Abstract
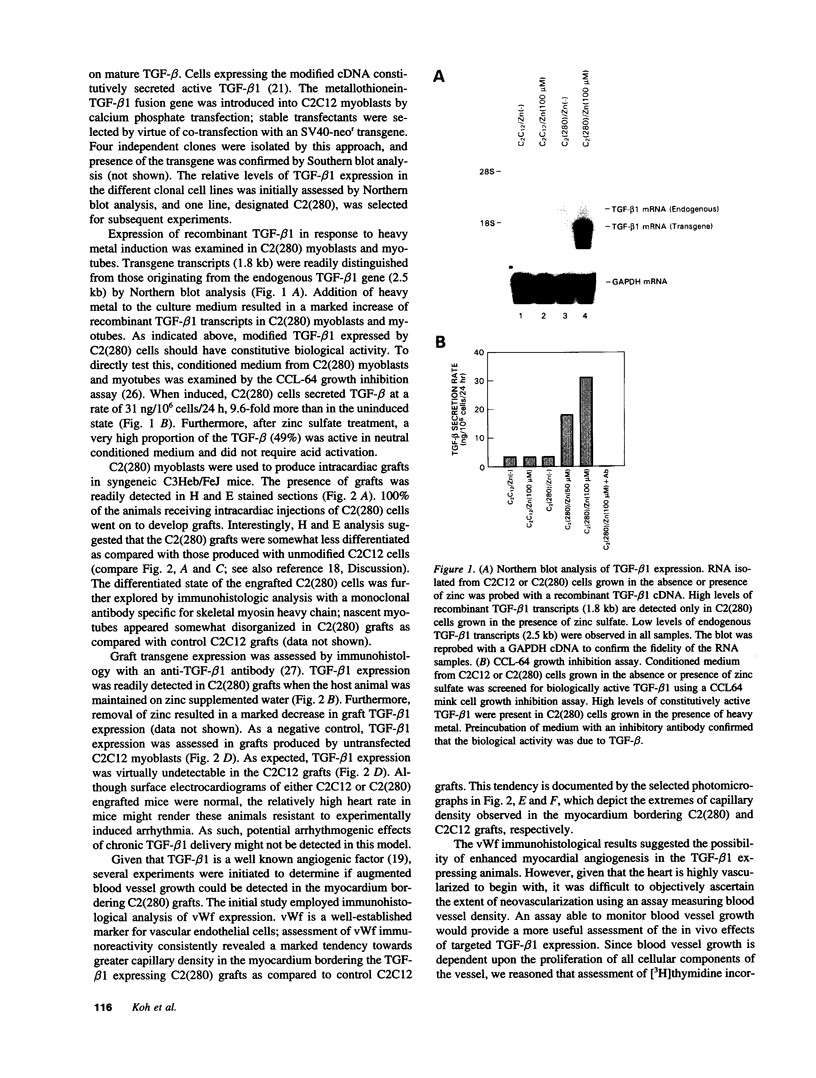
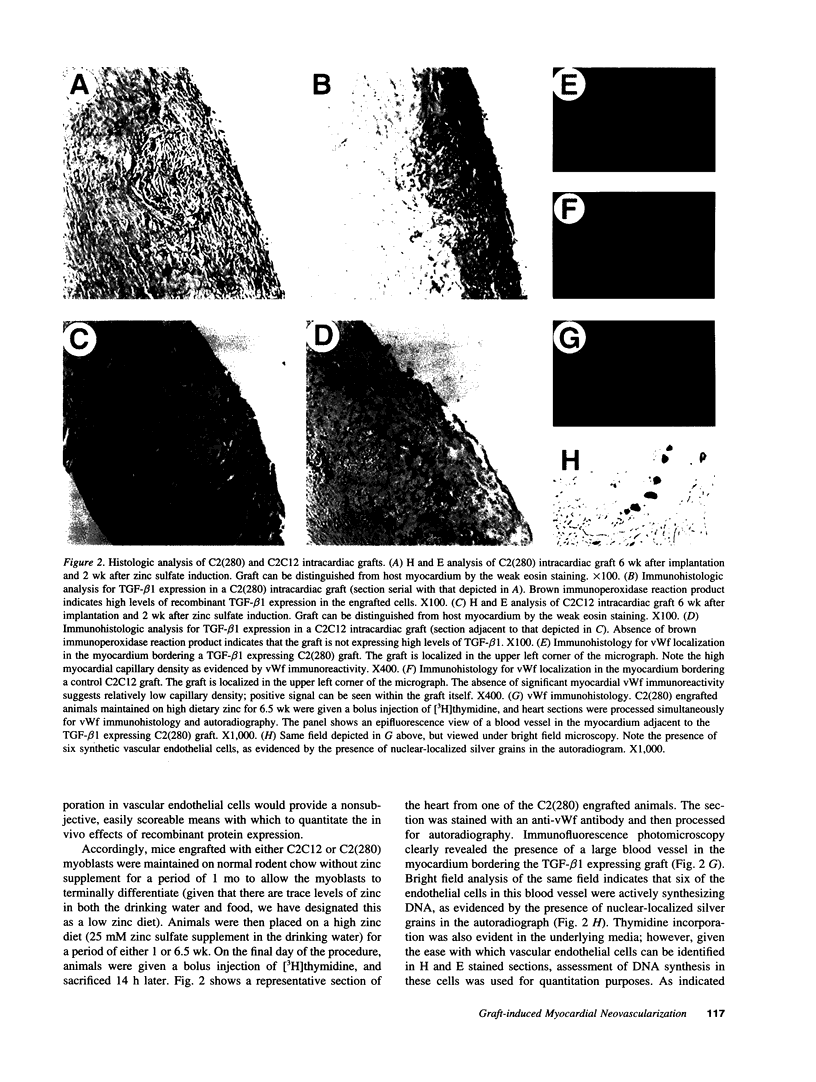
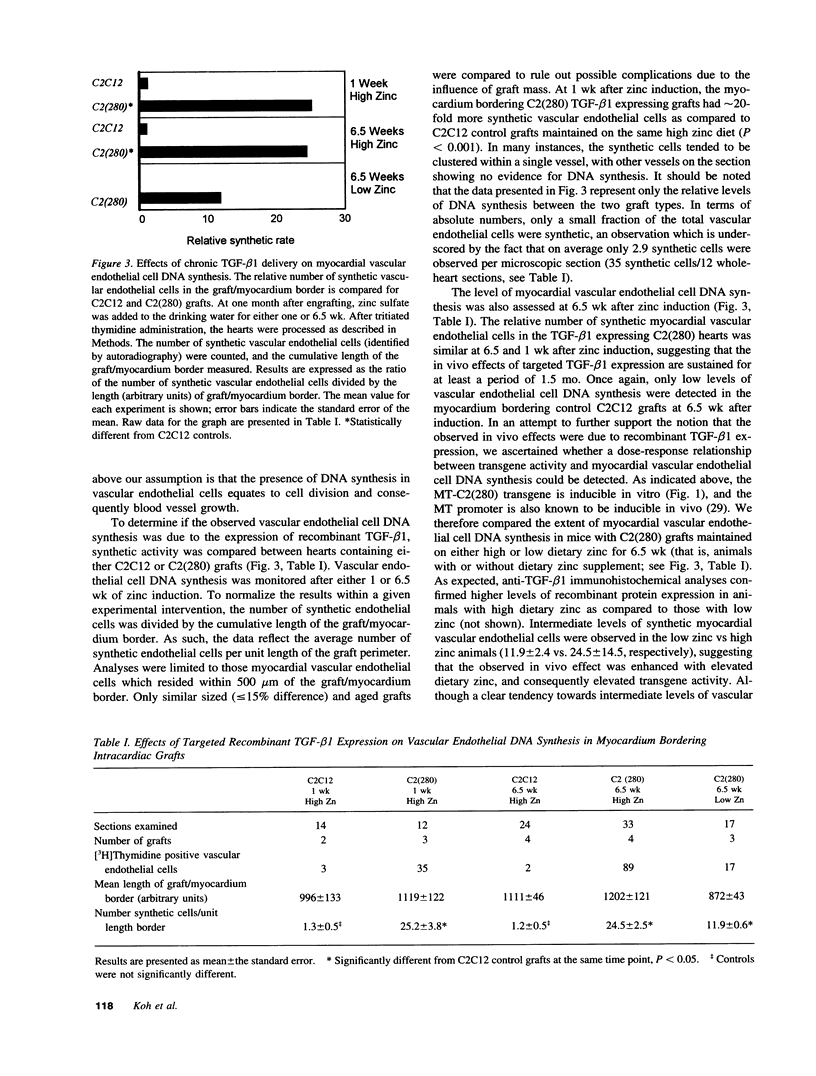
Intracardiac grafts comprised of genetically modified skeletal myoblasts were assessed for their ability to effect long-term delivery of recombinant transforming growth factor-beta (TGF-beta) to the heart. C2C12 myoblasts were stably transfected with a construct comprised of an inducible metallothionein promoter fused to a modified TGF-beta 1 cDNA. When cultured in medium supplemented with zinc sulfate, cells carrying this transgene constitutively secrete active TGF-beta 1. These genetically modified myoblasts were used to produce intracardiac grafts in syngeneic C3Heb/FeJ hosts. Viable grafts were observed as long as three months after implantation, and immunohistological analyses of mice maintained on water supplemented with zinc sulfate revealed the presence of grafted cells which stably expressed TGF-beta 1. Regions of apparent neovascularization, as evidenced by tritiated thymidine incorporation into vascular endothelial cells, were observed in the myocardium which bordered grafts expressing TGF-beta 1. The extent of vascular endothelial cell DNA synthesis could be modulated by altering dietary zinc. Similar effects on the vascular endothelial cells were not seen in mice with grafts comprised of nontransfected cells. This study indicates that genetically modified skeletal myoblast grafts can be used to effect the local, long-term delivery of recombinant molecules to the heart.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr E., Leiden J. M. Systemic delivery of recombinant proteins by genetically modified myoblasts. Science. 1991 Dec 6;254(5037):1507–1509. doi: 10.1126/science.1962212. [DOI] [PubMed] [Google Scholar]
- Behara A. M., Westcott A. J., Chang P. L. Intrathymic implants of genetically modified fibroblasts. FASEB J. 1992 Jul;6(10):2853–2858. doi: 10.1096/fasebj.6.10.1634049. [DOI] [PubMed] [Google Scholar]
- Brunner A. M., Marquardt H., Malacko A. R., Lioubin M. N., Purchio A. F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989 Aug 15;264(23):13660–13664. [PubMed] [Google Scholar]
- Buttrick P. M., Kass A., Kitsis R. N., Kaplan M. L., Leinwand L. A. Behavior of genes directly injected into the rat heart in vivo. Circ Res. 1992 Jan;70(1):193–198. doi: 10.1161/01.res.70.1.193. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Dhawan J., Pan L. C., Pavlath G. K., Travis M. A., Lanctot A. M., Blau H. M. Systemic delivery of human growth hormone by injection of genetically engineered myoblasts. Science. 1991 Dec 6;254(5037):1509–1512. doi: 10.1126/science.1962213. [DOI] [PubMed] [Google Scholar]
- Epstein H. F., Fischman D. A., Bader D., Changeux J. P., Buckhold K., Ordahl C. P., Hoffman E., Kedes L. H., Konieczny S., Leinwand L. A. Myoblast therapy. Science. 1992 Aug 7;257(5071):738–738. doi: 10.1126/science.1496388. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Houck K., Jakeman L., Leung D. W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992 Feb;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Field L. J. Transgenic mice in cardiovascular research. Annu Rev Physiol. 1993;55:97–114. doi: 10.1146/annurev.ph.55.030193.000525. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Kawaja M. D., Fisher L. J. Genetically modified cells: applications for intracerebral grafting. Trends Neurosci. 1991 Aug;14(8):328–333. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- Grossman M., Raper S. E., Kozarsky K., Stein E. A., Engelhardt J. F., Muller D., Lupien P. J., Wilson J. M. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet. 1994 Apr;6(4):335–341. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Pavlath G. K., Lanctot A. M., Sharma K. R., Miller R. G., Steinman L., Blau H. M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992 Apr 2;356(6368):435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Vossen J. M., v Beusechem V. W., Valerio D. Treatment of patients with severe combined immunodeficiency due to adenosine deaminase (ADA) deficiency by autologous transplantation of genetically modified bone marrow cells. Hum Gene Ther. 1992 Oct;3(5):553–558. doi: 10.1089/hum.1992.3.5-553. [DOI] [PubMed] [Google Scholar]
- Hughes S. M., Blau H. M. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990 May 24;345(6273):350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- Jiao S., Gurevich V., Wolff J. A. Long-term correction of rat model of Parkinson's disease by gene therapy. Nature. 1993 Apr 1;362(6419):450–453. doi: 10.1038/362450a0. [DOI] [PubMed] [Google Scholar]
- Jiao S., Wolff J. A. Long-term survival of autologous muscle grafts in rat brain. Neurosci Lett. 1992 Mar 30;137(2):207–210. doi: 10.1016/0304-3940(92)90405-v. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum L. A., MacLellan W. R., Mazur W., French B. A., Schneider M. D. Highly efficient gene transfer into adult ventricular myocytes by recombinant adenovirus. J Clin Invest. 1993 Jul;92(1):381–387. doi: 10.1172/JCI116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh G. Y., Klug M. G., Soonpaa M. H., Field L. J. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993 Sep;92(3):1548–1554. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh G. Y., Soonpaa M. H., Klug M. G., Field L. J. Long-term survival of AT-1 cardiomyocyte grafts in syngeneic myocardium. Am J Physiol. 1993 May;264(5 Pt 2):H1727–H1733. doi: 10.1152/ajpheart.1993.264.5.H1727. [DOI] [PubMed] [Google Scholar]
- Li L., Zhou J., James G., Heller-Harrison R., Czech M. P., Olson E. N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992 Dec 24;71(7):1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- Lin H., Parmacek M. S., Morle G., Bolling S., Leiden J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Molecular biology of metallothionein gene expression. Experientia Suppl. 1987;52:63–80. doi: 10.1007/978-3-0348-6784-9_4. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Roberts A. B., Sporn M. B. cDNA cloning by PCR of rat transforming growth factor beta-1. Nucleic Acids Res. 1990 May 25;18(10):3059–3059. doi: 10.1093/nar/18.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S. K., Hurta R. A., Kondaiah P., Khalil N., Turley E. A., Wright J. A., Greenberg A. H. Autocrine induction of tumor protease production and invasion by a metallothionein-regulated TGF-beta 1 (Ser223, 225). EMBO J. 1992 Apr;11(4):1599–1605. doi: 10.1002/j.1460-2075.1992.tb05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Ludtke J. J., Acsadi G., Williams P., Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992 Sep;1(6):363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Zipes D. P. Influence of myocardial ischemia and infarction on autonomic innervation of heart. Circulation. 1990 Oct;82(4):1095–1105. doi: 10.1161/01.cir.82.4.1095. [DOI] [PubMed] [Google Scholar]
- Zwiebel J. A., Freeman S. M., Newman K., Dichek D., Ryan U. S., Anderson W. F. Drug delivery by genetically engineered cell implants. Ann N Y Acad Sci. 1991;618:394–404. doi: 10.1111/j.1749-6632.1991.tb27259.x. [DOI] [PubMed] [Google Scholar]






