Abstract
We report the results of anatomical and vibrometric studies of the middle ear of the African clawed frog, Xenopus laevis. The cartilaginous tympanic disk of Xenopus shows pronounced sexual dimorphism, that of male frogs being much larger than that of females, relative to body size. The stapes footplate, however, is not enlarged in males. The cucullaris muscle was found to insert on the stapes in frogs of both sexes. Using laser interferometry to examine the response of middle ear structures to airborne sound, the stapes footplate was found to vibrate close to 180° out-of-phase with the tympanic disk across a range of frequencies, this resembling the relationship between tympanic membrane and footplate movement previously described in ranid frogs. By contrast, whereas there is a pronounced difference in vibration velocity between tympanic membrane and footplate in ranids, the footplate vibration velocity in Xenopus was found to be similar to that of the tympanic disk. This may be interpreted as an adaptation to improve the detection of sound underwater.
2 INTRODUCTION
Xenopus laevis (family Pipidae) is a well-known laboratory animal, used primarily in developmental biology studies [1]. Wild Xenopus have a widespread distribution in sub-Saharan Africa, where they live in a range of different water bodies, favouring stagnant ponds in open, savanna habitats [2]. Although it may travel overland when necessary, for instance to escape a drying pond, Xenopus is regarded as an aquatic (rather than an amphibious) frog, spending nearly all of its time in water [3,4]. Its anatomy, including that of its sensory systems, is specialised towards an aquatic existence: unlike most frogs, Xenopus retains its lateral-line systems even after metamorphosis [5], while its eyes are dorsally situated, probably so as to watch for predators as it floats at the water surface [4]. The middle ear is also unusual compared to that of most other frogs: lacking a tympanic membrane, pipids possess instead a cartilaginous “tympanic disk”, located just behind the eye and covered by skin and fatty tissue [6].
In many vertebrates, sensitive hearing underwater is associated with the presence of a bubble of air somewhere in the body, which, being compressible, will expand and contract in response to the sound-induced pressure fluctuations in the surrounding tissue [7,8]. The resulting displacements of the wall of the bubble are coupled in some way to the inner ear, resulting in the activation of auditory mechanoreceptors. In fishes, the presence of such an air-bubble (typically the swim-bladder) seems to be associated with enhanced hearing sensitivity or an expanded audible frequency range, in comparison with species which must rely on the detection of acoustic particle displacement directly [9]. Inner ear microphonic studies of both ranid frogs and Xenopus showed that these amphibians are sensitive to the pressure component of underwater sound, at frequencies from 0.2–3 kHz, and it is believed that the middle ear cavity represents the pulsating air-bubble which allows this [10]. Vibrations of the middle ear cavity are thought to result in movement of the tympanic membrane or disk, coupled via the stapes to the inner ear [10].
Christensen-Dalsgaard et al. [11,12] used laser interferometry to measure directly the vibration velocity of the surgically-exposed tympanic disk in Xenopus. The velocity amplitude of the disk was found to be up to around 40 dB higher than that of the water particles or surrounding tissue, but it was greatly reduced when the middle ear cavities were filled with water. There were two peaks in the tympanic disk response curve, one at 0.6–1.1 kHz corresponding to a response peak in the vibrations of the body wall overlying the lungs, and one at 1.6–2.2 kHz, believed to result from pulsations of the air contained within the middle ear cavity. Expansion of the middle ear cavity volume as Xenopus grows is associated with a reduced frequency of best hearing sensitivity, measured as the auditory brainstem response, conforming to the predictions of an “air-bubble” model [13]. The mouth cavity is believed to be water-filled when Xenopus is underwater [6], but pressure-induced pulsations of the lungs appear to be coupled through to the middle ear cavity via the larynx, which unusually in this frog fits within a recess in the roof of the mouth representing the shared opening of the Eustachian tubes [12]. The connection between the two middle ear cavities, via this recess, might in principle allow the ears to work as a pressure difference receiver, facilitating sound localization [12].
Christensen-Dalsgaard et al. [11,12] found that although the sound pressure sensitivities of the Xenopus ear are similar in air and water, sensitivity to sound intensity, a more appropriate comparison, is 30 dB lower in air. These authors also calculated that the peak vibration amplitude of the Xenopus tympanic disk underwater is likely to be similar to that of ranid frogs, which have much better hearing in air. The paradox that Xenopus appears not to benefit from its “aquatic” specialization relative to other frogs could be resolved, they suggested, if the ossicular coupling between tympanic disk and inner ear in Xenopus is tighter than in ranids, such that a given disk vibration results in a greater stapes footplate movement. We here report the results of anatomical and laser interferometric studies of the Xenopus middle ear, extending our knowledge of its structure and function. Preliminary results were presented by Wang [14].
3 MATERIALS AND METHODS
Anatomical measurements were taken from the left ears of 20 female and 10 male Xenopus laevis specimens, obtained as corpses from this and other experimental projects. Snout-vent length, used as an index of body size, was measured to the nearest 0.1 mm using calipers, and the specimens were then dissected and examined under light microscopy. The areas of the stapes footplate and tympanic disk were either obtained manually from scale drawings constructed using a microscope fitted with a graticule eyepiece, or from photomicrographs taken with a Moticam 2000 digital camera, using image analysis software (Motic Images Plus 2.0).
Laser vibrometric measurements were made on 12 healthy adult Xenopus laevis specimens, 7 females and 5 males, obtained from a commercial supplier. The frogs were deeply anaesthetized with intramuscular injections of pentobarbital sodium solution (Nembutal, Abbott Laboratories, 50 mg/ml: 1 µl/g body mass) and ketamine (Ketaject, Phoenix Scientific, 100 mg/ml: 1 µl/g body mass). Supplementary doses were given as necessary to maintain areflexia. The frogs were sprayed regularly with water to facilitate cutaneous respiration. After the experiments the animals were euthanized by an overdose of Nembutal, and the corpses were dissected. The “Principles of Animal Care” (NIH publication 85-23, revised 1985) and USA regulations were followed throughout this study, and protocols were approved by the University of California Animal Research Committee.
The laser vibrometric measurements were made on the left middle ear structures. One of two experimental procedures was chosen. In procedure one, the tympanic disk was surgically exposed and its vibratory response measured before and after stapes footplate exposure. In procedure two, the footplate was surgically exposed first and its response was examined before and after tympanic disk exposure. To expose the tympanic disk, the overlying skin and fat were carefully removed, and a small square of reflective beads (Polytec) was placed on its centre, at the position of the distal tip of the pars media of the stapes. To expose the stapes footplate, a piece of skin (about 5×5 mm) above its approximate location was removed. The m. depressor mandibulae was either moved aside or partially excised to reveal the caudal edge of the parotic crest (part of the skull near to the stapes) and the stapes, over which runs a nerve branch and the vena capitis lateralis. The vein was carefully moved aside, whereupon individual reflective beads (40–60 µm diameter) were placed upon the footplate, approximately at the junction between pars media and pars interna. A reflective bead was also placed on the parotic crest: vibrations of this region, representing background vibrations of the whole head, were recorded as a control.
The animal was placed on its belly on a vibration-isolated table (Backer-Loring Micro-g) within a double-walled, sound-attenuating chamber (IAC 1202-A). A single point, He-Ne laser vibrometer sensor head (Polytec OFV-303) was positioned about 60 cm from the animal’s head. The beam could be aimed either at the tympanic disk directly, or deflected vertically downwards onto the stapes footplate or parotic crest via a prism mounted in a binocular light microscope (Zeiss OP-1). A 10 cm diameter speaker (Analog and Digital Systems Inc. 300) was positioned 75 cm from the centre of the frog’s interaural axis, at an azimuth of 30° to the midline. The speaker was not resting on the same table as the frog. The rest of the apparatus was located outside the chamber.
Two-second duration pure tones, running from 150 Hz to 3 kHz in 30 Hz steps, were synthesized by a custom program (Acoustic Analyzer 0.20β: A. Purgue, 1999) running on an Apple Macintosh iMac computer. The output of the computer’s 16-bit D/A board was amplified (Optimus MPA-50) and sent to the speaker; a returning signal from the amplifier was used as a reference signal for the phase measurements. A probe microphone (Knowles Electronics, EK-3033), positioned around 3 mm from the tympanic disk of the frog, was used to calibrate the sound pressure level at 90±3 dB SPL across frequency at this position. The microphone was then removed and the laser sensor head was aimed at the structures of interest in the frog ear. The sensor head was connected to a vibrometer controller processor (Polytec OFV-3001), the output of which was fed to the computer via a band-pass filter (Krohn-Hite 3323 active filter) and an attenuator (Hewlett-Packard 350D). The sampling frequency was 44.1 kHz. The response of each structure was usually measured three times and compared, to ensure consistency of response.
The stapes footplate is angled at around 45° to the horizontal, but only the vertical components of its vibrations were measured. The measured amplitude was therefore expected to be around 3 dB below the true amplitude, so all measured stapes responses were increased by 3 dB. It was estimated that a line normal to the centre of the tympanic disk, representing its direction of motion in response to sound, would extend at about 30° to the horizontal, while the angle of the laser when pointed at the disk centre was around 25–28° to the horizontal: it was assumed that the very small difference in these angles could safely be ignored.
4 RESULTS
4.1 ANATOMICAL RESULTS
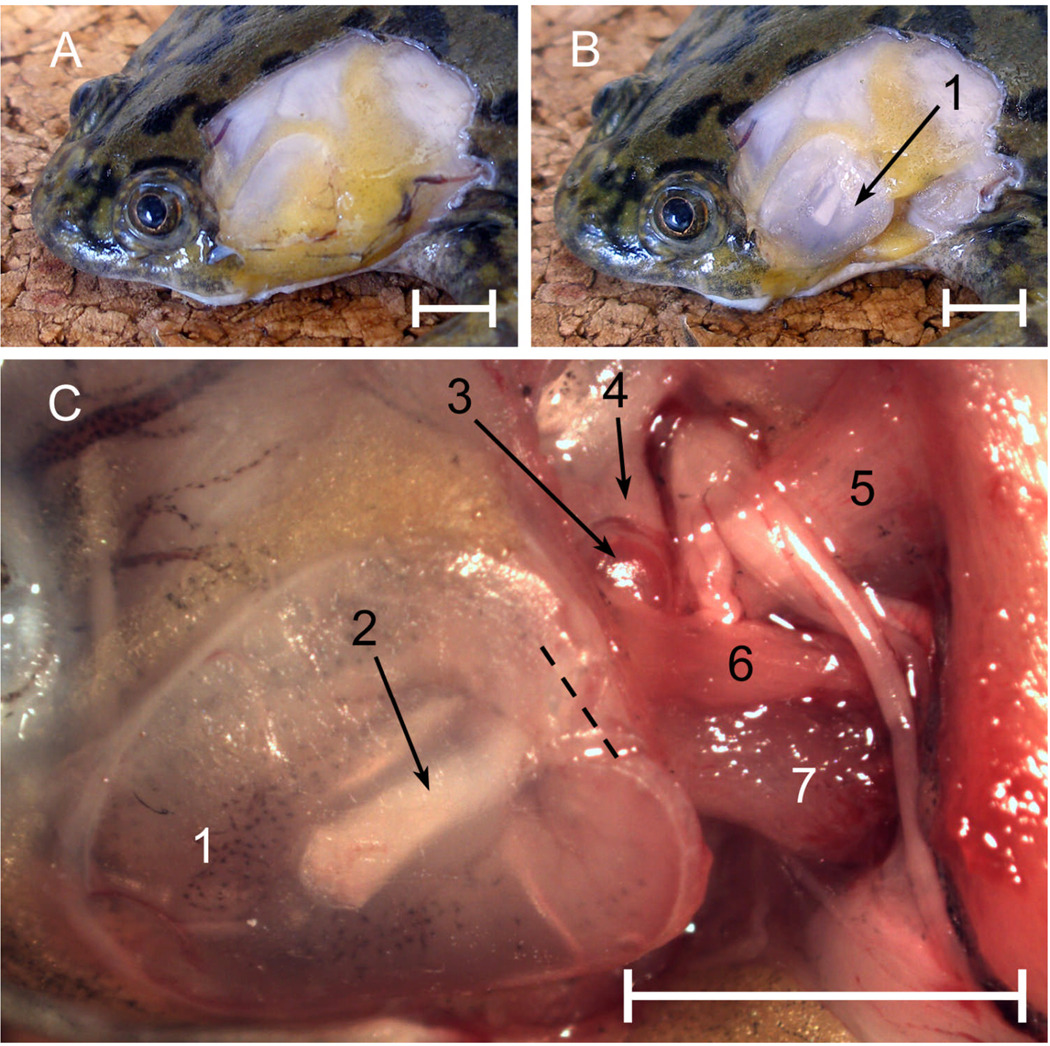
Immediately under the skin, the auditory region of Xenopus is covered with a layer of bright yellow fat, which extends under the throat and is continuous with that on the other side (Fig. 1A). This fat covers the tympanic disk to a variable extent in different individuals, the dorsorostral part of the disk often remaining uncovered. The fat is not firmly attached to the disk itself and can easily be removed, revealing the translucent disk cartilage through which the pars media of the stapes can be seen (Fig. 1B). The tympanic disk is slightly cardioid in shape: in males it is slightly concave externally, whereas in females it is convex. The cartilaginous tympanic annulus, to which the disk is attached around its circumference by connective tissue, is more extensive and flatter in male frogs, while in females the annulus encircles the disk. The disk is also tethered to the parotic crest dorsocaudally by a short band of cartilage probably representing the ascending process (processus ascendens plectri), which arises from its medial side. Removing the disk reveals the shallow, air-filled middle ear cavity, which tapers to form the Eustachian tube. The single, shared opening of the right and left tubes is obvious in the roof of the mouth.
Figure 1.
Left middle ear structures in male Xenopus laevis. A: Skin removed to reveal fatty pad. B: Tympanic disk, after removal of some of the fat. C: Ear region after further dissection, dorsolateral view. Key: 1, tympanic disk; 2, pars media of stapes, seen through disk cartilage; 3, pars interna of stapes; 4, parotic crest; 5, m. levator scapulae superior; 6, m. petrohyoideus; 7, m. cucullaris. Dashed line = approximate position of the rotatory axis. Scale bars represent 5 mm.
Caudal to the tympanic disk and originating in part from the annulus is the m. depressor mandibulae, which must be removed together with some underlying fat and connective tissue to reveal the stapes. The bony pars media of the stapes is shaped like a bent rod. Its distal half is firmly embedded within the tympanic disk, extending from the disk centre caudally and dorsally (Fig. 1C). Turning inwards, it reaches the oval window at a shallow angle. The footplate has two components, the proximal end of the pars media and the cartilaginous pars interna, which covers the inner surface and forms the footplate tip. The footplate is only loosely connected within the oval window. Prodding the disk revealed that the stapes moves as if hinged somewhere near the middle of the pars media, where it passes through the tympanic annulus: the approximate position of this axis is indicated in Fig. 1C. There was no operculum in any of the specimens examined.
There is a small, bony projection on the ventral side of the stapes in the hinge region, just proximal to which inserts a component of the m. cucullaris; the majority of this muscle arises from the adjacent otic capsule and tympanic annulus. Other muscles associated with the middle ear region include the superior and inferior components of the m. levator scapulae, which insert on the otic capsule caudal to the oval window, and the long, strap-like m. petrohyoideus, which originates on the parotic crest and annulus, passes over the stapes and ultimately inserts on the ventral surface of the larynx. In some of the female specimens, branches of the petrohyoideus were found in the ear region: a few fibres arose from the pars media of the stapes and others with the m. cucullaris.
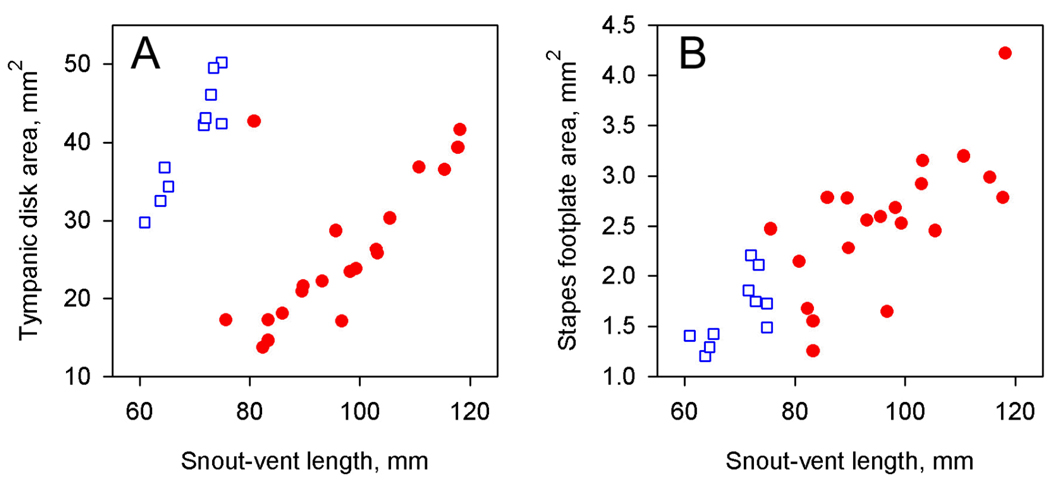
The tympanic disk is relatively much larger in male Xenopus than in females, given the small body size of male animals, although in absolute terms the areas are similar (Fig. 2A). One female data-point clusters with the males in Fig. 2A: it is likely that this animal was wrongly sexed. In both male and female frogs, the disk area increases with snout-vent length. Although male frogs tend to have smaller footplates than females on average (Fig. 2B), there is no evidence to suggest that this is anything other than a function of their smaller body size. The mean anatomical area ratio of male frogs, defined as disk area divided by footplate area, is 25.0 (n=10), compared with only 10.3 in females (n=20).
Figure 2.
Measurements of (A) tympanic disk area and (B) stapes footplate area in Xenopus laevis, plotted against snout-vent length. Blue squares = males, red circles = females.
4.2 LASER VIBROMETRIC RESULTS
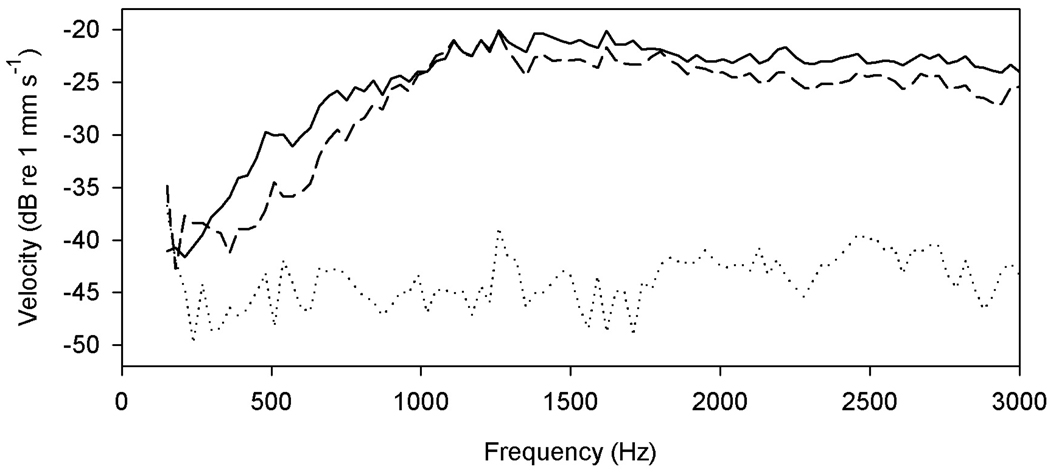
The velocity response of the exposed tympanic disk was quite variable between the 12 frogs examined. The typical pattern was an approximately high-pass response curve over the frequency range examined, the response rising at low frequencies and flattening off somewhere between 1 and 1.5 kHz (Fig. 3). In some individuals the response dropped somewhat at high frequencies, while in one case the disk response rose above that of the parotic crest only at the very highest frequencies. The stapes footplate response was typically similar to that of the tympanic disk in shape but was usually of lower amplitude: it was always very close to the “background” response of the parotic crest at the lowest frequencies examined, and sometimes at the highest frequencies as well. In two frogs, the footplate response never rose significantly above that of the parotic crest. There was no large or consistent change in the response of either the disk to stapes exposure (n=6 experiments), or the stapes to disk exposure (n=6 experiments). To ensure that further analysis was based on clear stapedial movement relative to the skull, lever and phase data were calculated only when the stapes response was more than an arbitrary value of 10 dB higher than that of the parotic crest. This never occurred at frequencies below 500 Hz.
Figure 3.
Responses to 90 dB SPL airborne sound of the exposed tympanic disk (solid line), stapes footplate (dashed line) and parotic crest (dotted line) in a female Xenopus, as measured with laser vibrometry. The responses of disk and stapes in other experiments were often lower in amplitude than this (closer to the crest), and the stapes did not always follow the tympanic disk so faithfully.
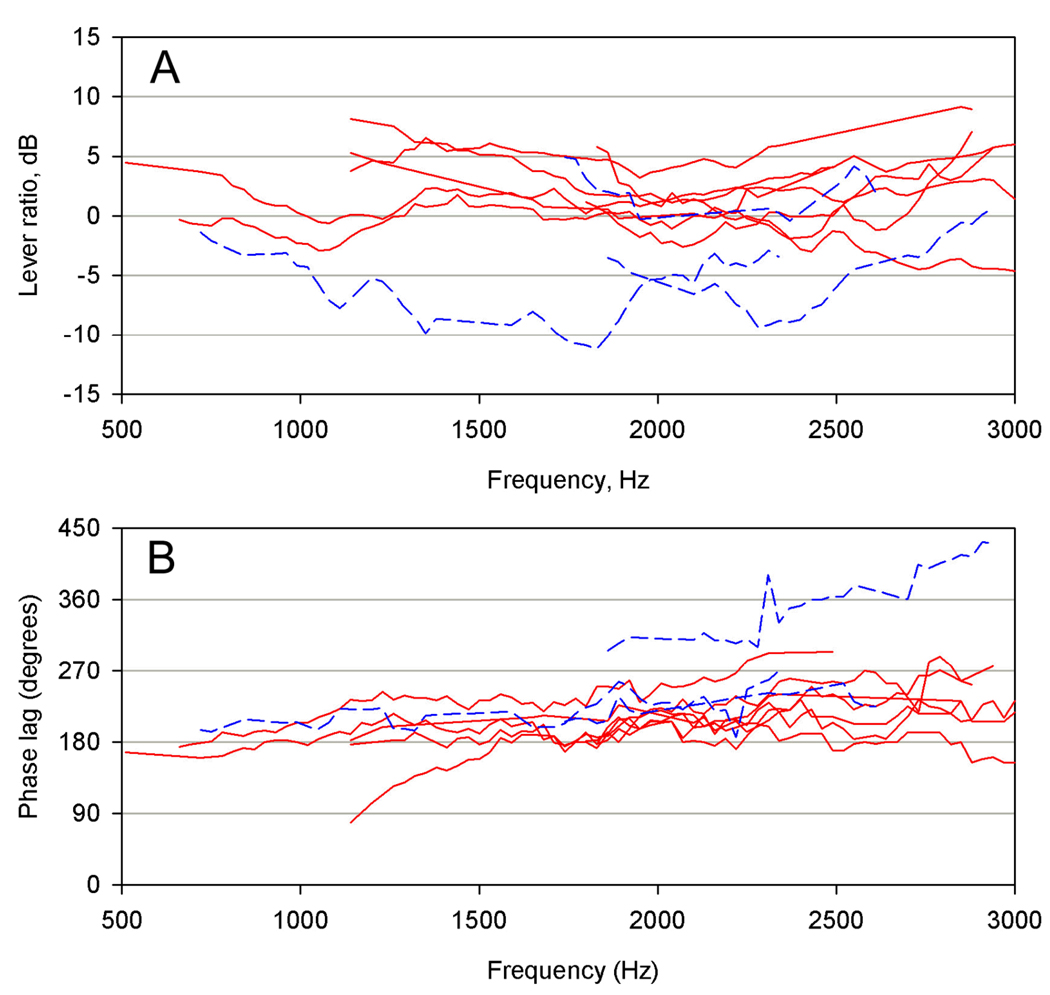
The lever ratio was defined as the dB difference between the velocity of the tympanic disk and that of the stapes footplate. Although quite variable between animals, the ratio was typically from 0–5 dB (Fig. 4A). Negative values, which were occasionally obtained, indicated that the footplate sometimes moved with a higher amplitude than the disk: this was the case in two of the three male frogs for which data were available. The phase lag between tympanic disk and stapes footplate responses typically began near 180° at low frequencies, rising slightly with frequency (Fig. 4B).
Figure 4.
Results of vibrometric experiments on 10 Xenopus frogs. Data were only included when the stapes response was >10 dB above that of the parotic crest. A: Lever ratios, calculated as the dB difference between tympanic disk and stapes footplate responses. B: Phase lags between tympanic disk and stapes footplate. Solid red lines = females, dashed blue lines = males.
5 DISCUSSION
The unusual ear structures of Xenopus are thought to have evolved from a more typical anuran morphology, adapted for hearing in air [6,15]. Wever [6] believed that the tympanic disk of pipids corresponds to the tympanic membrane of other frogs, but others e.g. [16,17,18] have considered this structure to be an expansion of the cartilaginous extrastapes, or pars externa plectri. The latter interpretation is supported by our discovery of a small ascending process arising from the disk, the presence of which was denied by de Villiers [19]. Wever described an “ossicular muscle” in Xenopus inserting (in part) on the pars media, but he was unable to establish its affinities. In the present study, this muscle was identified as the m. cucullaris, which has apparently not previously been described as inserting on the stapes of a frog, although it does insert on the fenestral plate occupying the oval window in plethodontid salamanders [20]. Its function in Xenopus is unclear, but Wever suggested that it might have a protective role. Elements of the opercular apparatus have been identified in the Xenopus embryo see e.g. [17,18,19], but the loss of the operculum in adult pipids might reflect their aquatic habits, given that this apparatus is thought to be involved in the detection of ground-borne vibrations transmitted via the forelimb see [21] for a review.
The difference in relative tympanic disk area in male and female Xenopus has been briefly noted before [15], but no quantitative data were presented. Sexual dimorphism is also pronounced in the ear of the bullfrog, Rana catesbeiana [22]. Male and female bullfrogs are of similar body size; males have larger tympanic membranes but their stapes footplate areas are not correspondingly enlarged, so male area ratios are larger than those of females [22]. In Xenopus, male frogs are much smaller than females, so although their tympanic disks are similar in absolute size to those of larger females, they are much larger in relation to body size. Stapes footplate areas, however, are not enlarged in males, and so male Xenopus, like bullfrogs, have larger area ratios than females. It is tempting to speculate that larger area ratios in male Xenopus compensate for reduced lever ratios, given that the two lowest lever ratios recorded in the present study were both from male frogs (Fig. 4A), but these particular lever measurements were unusually variable and so this requires further investigation.
The expanded eardrum of male bullfrogs has been proposed to radiate high-frequency components of their vocalizations [23]. It is notable that the larynx of Xenopus is sexually dimorphic, the enlarged male larynx being implicated in the production and radiation of click-like calls underwater [24,25]. It is at least possible that the (relatively) enlarged tympanic disks of male Xenopus are involved in the radiation of some component of this vocalization underwater, in a manner analogous to tympanic sound radiation in the bullfrog.
Comparison with data from Mason & Narins [26], obtained using a very similar experimental set-up, shows that the peak response of the exposed tympanic disk of Xenopus to airborne sound was typically around 25 dB lower than that of the tympanic membrane of the bullfrog. Although the frequency range examined here closely corresponds with the Xenopus hearing range [27], Xenopus is evidently relatively insensitive to airborne sound. Together with difficulties arising from the invasive surgery needed to expose the middle ear structures, this insensitivity presumably explains the rather variable and in some cases noisy recordings.
Ossicular lever ratios of up to around 30 dB have been experimentally measured in both bullfrogs and the grass frog, Rana temporaria [26,28]. These large values appear to be due to a rotatory axis located very close to the stapes footplate, together with flexion at the extrastapes/pars media junction [26,29]. In the present study, the lever ratio in Xenopus was found to be much lower, under 10 dB in all frogs and averaging around 1 dB. The lower values must relate in part to the fact that the extrastapes in this species does not take the form of an elongated lever element but instead forms the flattened tympanic disk, to which the pars media is firmly attached. Both ranids and Xenopus show a 180° phase difference between tympanic membrane/disk and stapes footplate at low frequencies, suggesting a rocking motion of the stapes, but in Xenopus the apparent location of the rotatory axis, roughly in the centre of the pars media, is further from the footplate than it is in the ranids, contributing to the lower lever ratio. The discovery of a rocking motion of the Xenopus stapes is contrary to the findings of Wever [6], who measured inner ear potentials in response to a vibrating needle pressed onto different parts of the middle ear apparatus, concluding that the disk and stapes simply move in and out together. It is possible that the surgery used by Wever to expose the middle ear structures, or the direction of the needle applied to the structures, somehow influenced his results.
Although the tympanic membrane of ranid frogs is expected to vibrate with similar amplitudes underwater to that of the tympanic disk of Xenopus, Christensen-Dalsgaard & Elepfandt [12] suggested that tighter coupling between the disk and stapes in Xenopus might result in greater footplate vibrations, and thus a more efficient transfer of sound to the inner ear. The results of the present study show that the lever ratio is much lower in Xenopus than in ranids, so a given disk vibration would indeed result in a greater footplate vibration. The Xenopus middle ear can therefore be regarded as specialized for underwater hearing.
ACKNOWLEDGEMENTS
The authors would like to express their thanks to P.S. Mischel, D.E. Simpson and F.C. Wardle for provision of Xenopus corpses for dissection. This work was supported by grant no. R01 DC00222 from the NIDCD, National Institutes of Health, to PMN, and a grant from St. Catharine’s College, Cambridge, to MJM.
Contributor Information
MJ Mason, Current address: Department of Physiology, Development & Neuroscience, University of Cambridge, Cambridge, UK.
M Wang, Current address: Kaiser Permanente, Fontana, USA.
PM Narins, Department of Physiological Science, UCLA, Los Angeles, USA.
REFERENCES
- 1.Gurdon JB, Hopwood N. The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 2000;44:43–50. [PubMed] [Google Scholar]
- 2.Tinsley RC, Loumont C, Kobel HR. Geographical distribution and ecology. In: Tinsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford: Clarendon Press; 1996. pp. 35–59. [Google Scholar]
- 3.Trueb L. Historical constraints and morphological novelties in the evolution of the skeletal system of pipid frogs (Anura: Pipidae) In: Tinsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford: Clarendon Press; 1996. pp. 349–377. [Google Scholar]
- 4.Deuchar EM. Xenopus: the South African Clawed Frog. London: John Wiley & Sons; 1975. [Google Scholar]
- 5.Elepfandt A. Sensory perception and the lateral line system in the clawed frog, Xenopus. In: Tinsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford: Clarendon Press; 1996. pp. 97–120. [Google Scholar]
- 6.Wever EG. The Amphibian Ear. Princeton: Princeton University Press; 1985. [Google Scholar]
- 7.Rogers PH, Cox M. Underwater sound as a biological stimulus. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory Biology of Aquatic Animals. New York: Springer-Verlag; 1988. pp. 131–149. [Google Scholar]
- 8.van Bergeijk WA. The evolution of vertebrate hearing. In: Neff WD, editor. Contributions to Sensory Physiology. vol 2. New York: Academic Press; 1967. pp. 1–49. [DOI] [PubMed] [Google Scholar]
- 9.Fay RR, Popper AN. The octavolateralis system. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. London: Belknap Press; 1985. pp. 291–316. [Google Scholar]
- 10.Hetherington TE, Lombard RE. Biophysics of underwater hearing in anuran amphibians. J. Exp. Biol. 1982;98:49–66. doi: 10.1242/jeb.98.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Christensen-Dalsgaard J, Breithaupt T, Elepfandt A. Underwater hearing in the clawed frog, Xenopus laevis. Tympanic motion studied with laser vibrometry. Naturwissenschaften. 1990;77:135–137. doi: 10.1007/BF01134478. [DOI] [PubMed] [Google Scholar]
- 12.Christensen-Dalsgaard J, Elepfandt A. Biophysics of underwater hearing in the clawed frog, Xenopus laevis. J. Comp. Physiol. [A] 1995;176:317–324. doi: 10.1007/BF00219057. [DOI] [PubMed] [Google Scholar]
- 13.Katbamna B, Brown JA, Collard M, Ide CF. Auditory brainstem responses to airborne sounds in the aquatic frog Xenopus laevis: correlation with middle ear characteristics. J. Comp. Physiol. [A] 2006;192:381–387. doi: 10.1007/s00359-005-0076-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang M. Middle ear structure and function in an aquatic frog, Xenopus laevis. UCLA Undergrad. Sci. J. 2001;14:110–116. [Google Scholar]
- 15.Elepfandt A. Underwater acoustics and hearing in the clawed frog, Xenopus. In: Tinsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford: Clarendon Press; 1996. pp. 177–193. [Google Scholar]
- 16.Trueb L, Hanken J. Skeletal development in Xenopus laevis (Anura: Pipidae) J. Morphol. 1992;214:1–41. doi: 10.1002/jmor.1052140102. [DOI] [PubMed] [Google Scholar]
- 17.Sedra SN, Michael MI. The development of the skull, visceral arches, larynx and visceral muscles of the South African clawed toad, Xenopus laevis (Daudin) during the process of metamorphosis (from stage 55 to stage 66) Verh. K. Acad. Wetenschappen, Afdeeling Natuurkunde, 2e Reeks. 1957:1–80. [Google Scholar]
- 18.Spannhof L. Die Entwicklung des Mittelohres und des schalleitenden Apparates bei Xenopus laevis Daudin. Z. Wiss. Zool. 1954;158:1–30. [Google Scholar]
- 19.de Villiers CGS. Über das Gehörskelett der aglossen Anuren. Anat. Anz. 1932;74:33–55. [Google Scholar]
- 20.Hetherington TE, Jaslow AP, Lombard RE. Comparative morphology of the amphibian opercularis system: I. General design features and functional interpretation. J. Morphol. 1986;190:43–61. doi: 10.1002/jmor.1051900105. [DOI] [PubMed] [Google Scholar]
- 21.Mason MJ. Pathways for sound transmission to the inner ear in amphibians. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. vol 28. New York: Springer; 2007. pp. 147–183. [Google Scholar]
- 22.Mason MJ, Lin CC, Narins PM. Sex differences in the middle ear of the bullfrog (Rana catesbeiana) Brain. Behav. Evol. 2003;61:91–101. doi: 10.1159/000069354. [DOI] [PubMed] [Google Scholar]
- 23.Purgue AP. Tympanic sound radiation in the bullfrog Rana catesbeiana. J. Comp. Physiol. [A] 1997;181:438–445. doi: 10.1007/s003590050127. [DOI] [PubMed] [Google Scholar]
- 24.Yager DD. Sound production and acoustic communication in Xenopus borealis. In: Tinsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford: Clarendon Press; 1996. pp. 121–141. [Google Scholar]
- 25.Kelley DB. Sexual differentiation in Xenopus laevis. In: Tisnsley RC, Kobel HR, editors. The Biology of Xenopus. Oxford: Clarendon Press; 1996. pp. 143–176. [Google Scholar]
- 26.Mason MJ, Narins PM. Vibrometric studies of the middle ear of the bullfrog Rana catesbeiana I. The extrastapes. J. Exp. Biol. 2002;205:3153–3165. doi: 10.1242/jeb.205.20.3153. [DOI] [PubMed] [Google Scholar]
- 27.Elliott TM, Christensen-Dalsgaard J, Kelley DB. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis. J. Comp. Physiol. [A] 2007;193:1243–1257. doi: 10.1007/s00359-007-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jørgensen MB, Kanneworff M. Middle ear transmission in the grass frog, Rana temporaria. J. Comp. Physiol. [A] 1998;182:59–64. doi: 10.1007/s003590050158. [DOI] [PubMed] [Google Scholar]
- 29.Werner YL. Mechanical leverage in the middle ear of the American bullfrog, Rana catesbeiana. Hear. Res. 2003;175:54–65. doi: 10.1016/s0378-5955(02)00709-8. [DOI] [PubMed] [Google Scholar]