli tested. Although call rate has been found to be highly temperature-dependent (Zweifel 1968, Gerhardt & Huber 2002, Pröhl 2003, Pröhl et al. 2007), this was not a significant factor in our study since the temperature variation was minimal throughout the experimental trials (mean ± S.D.: 28.4° ± 0.2°C).
Snout-vent length did produce significant effects on call rate. Snout-vent length was significant only for responses to cicada playback. Snout-vent length (SVL) has previously been found to be a non-significant factor in determining calling characteristics in O. pumilio (Pröhl 2003, Graves et al. 2005). At present we can offer no biological explanation for the effect of SVL on call rate in response to cicada playback.
Terrestrial frogs such as Oophaga pumilio, which inhabit lowland wet tropical forests characterized by high levels of background noise, must contend with both conspecific and heterospecific acoustic interference on a day-to-day, minute-to-minute and even second-to-second basis (Gerhardt & Huber 2002, Wells 2007). Páez et al. (2003) were the first to document a case of heterospecific acoustic interference on frog calling from a non-anuran species. Our study was able to confirm their findings and expand them to include the effects of other co-occurring species. Furthermore, we found that O. pumilio has the ability to selectively alter particular call parameters to differentially respond to stimuli. The study of non-anuran acoustic interference on anuran vocalizations is relatively novel. Call rate, call bout duration, percent time calling, dominant frequency and first-call latency are just a few of the many parameters that could be examined in order to characterize this phenomenon more closely. In males of O. pumilio, call suppression resulted when the interfering stimulus lasted for 50% of the mean spontaneous call length. Eleutherodactylus coqui and E. portoricensis males have been shown to suppress vocalizations during interfering tone bursts as well, however when the length of the tone burst was increased, a decrease in suppression was observed (Zelick & Narins 1983). It would be interesting to compare the response of males of O. pumilio to experimental stimuli of increasing durations.
Lastly, one of the inevitable consequences of increasing deforestation and human development in Central America is higher levels of anthropogenic noise - potential competition for a wide variety of acoustically communicating organisms. We believe that a fruitful field for future bioacoustics research should include the study of the effects of anthropogenic noise on New World amphibians.
Acknowledgments
We would like to thank Greg Grether for his advice in the initial project design, and Kris Kaiser for her invaluable wisdom, help and encouragement. Additional thanks go to the staff at Refúgio Bartola, for their kindness and hospitality. Michael Greenfield and Scott Brandes aided with the identification of the invertebrate species. This project was carried out in part under the auspices of the Field Biology Quarter at UCLA. Supported by the Office for Instructional Development at UCLA and by NIH grant DC00222 to PMN.
LITERATURE CITED
- Awbrey FT. Social interactions among chorusing Pacific tree frogs, Hyla regilla. Copeia. 1978;1978:208–214. [Google Scholar]
- Bee MA, Perrill SA, Owen PC. Size assessment in simulated territorial encounters between male green frogs (Rana clamitans) Behav Ecol Sociobiol. 1999;45:177–184. [Google Scholar]
- Bennet-Clark HC. Resonators in insect sound production: How insects produce loud pure-tone songs. J Exp Biol. 1999;202:3347–3357. doi: 10.1242/jeb.202.23.3347. [DOI] [PubMed] [Google Scholar]
- Brandes TS, Naskrecki P, Figueroa HK. Using image processing to detect and classify narrow-band cricket and frog calls. J Acoust Soc Am. 2006;120:2950–2957. doi: 10.1121/1.2355479. [DOI] [PubMed] [Google Scholar]
- Bucher TL, Ryan MJ, Bartholomew GA. Oxygen consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiol Zool. 1982;55:10–22. [Google Scholar]
- Bunnell P. Vocalizations in the territorial behavior of the frog Dendrobates pumilio. Copeia. 1973;1973:277–284. [Google Scholar]
- Capranica RR, Moffat AJ. Neurobehavioral correlates of sound communication in anurans. In: Ewert JP, Capranica RR, Ingle DJ, editors. Advances in Vertebrate Neuroethology. Plenum Press; New York, USA: 1983. pp. 701–730. [Google Scholar]
- Davies NB, Halliday TR. Deep croaks and fighting assessment in toads Bufo bufo. Nature. 1978;274:683–685. [Google Scholar]
- Drewry GE. Puerto Rico Nuclear Center Rain Forest Project Annual Report. 1970. The role of amphibians in the ecology of Puerto Rican rainforest; pp. 16–63. [Google Scholar]
- Gerhardt HC. The evolution of the vocalization in frogs and toads. Ann Rev Ecol Syst. 1994;25:293–324. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans. The University of Chicago Press; Chicago: 2002. [Google Scholar]
- Gerhardt HC, Schwartz JJ. Interspecific interactions in anuran courtship. In: Heatwole H, Sullivan B, editors. Amphibian Biology. Vol. 2. Surrey Beatty & Sons, Chipping Norton; Australia: 1995. pp. 603–632. [Google Scholar]
- Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC. Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae) 299. Bulletin of the American Museum of Natural History; New York, USA: 2006. pp. 1–262. [Google Scholar]
- Graves BM, Stanley KA, Gardner EA. Correlates of vocal display in a Costa Rican population of Strawberry Poison-Dart Frogs, Dendrobates pumilio. J Herpetol. 2005;39:101–107. [Google Scholar]
- Hau LW, Narins PM. Program No. 530.6 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. Auditory-nerve fiber response latencies in the northern leopard frog, Rana pipiens. [Google Scholar]
- IRENA, Instituto de Recursos Naturales. Plan de acción forestal. Managua, Nicaragua: IRENA; 1992. [Google Scholar]
- Littlejohn MJ, Martin AA. Acoustic interaction between two species of Leptodactylid frogs. Anim Behav. 1969;17:785–791. [Google Scholar]
- Lopez PT, Narins PM. Mate choice in the neotropical frog, Eleutherodactylus coqui. Anim Behav. 1991;41:757–772. [Google Scholar]
- Lopez PT, Narins PM, Lewis ER, Moore SW. Acoustically-induced call modification in the white-lipped frog, Leptodactylus albilabris. Anim Behav. 1988;36:1295–1308. [Google Scholar]
- McVey ME, Zahary RG, Perry D, MacDougal J. Territoriality and homing behavior in the poison dart frog (Dendrobates pumilio) Copeia. 1981;1981:1–8. [Google Scholar]
- Narins PM. Effects of masking noise on evoked calling in the Puerto Rican Coqui (Anura: Leptodactylidae) J Comp Physiol. 1982;147:438–446. [Google Scholar]
- Narins PM. Frog Communication. Sci Am. 1995;273:78–83. [Google Scholar]
- Narins PM, Capranica RR. Communicative significance of the two-note call of the treefrog Eleutherodactylus coqui. J Comp Physiol. 1978;127:1–9. [Google Scholar]
- Narins PM, Zelick R. The effects of noise on auditory processing and behavior in amphibians. In: Fritszch B, Ryan M, Wilczynski W, Hetherington T, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. John Wiley & Sons; New York, USA: 1988. pp. 511–536. [Google Scholar]
- Nygren A. Contested lands and incompatible images: The political ecology of struggles over resources in Nicaragua’s Indio Maíz Reserve. Soc Nat Res. 2004;17:189–205. [Google Scholar]
- Páez V, Bock BC, Rand AS. Inhibition of evoked calling of Dendrobates pumilio due to acoustic interference from cicada calling. Biotropica. 1993;25(2):242–245. [Google Scholar]
- Penna M, Hamilton-West C. Susceptibility of evoked vocal responses to noise exposure in a frog of the temperate austral forest. Anim Behav. 2007;74:45–56. [Google Scholar]
- Portig WH. Central American rainfall. Geog Rev. 1965;55:68–90. [Google Scholar]
- Pröhl H. Variation in male calling behaviour and relation to male mating success in the strawberry poison frog (Dendrobates pumilio) Ethol. 2003;109:273–290. [Google Scholar]
- Pröhl H. Territorial behavior in dendrobatid frogs. J Herpetol. 2005;39:354–365. [Google Scholar]
- Pröhl H, Hagemann S, Karsch J, Höbel G. Geographic variation in male sexual signals in Strawberry Poison Frogs (Dendrobates pumilio) Ethol. 2007;113:825–837. [Google Scholar]
- Roithmair ME. Male territoriality and female mate selection in the dart-poison frog Epipedobates trivittatus (Dendrobatidae, Anura) Copeia. 1994;1994:107–115. [Google Scholar]
- Ryan MJ. Synchronized calling in a treefrog (Smilisca sila) Brain Behav Evol. 1986;29:196–206. doi: 10.1159/000118681. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Rand AS. The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (sexual selection for sensory exploitation) Evolution. 1990;44:305–314. doi: 10.1111/j.1558-5646.1990.tb05200.x. [DOI] [PubMed] [Google Scholar]
- Savage JM. The Dendrobatid frogs of Central America. Copeia. 1968;1968:745–776. [Google Scholar]
- Schwartz JJ, Wells KD. An experimental study of acoustic interference between two species of neotropical frogs. Anim Behav. 1983;31:181–190. [Google Scholar]
- Taigen TL, Wells KD. The effect of social interactions on calling energetics in the gray treefrog Hyla versicolor. Behav Ecol Sociobiol. 1986;19:9–18. [Google Scholar]
- Taigen TL, Wells KD. Calling energetics of a neotropical treefrog Hyla microcephala. Behav Ecol Sociobiol. 1989;25:13–22. [Google Scholar]
- Taigen TL, Wells KD, Marsh RL. The enzymatic basis of high metabolic rates in calling frogs. Physiol Zool. 1985;58:719–726. [Google Scholar]
- Van Staaden MJ, Rómer H. Sexual signaling in bladder grasshoppers: Tactical design for maximizing calling range. J Exp Biol. 1997;200:2597–2608. doi: 10.1242/jeb.200.20.2597. [DOI] [PubMed] [Google Scholar]
- Wagner WE. Graded aggressive signals in Blanchard's cricket frog: vocal responses to opponent proximity and size. Anim Behav. 1989;38:1025–1038. [Google Scholar]
- Wells KD. The social behaviour of anuran amphibians. Anim Behav. 1977;25:666–693. [Google Scholar]
- Wells KD. The Ecology and Behavior of Amphibians. The University of Chicago Press; Chicago: 2007. [Google Scholar]
- Wollerman L. Acoustic interference limits call detection in a neotropical frog Hyla ebraccata. Anim Behav. 1999;57:529–536. doi: 10.1006/anbe.1998.1013. [DOI] [PubMed] [Google Scholar]
- Zelick RD, Narins PM. Analysis of acoustically evoked call suppression behaviour in a neotropical treefrog. Anim Behav. 1982;30:728–733. [Google Scholar]
- Zelick RD, Narins PM. Intensity discrimination and the precision of call timing in two species of neotropical tree frogs. J Comp Physiol. 1983;153:403–412. [Google Scholar]
- Zelick RD, Narins PM. Characterization of the advertisement call oscillator in the frog Eleutherodactylus coqui. J Comp Physiol. 1985;156:223–229. [Google Scholar]
- Zweifel RG. Effects of temperature, body size, and hybridization on mating calls of toads, Bufo americanus and Bufo woodhousii fowleri. Copeia. 1968;1968:269–285. [Google Scholar]