Abstract
The identification of a new T-cell subset referred to as T helper 17 (Th17) cells and its role in protective immunity against extracellular bacterial infections is well established. In contrast, initial studies suggested that the IL-23-IL-17 pathway was not required for protection against intracellular pathogens such as mycobacterial infections. However, recent studies demonstrate that Th17-IL -23 pathway may play a crucial role in protective immunity against other intracellular pathogens by regulating the innate and adaptive immune responses. The current outlook on the role of IL-23-IL-17 pathway in protective immunity to intracellular pathogens is discussed here.
Key words: IL-17, Th1, Th17, intracellular pathogens
Introduction
Until recently, effector CD4 T helper cells have been classified into T helper1 (Th1) and T helper2 (Th2) effectors1 based on the signature cytokines that they produce. Th1 effector cells produce the cytokine Interferon-gamma (IFNγ) and are known to regulate immunity against intracellular infections, whereas Th2 effector cells produce the cytokines Interleukin (IL)-4, IL-5 and IL-13 and are known to mediate humoral immunity against parasite infections. Recent functional evidence has changed the Th1/Th2 cell dichotomy to include a new T cell subset referred to as T helper 17 (Th17) cells.2,3 Studies suggest that the IL-23-Th17 pathway has evolved to confer protective immunity against extracellular bacterial infections.4–6 In contrast, initial studies by us and others suggested that the IL-23-Th17 pathway was not critical for protection against intracellular pathogens such as Mycobacterium tuberculosis,7 and M. bovis BCG.8 However, recent studies demonstrate that IL-23-IL-17 pathway may in fact play a crucial role in protective immunity against other intracellular pathogens. The current consensus on the role of IL-23-IL-17 pathway in protective immunity to intracellular pathogens is discussed here.
IL-17-producing Cells and Th17 Cells in Intracellular Infectious Diseases
Th17 CD4 T cells have been characterized to produce the cytokines IL-17A (IL-17) and IL-17F, as well as IL-21 and IL-22 (reviewed in ref. 9). The differentiation of Th1 or Th17 cells occurs following exposure to APC-derived polarizing cytokines such as IL-12,10 for Th1 cells and TGFβ, IL-6, IL-1β and IL-23 for Th17 cells.2,3,11–13 Although IL-23 is not required for generation of murine Th17 cells in vitro,11 it is critical for in vivo Th17 responses in mice.4,6,7,14–16 In contrast, generation of human Th17 cells is dependent on IL-23,13,17 IL-1β,13,18,19 TGFβ17 and IL-6.19 These polarizing cytokines further induce the expression of the transcription factors T-bet or RORγt and RORα for Th1 and Th17 differentiation respectively.20,21 Dendritic cells activated by signals from Pathogen Associated Recognition Receptors (PRRs) recognize components of intracellular pathogens such as M. tuberculosis,22 Francisella tularensis23 and Salmonella enterica24 and induce the production of polarizing cytokines and drive generation of Th17 responses. Consistent with these findings, Th17 cells are induced following infection with M. tuberculosis,7 F. tularensis15,25 and Chlamydia muridarum,26 suggesting a role for Th17 cells in immunity against intracellular pathogens.
Although most of the recent studies have focused on IL-17 produced by CD4 αβ T cells, γδ T cells are potent producers of IL-17 during the early immune response following intracellular infections. In murine models of infection with M. tuberculosis,27 M. bovis BCG,8 S. enterica,28 S. typhimurium,29 Listeria monocytogenes30,31 and F. tularensis LVS,15,25 γδ T cells are a major producer of IL-17. Consistent with animal models, γδ T cells are also a major source of IL-17 in human tuberculosis patients.32 Furthermore, a αβ-TCR+ CD4−CD8− T-cell population that produces IL-17 in response to L. monocytogenes30 and F. tularensis LVS infection30,33 has been documented. These studies suggest that IL-17 production by innate cells may function as a bridge between innate and adaptive immunity and contribute to protective immunity against intracellular pathogens.
IL-17 is not Critical for Overall Protection, but Mediates Inflammatory Responses Against Some Intracellular Pathogens
A clear role for IL-17 in generation of chemokine responses, induction of anti-microbial proteins and recruitment for neutrophils for control of extracellular pathogens has emerged (reviewed in ref. 34). In contrast, early studies suggested that the Th17 effector cytokines were not required for overall protection against some intracellular infections.7,8 For example, although the absence of the IL-23/IL-17 axis resulted in reduced inflammation following M. tuberculosis pulmonary infections,7 IL-17Receptor Knock Out (IL-17RKO)5 and IL-23KO7 mice were not more susceptible than wild-type control mice to M. tuberculosis pulmonary infection. However, IL-23 was shown to provide some protective immunity in the absence of functional IL-12 during murine tuberculosis.7 Also in pulmonary infection with M. bovis BCG, absence of IL-17 did not impact overall survival or susceptibility to infection, but impacted the formation of granulomas in the lung.8,35 Interestingly, in M. bovis BCG infected mice, absence of IL-17 also resulted in reduced generation of IFNγ-producing Th1 cells and impaired neutrophil recruitment to the lungs, without increase in bacterial burdens.8 In contrast, there were no defects in Th1 responses in the absence of IL-23 in a systemic M. bovis BCG infection model.36 These murine models suggest that in the presence of functional IL-12/Th1 pathway, IL-23/Th17 pathway is not crucial for primary protection against mycobacterial infections, but may play a role in granuloma formation and inflammation7,8 (Fig. 1A). Therefore, although mycobacteriaspecific human Th17 cells have been described,37,38 the actual role of the Th17 cells during human mycobacterial infections is still not completely understood. In contrast, in murine models, Th17 cells may have an important role to play in vaccine-induced immunity against mycobacterial infections.14
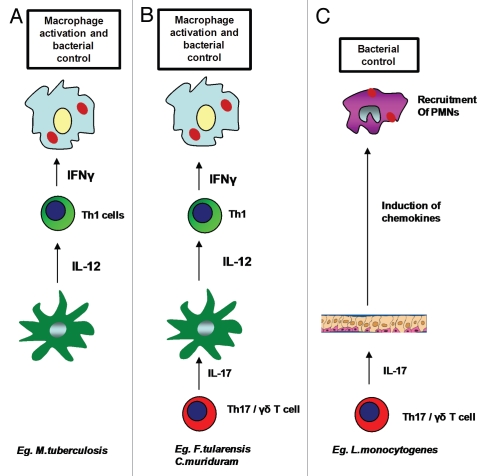
Figure 1.
Role of IL-17 in protective immunity against intracellular pathogens. Involvement of Th17 pathway is not required for overall protective immunity against some intracellular pathogens such as M. tuberculosis, where activation of macrophages and bacterial killing takes place in the presence of functional IL-12 mediated Th1 responses (A). Th17 pathway is required for induction of IL-12 from DCs and generation of Th1 responses for macrophage activation and bacterial control in some pathogens such as F. tularensis LVS and C. muridarum (B). Th17 pathway is required for recruitment of neutrophils and bacterial killing in intracellular pathogens such as L. monocytogenes and S. typhimurium (C).
Similarly, infection with S. enterica in IL-23KO mice did not result in increased susceptibility,39 suggesting that IL-23/IL-17 pathway is not critical for protection against this intracellular pathogen in mouse models.
IL-17 is Required for Generation of Th1 Responses and Protective Immunity Against some Intracellular Pathogens
Pulmonary infection in mice with the intracellular bacterium F. tularensis LVS induced Th17 cells,40 suggesting a role for IL-17 in protective immunity against this pathogen. Accordingly, the absence of IL-23/IL-17 pathway in mice resulted in increased susceptibility to pulmonary tularemia and correlated with decreased Th1 responses.15 The mechanism by which IL-17 regulates the Th1 pathway appears to be via induction of IL-12 and IFNγ in APCs. Following IL-17 stimulation, both DCs and macrophages produced IL-12 and IFNγ and regulated downstream immune responses.15 For example, IL-17 dependent-DC-derived IL-12 was able to drive the differentiation of naive T cells into Th1 cells, while IL-17-dependent macrophage-derived IFNγ was able to activate macrophages for control of intracellular Francisella.15 Although until recently, non-hematopoietic cells such as fibroblasts and epithelial cells were thought to be the primary responders to IL-17,41 more recent evidence suggests that macrophages and dendritic cells express the receptors for IL-17 (IL-17RA and IL-17RC), and can respond to IL-17 and produce cytokines and chemokines.15,42 These data suggest that IL-17 responses can regulate the induction and generation of Th1 responses and define the outcome of protective immunity during pulmonary tularemia. Similarly, in a sub lethal pulmonary tularemia infection model, the IL-17 gene deficient mice had delayed clearance of F. tularensis LVS suggesting a crucial role for IL-17 in protective immunity against this pathogen.33 Consistent with the pulmonary tularemia model, studies with a pulmonary C. muridarum infection model have demonstrated that blocking IL-17 responses during infection results in increased susceptibility to infection.26,43 One mechanism by which IL-17 can modulate adaptive response to Chlamydia was shown to be mediated via induction of IL-12 responses and driving Th1 responses.26 However, it is likely that the impaired neutrophilic recruitment seen in both the pulmonary tularemia15 and Chlamydia infection43 may also contribute to protection. These studies suggest that IL-17 can regulate the adaptive Th1 immune response by modulating the production of Th1 polarizing cytokines by APCs and contribute to Th1 immunity and protection (Fig. 1B).
The above studies suggest an unique requirement for the IL-23-Th17 cell pathway in induction of Th1 cell responses during F. tularensis LVS,15 M. bovis BCG8 and C. muriduram26 but not other intracellular infections such as M. tuberculosis (Table 1).7 Since these different bacteria use a variety of specific PRR signaling, it is likely that they stimulate the production of distinct polarizing cytokines that influences subsequent adaptive immune responses. We propose that some intracellular bacterial infections can effectively induce IL-12-IFNγ responses in the host, while other pathogens require the IL-23-IL-17 pathway for effective induction of host IL-12-IFNγ responses for intracellular pathogen control.
Table 1.
Involvement of IL-17 in protective immunity to intracellular pathogens
| Organism | Effect of lack of Th17 pathway | References |
| M. tuberculosis | Impacts inflammation, but not required for overall protection | 5, 7 |
| M. bovis BCG | Impacts formation of granulomas, but not required for overall protection | 8, 35 |
| S. enterica | Not required for protection | 39 |
| F. tularensis | LVS Increased susceptibility due to impaired Th1 responses | 15 |
| C. muriduram | Increased susceptibility due to impaired Th1 responses | 26 |
| L. monocytogenes | Increased susceptibility due to impaired neutrophilic recruitment | 31, 45 |
| S. typhimurium | Increased dissemination due to impaired neutrophilic responses | 29, 46 |
| C. muriduram | Increased susceptibility due to impaired neutrophilic responses | 43, 47 |
Intracellular Pathogens that may Require IL-17-dependent Neutrophilic Recruitment for Protective Immunity
Intracellular pathogens that require neutrophilic contribution to mediate protective immunity are dependent on the IL-23/IL-17 axis for induction of neutrophil attracting chemokines (Fig. 1C). IL-17 induces granulopoietic factors such as Granulocyte colony-stimulating factor (G-CSF) as well as CXC- chemokines such as CXCL-1, CXCL-2, CXCL-5 and CXCL-8, therefore implicating a crucial role for IL-17 in generation and recruitment of neutrophils in response to inflammation and infection (reviewed in ref. 9). Accordingly, induction of IL-17 and IL-17F production following acute Mycoplasma pneumonia pulmonary infection is IL-23-dependent and is required for neutrophil recruitment and protective immunity against this intracellular pathogen.44 Neutrophils are a critical component of protective immunity against the gram positive intracellular bacteria L. monocytogenes. Accordingly, IL-23KO mice and IL-17RKO mice are more susceptible to L. monocytogenes infection and have reduced neutrophil recruitment to the liver.31,45 γδ T cells and αβ TCR CD4− CD8− DN cells are documented to produce IL-17 during the early phase following L. monocytogenes infection and regulate the recruitment of neutrophils31,45 and formation of granulomas.45 Importantly, reconstitution of IL-17 by cytokine therapy31 or adoptive transfer of αβ TCR CD4− CD8− IL-17 producing cells30 reduced bacterial burden, confirming a role for IL-17 in protective immunity against L. monocytogenes.
Studies using the intracellular pathogen S. typhimurium have also shown that IL-17 and IL-22 are induced in the ileal mucosa in response to infection.46 Absence of IL-23 and IL-17R signaling resulted in reduced induction of chemokines Macrophage inflammatory protein-2 (MIP-2) and keratinocyte-derived chemokine (KC) as well as anti-microbials, reduced neutrophilic recruitment to the ileal mucosa and increased dissemination of the bacteria to the lymph nodes.29,46
Similar observations were also seen in the C. muridarum pulmonary infection model where IL-17 was required for the induction of chemokines and recruitment of neutrophils for bacterial control.43,47 These studies show that IL-17 made by both Th17 cells and γδ T cells29,48 may have an important role in immunity against intracellular infections by mediating the induction of chemokines required for neutrophilic recruitment and the control of bacteria.
Summary and Outlook
Our current understanding on the role of IL-17 and Th17 cells in the protective immunity to intracellular pathogens is evolving rapidly. Although initial studies in the mycobacterial infection models suggested that IL-17 was not required for protection, more recent studies have provided new insights into how IL-17 can regulate both innate and adaptive responses against other intracellular pathogens. Further studies to determine which PRRs used by intracellular pathogens trigger specific Th1 and Th17 polarizing cytokines will be critical in understanding why some, but not all intracellular pathogens require IL-17 for protective immunity.
Acknowledgements
This work was supported by Children's Hospital of Pittsburgh, Pennsylvania Department of Health Formula Funding, AI075106, A1083541 from National Institute of Health, USA to S.A.K. The authors have no conflicting financial interests.
Abbreviations
- APC
antigen presenting cell
- IFN
interferon
- IL
interleukin
- Th
T helper cell
- KO
knock out
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/12862
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:11–13. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 7.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFNgamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 8.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. In Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DS, O'Garra A. Further checkpoints in Th1 development. Immunity. 2002;16:755–758. doi: 10.1016/s1074-7613(02)00331-x. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 18.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORalpha and RORgamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, et al. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 24.Siegemund S, Schütze N, Freudenberg MA, Lutz MB, Straubinger RK, Alber G. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella enteritidis. Immunobiology. 2007;212:739–750. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, et al. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J Immunol. 2010;184:3755–3767. doi: 10.4049/jimmunol.0902065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 27.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 28.Siegemund S, Schütze N, Schulz S, Wolk K, Nasilowska K, Straubinger RK, et al. Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis. Int Immunol. 2009;21:555–565. doi: 10.1093/intimm/dxp025. [DOI] [PubMed] [Google Scholar]
- 29.Godinez I, Raffatellu M, Chu H, Paixão TA, Haneda T, Santos RL, et al. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2009;77:387–398. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, et al. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 32.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowley SC, Meierovics AI, Frelinger JA, Iwakura Y, Elkins KL. Lung CD4− CD8− double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. J Immunol. 2010;184:5791–5801. doi: 10.4049/jimmunol.1000362. [DOI] [PubMed] [Google Scholar]
- 34.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 36.Chackerian AA, Chen SJ, Brodie SJ, Mattson JD, McClanahan TK, Kastelein RA, et al. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect Immun. 2006;74:6092–6099. doi: 10.1128/IAI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008;198:544–552. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz SM, Köhler G, Schütze N, Knauer J, Straubinger RK, Chackerian AA, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 40.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008;76:2651–2659. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol. 2009;183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X, Chen Q, Moore J, Kolls JK, Halperin S, Wang J, et al. Critical role of the interleukin-17/interleukin-17 receptor axis in regulating host susceptibility to respiratory infection with Chlamydia species. Infect Immun. 2009;77:5059–5070. doi: 10.1128/IAI.00403-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godinez I, Haneda T, Raffatellu M, George MD, Paixão TA, Rolán HG, et al. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun. 2008;76:2008–2017. doi: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]