Abstract
Nicotine underlies tobacco addiction, influences tobacco use patterns, and is used as a pharmacological aid to smoking cessation. The absorption, distribution and disposition characteristics of nicotine from tobacco and medicinal products are reviewed. Nicotine is metabolized primarily by the liver enzymes CYP2A6, UDP-glucuronosyltransfease (UGT), and flavin-containing monooxygenase (FMO). In addition to genetic factors, nicotine metabolism is influenced by diet and meals, age, sex, use of estrogen-containing hormone preparations, pregnancy and kidney disease, other medications, and smoking itself. Substantial racial/ethnic differences are observed in nicotine metabolism, which are likely influenced by both genetic and environmental factors. The most widely used biomarker of nicotine intake is cotinine, which may be measured in blood, urine, saliva, hair, or nails. The current optimal plasma cotinine cut-point to distinguish smokers from non-smokers in the general US population is 3 ng ml−1. This cut-point is much lower than that established 20 years ago, reflecting less secondhand smoke exposure due to clear air policies and more light or occasional smoking.
1 Introduction
An understanding of the pharmacology of nicotine and how nicotine produces addiction and influences smoking behavior provides a necessary basis for therapeutic advances in smoking cessation interventions. This chapter provides a review of several aspects of the human pharmacology of nicotine. These include the presence and levels of nicotine and related alkaloids in tobacco products, the absorption of nicotine from tobacco products and nicotine medications, the distribution of nicotine in body tissues, the metabolism and renal excretion of nicotine, nicotine and cotinine blood levels during tobacco use or nicotine replacement therapy, and biomarkers of nicotine exposure. For more details and references on the pharmacokinetics and metabolism of nicotine, the reader is referred to Hukkanen et al. (2005c).
2 Nicotine and Related Alkaloids in Tobacco Products
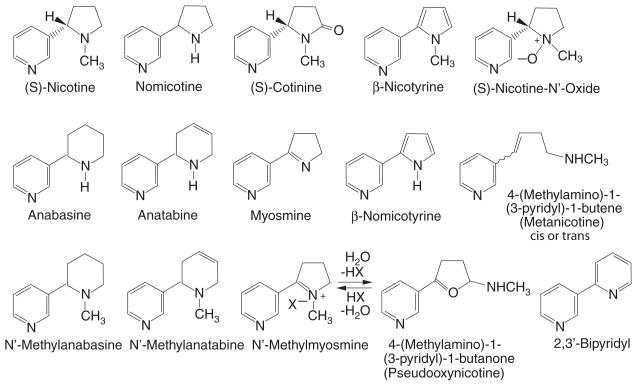
Nicotine (Fig. 1) is a natural ingredient acting as a botanical insecticide in tobacco leaves. It is the principal tobacco alkaloid, occurring to the extent of about 1.5% by weight in commercial cigarette tobacco and comprising about 95% of the total alkaloid content. Oral snuff and pipe tobacco contain concentrations of nicotine similar to cigarette tobacco, whereas cigar and chewing tobacco have only about half the nicotine concentration of cigarette tobacco. An average tobacco rod contains 10–14 mg of nicotine (Kozlowski et al. 1998), and on average about 1–1.5 mg of nicotine is absorbed systemically during smoking (Benowitz and Jacob 1984). Nicotine in tobacco is largely the levorotary (S)-isomer; only 0.1–0.6% of total nicotine content is (R)-nicotine (Armstrong et al. 1998). Chemical reagents and pharmaceutical formulations of (S)-nicotine have a similar content of (R)-nicotine (0.1–1.2%) as impurity since plant-derived nicotine is used for their manufacture.
Fig. 1.
Structures of tobacco alkaloids. Reprinted from Benowitz and Jacob (1998) with permission of Wiley-Liss, a subsidiary of Wiley
In most tobacco strains, nornicotine and anatabine are the most abundant of minor alkaloids, followed by anabasine (Fig. 1). This order of abundance is the same in cigarette tobacco and oral snuff, chewing, pipe, and cigar tobacco (Jacob et al. 1999). However, nornicotine levels are highest in cigar tobacco, anatabine levels are lowest in chewing tobacco and oral snuff, and anabasine levels are lowest in chewing tobacco (Jacob et al. 1999). Small amounts of the N′-methyl derivatives of anabasine and anatabine are found in tobacco and tobacco smoke. Several of the minor alkaloids are thought to arise by bacterial action or oxidation during tobacco processing rather than by biosynthetic processes in the living plant (Leete 1983). These include myosmine, N′-methylmyosmine, cotinine, nicotyrine, nornicotyrine, nicotine N′-oxide, 2, 3′-bipyridyl, and metanicotine (Fig. 1). Myosmine is found not only in tobacco but also in a variety of foods including nuts, cereals, milk, and potatoes (Tyroller et al. 2002). Also, nicotine is found in low levels in vegetables such as potatoes, tomatoes, and eggplants (Siegmund et al. 1999).
3 Absorption of Nicotine
Nicotine is distilled from burning tobacco and carried proximally on tar droplets (also called particulate matter), which are inhaled. Absorption of nicotine across biological membranes depends on pH. Nicotine is a weak base with a pKa of 8.0. In its ionized state, such as in acidic environments, nicotine does not rapidly cross membranes. The pH of smoke from flue-cured tobaccos, found in most cigarettes, is acidic (pH 5.5–6.0). At this pH, nicotine is primarily ionized. As a consequence, there is little buccal absorption of nicotine from flue-cured tobacco smoke, even when it is held in the mouth (Gori et al. 1986). Smoke from air-cured tobaccos, the predominant tobacco used in pipes, cigars, and some European cigarettes, is more alkaline (pH 6.5 or higher) and, considerable nicotine is unionized. Smoke from these products is well absorbed through the mouth (Armitage et al. 1978). It has recently been proposed that the pH of cigarette smoke particulate matter is higher than previously thought, and thus, a larger portion of nicotine would be in the unionized form, facilitating rapid pulmonary absorption (Pankow 2001).
When tobacco smoke reaches the small airways and alveoli of the lung, nicotine is rapidly absorbed. Blood concentrations of nicotine rise quickly during a smoke and peak at the completion of smoking (Fig. 2). The rapid absorption of nicotine from cigarette smoke through the lungs, presumably because of the huge surface area of the alveoli and small airways, and dissolution of nicotine in the fluid of pH 7.4 in the human lung facilitate transfer across membranes. After a puff, high levels of nicotine reach the brain in 10–20 s, faster than with intravenous administration, producing rapid behavioral reinforcement (Benowitz 1990). The rapidity of rise in nicotine levels permits the smoker to titrate the level of nicotine and related effects during smoking, and makes smoking the most reinforcing and dependence-producing form of nicotine administration (Henningfield and Keenan 1993).
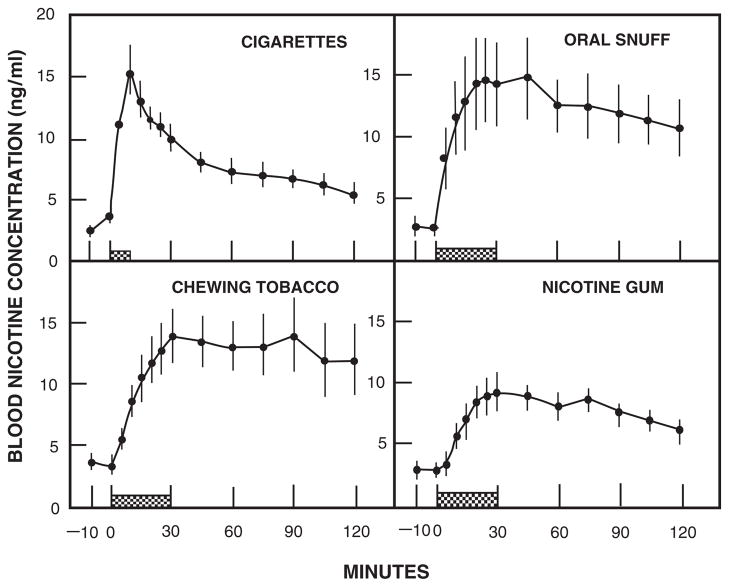
Fig. 2.
Blood nicotine concentrations during and after cigarette smoking for 9 min, oral snuff (2.5 g), chewing tobacco (average 7.9 g), and nicotine gum (two 2-mg pieces). Average values for 10 subjects (±SEM). Reprinted from Benowitz et al. (1988) with permission from American Society for Clinical Pharmacology and Therapeutics
The process of cigarette smoking is complex and, as mentioned above, the smoker can manipulate the dose of nicotine and nicotine brain levels on a puff-by-puff basis. Intake of nicotine during smoking depends on puff volume, depth of inhalation, the extent of dilution with room air, and the rate and intensity of puffing (USDHHS 2001). For this reason, machine-determined nicotine yields of cigarettes cannot be used to estimate the dose of nicotine by a smoker (Jarvis et al. 2001). In general, cigarette smokers switching from a higher to a lower-yield cigarette will compensate, i.e., will change their smoking pattern to gain more nicotine (USDHHS 2001).
Chewing tobacco and snuff are buffered to alkaline pH to facilitate absorption of nicotine through oral mucosa. Although absorption through cell membranes is rapid for these more alkaline tobacco products, the rise in the brain nicotine level is slower than with smoking (Fig. 2). Concentrations of nicotine in the blood rise gradually with the use of smokeless tobacco and plateau at about 30 min, with levels persisting and declining only slowly over 2 h or more (Benowitz et al. 1988).
Various formulations of nicotine replacement therapy (NRT), such as nicotine gum, transdermal patch, nasal spray, inhaler, sublingual tablets, and lozenges, are buffered to alkaline pH to facilitate absorption of nicotine through cell membranes. Absorption of nicotine from all NRTs is slower and the increase in nicotine blood levels is more gradual than from smoking. This slow increase in blood and especially in brain levels results in low abuse liability of NRTs (West et al. 2000). Only nasal spray provides a rapid delivery of nicotine that is closer to the rate of nicotine delivery achieved with smoking (Gourlay and Benowitz 1997; Guthrie et al. 1999). The absolute dose of nicotine absorbed systemically from nicotine gum is much less than the nicotine content of the gum, in part, because considerable nicotine is swallowed with subsequent first-pass metabolism (Benowitz et al. 1987). Some nicotine is also retained in chewed gum. A portion of the nicotine dose is swallowed and subjected to first-pass metabolism when using other NRTs, inhaler, sublingual tablets, nasal spray, and lozenges. Bioavailability for these products with absorption mainly through the mucosa of the oral cavity and a considerable swallowed portion is about 50–80%.
Nicotine base is well absorbed through skin. That is the reason for the occupational risk of nicotine poisoning (green tobacco sickness) in tobacco harvesters who are exposed to wet tobacco leaves (McBride et al. 1998). That is also the basis for transdermal delivery technology. Currently in the United States several different nicotine transdermal systems are marketed. All are multilayer patches. The rate of release of nicotine into the skin is controlled by the permeability of the skin, rate of diffusion through a polymer matrix, and/or rate of passage through a membrane in the various patches. Rates of nicotine delivery and plasma nicotine concentrations vary among different transdermal systems (Fant et al. 2000). In all cases, there is an initial lag time of about 1 h before nicotine appears in the bloodstream, and there is continued systemic absorption (about 10% of the total dose) after the patch is removed, the latter due to residual nicotine in the skin.
4 Distribution of Nicotine in Body Tissues
After absorption, nicotine enters the bloodstream where, at pH 7.4, it is about 69% ionized and 31% unionized. Binding to plasma proteins is less than 5% (Benowitz et al. 1982a). The drug is distributed extensively to body tissues with a steady-state volume of distribution averaging 2.6 L/Kg. Based on human autopsy samples from smokers, the highest affinity for nicotine is in the liver, kidney, spleen, and lung and lowest in adipose tissue. In skeletal muscle, concentrations of nicotine and cotinine are close to that of whole blood. Nicotine binds to brain tissues with high affinity, and the receptor binding capacity is increased in smokers compared with nonsmokers (Breese et al. 1997; Perry et al. 1999). Increase in the binding is caused by a higher number of nicotinic cholinergic receptors in the brain of the smokers. Nicotine accumulates markedly in gastric juice and saliva (Lindell et al. 1996). Gastric juice/plasma and saliva/plasma concentration ratios are 61 and 11 with transdermal nicotine administration, and 53 and 87 with smoking, respectively (Lindell et al. 1996). Accumulation is caused by ion-trapping of nicotine in gastric juice and saliva. Nicotine also accumulates in breast milk (milk/plasma ratio 2.9) (Dahlstrom et al. 1990). Nicotine crosses the placental barrier easily, and there is evidence for accumulation of nicotine in fetal serum and amnionic fluid in slightly higher concentrations than in maternal serum (Dempsey and Benowitz 2001).
The time course of nicotine accumulation in the brain and in other body organs and the resultant pharmacologic effects are highly dependent on the route and rate of dosing. Smoking a cigarette delivers nicotine rapidly to the pulmonary venous circulation, from which it moves quickly to the left ventricle of the heart and to the systemic arterial circulation and brain. The lag time between a puff of a cigarette and nicotine reaching the brain is 10–20 s. Although delivery of nicotine to the brain is rapid, there is nevertheless significant pulmonary uptake and some delayed release of nicotine as evidenced by pulmonary positron emission tomography data and the slow decrease in arterial concentrations of nicotine between puffs. (Rose et al. 1999) Nicotine concentrations in arterial blood after smoking a cigarette can be quite high, reaching up to 100 ng ml−1, but usually ranging between 20 and 60 ng ml−1 (Gourlay and Benowitz 1997; Henningfield and Keenan 1993; Lunell et al. 2000; Rose et al. 1999). The usual peak arterial nicotine concentration after the first puff is lower, averaging 7 ng ml−1. As high as tenfold arterial/venous nicotine concentration ratios have been measured (Henningfield et al. 1993), but the mean ratio is typically around 2.3–2.8 (Gourlay and Benowitz 1997; Rose et al. 1999). The rapid rate of delivery of nicotine by smoking (or intravenous injection, which presents similar distribution kinetics) results in high levels of nicotine in the central nervous system with little time for development of tolerance. The result is a more intense pharmacologic action. The short time interval between puffing and nicotine entering the brain also allows the smoker to titrate the dose of nicotine to a desired pharmacologic effect, further reinforcing drug self-administration and facilitating the development of addiction.
5 Metabolism of Nicotine
5.1 Pathways of Nicotine and Cotinine Metabolism
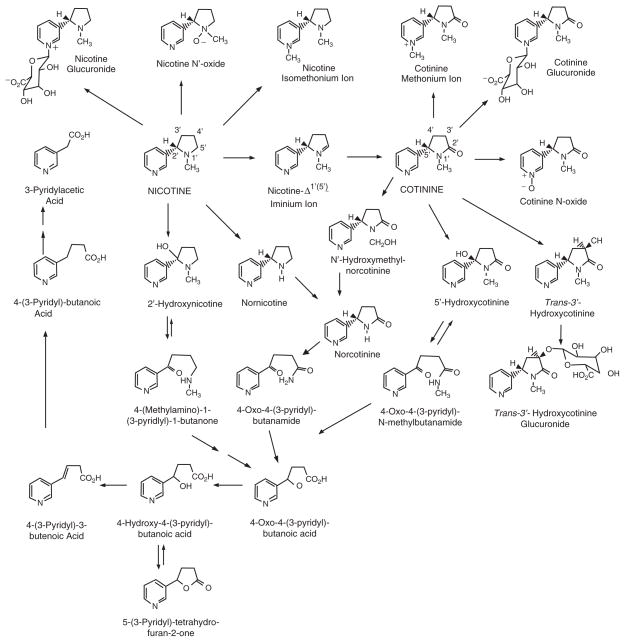
Nicotine is extensively metabolized to a number of metabolites (Fig. 3) by the liver. Six primary metabolites of nicotine have been identified. Quantitatively, the most important metabolite of nicotine in most mammalian species is the lactam derivative, cotinine. In humans, about 70–80% of nicotine is converted to cotinine. This transformation involves two steps. The first is mediated primarily by CYP2A6 to produce nicotine-Δ1′ (5′)-iminium ion, which is in equilibrium with 5′-hydroxynicotine. The second step is catalyzed by a cytoplasmic aldehyde oxidase. Nicotine iminium ion has received considerable interest since it is an alkylating agent and, as such, could play a role in the pharmacology of nicotine (Shigenaga et al. 1988).
Fig. 3.
Pathways of nicotine metabolism. Reprinted with permission from Hukkanen et al. 2005c
Nicotine N′-oxide is another primary metabolite of nicotine, although only about 4–7% of nicotine absorbed by smokers is metabolized via this route (Benowitz et al. 1994). The conversion of nicotine to nicotine N′-oxide involves a flavin-containing monooxygenase 3 (FMO3), which results in formation of both possible diasteriomers, the 1′-(R)-2′-(S)-cis and 1′-(S)-2′-(S)-trans-isomers in animals (Cashman et al. 1992; Park et al. 1993). In humans, this pathway is highly selective for the trans-isomer (Cashman et al. 1992). Only the trans-isomer of nicotine N′-oxide was detected in urine after administration of nicotine by intravenous infusion, trans-dermal patch or smoking (Park et al. 1993). It appears that nicotine N′-oxide is not further metabolized to any significant extent, except by reduction back to nicotine in the intestines, which may lead to recycling nicotine in the body.
In addition to oxidation of the pyrrolidine ring, nicotine is metabolized by two nonoxidative pathways, methylation of the pyridine nitrogen giving nicotine isomethonium ion (also called N -methylnicotinium ion) and glucuronidation.
Nicotine glucuronidation results in an N-quaternary glucuronide in humans (Benowitz et al. 1994). This reaction is catalyzed by uridine diphosphate-glucuronosyltransferase (UGT) enzyme(s) producing (S)-nicotine-N-β-glucuronide. About 3–5% of nicotine is converted to nicotine glucuronide and excreted in urine in humans.
Oxidative N-demethylation is frequently an important pathway in the metabolism of xenobiotics, but this route is, in most species, a minor pathway in the metabolism of nicotine. Conversion of nicotine to nornicotine in humans has been demonstrated. We found that small amounts of deuterium-labeled nornicotine are excreted in the urine of smokers administered deuterium-labeled nicotine (Jacob and Benowitz 1991). Metabolic formation of nornicotine from nicotine has also been reported (Neurath et al. 1991). Nornicotine is a constituent of tobacco leaves. However, most urine nornicotine is derived from metabolism of nicotine with less than 40% coming directly from tobacco, as estimated from the difference in nornicotine excretion in smokers during smoking and transdermal nicotine treatment (0.65 and 0.41%, respectively) (Benowitz et al. 1994). A new cytochrome P450-mediated metabolic pathway for nicotine metabolism was reported by Hecht et al. (2000). 2′-Hydroxylation of nicotine was shown to produce 4-(methylamino)-1-(3-pyridyl)-1-butanone with 2′-hydroxynicotine as an intermediate. 2′-Hydroxynicotine also yields nicotine-Δ1′(2′)-iminium ion. 4-(methylamino)-1-(3-pyridyl)-1-butanone is further metabolized to 4-oxo-4-(3-pyridyl)butanoic acid and 4-hydroxy-4-(3-pyridyl)butanoic acid. The new pathway is potentially significant since 4-(methylamino)-1-(3-pyridyl)-1-butanone can be converted to carcinogenic NNK. However, endogenous production of NNK from nicotine has not been detected in humans or rats (Hecht et al. 1999a).
Although on average about 70–80% of nicotine is metabolized via the cotinine pathway in humans, only 10–15% of nicotine absorbed by smokers appears in the urine as unchanged cotinine (Benowitz et al. 1994). Six primary metabolites of cotinine have been reported in humans: 3′-hydroxycotinine (McKennis et al. 1963; Neurath et al. 1987), 5′-hydroxycotinine (also called allohydroxycotinine) (Neurath 1994), which exists in tautomeric equilibrium with the open chain derivative 4-oxo-4-(3-pyridyl)-N-methylbutanamide, cotinine N-oxide, cotinine methonium ion, cotinine glucuronide, and norcotinine (also called demethylcotinine).
3′-Hydroxycotinine is the main nicotine metabolite detected in smokers’ urine. It is also excreted as a glucuronide conjugate (Benowitz et al. 1994). 3′-Hydroxycotinine and its glucuronide conjugate account for 40–60% of the nicotine dose in urine (Benowitz et al. 1994; Byrd et al. 1992). The conversion of cotinine to 3′-hydroxycotinine in humans is highly stereoselective for the trans-isomer, as less than 5% is detected as cis-3′-hydroxycotinine in urine (Jacob et al. 1990; Voncken et al. 1990). While nicotine and cotinine conjugates are N -glucuronides, the only 3′-hydroxycotinine conjugate detected in urine is O-glucuronide (Byrd et al. 1994).
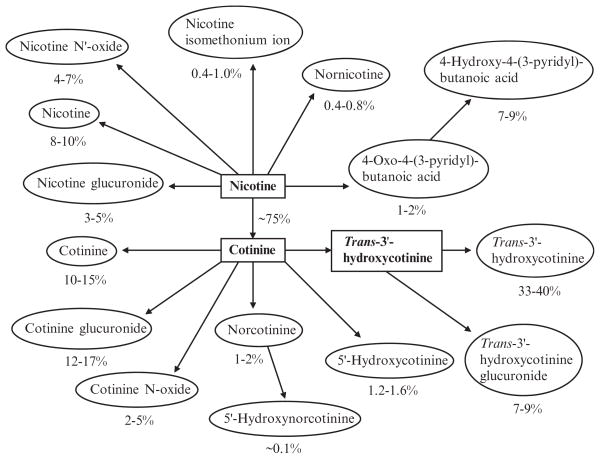
Quantitative aspects of the pattern of nicotine metabolism have been elucidated fairly well in people (Fig. 4). Approximately 90% of a systemic dose of nicotine can be accounted for as nicotine and metabolites in urine (Benowitz et al. 1994). Based on studies with simultaneous infusion of labeled nicotine and cotinine, it has been determined that 70–80% of nicotine is converted to cotinine (Benowitz and Jacob 1994). About 4–7% of nicotine is excreted as nicotine N′–oxide and 3–5% as nicotine glucuronide (Benowitz et al. 1994; Byrd et al. 1992). Cotinine is excreted unchanged in urine to a small degree (10–15% of the nicotine and metabolites in urine). The remainder is converted to metabolites, primarily trans–3′–hydroxycotinine (33–40%), cotinine glucuronide (12–17%), and trans–3′–hydroxycotinine glucuronide (7–9%).
Fig. 4.
Quantitative scheme of nicotine metabolism, based on estimates of average excretion of metabolites as percent of total urinary nicotine. Reprinted with permission from Hukkanen et al. 2005c
5.2 Rates of Nicotine and Cotinine Metabolism
The rate of metabolism of nicotine can be determined by measuring blood levels after administration of a known dose of nicotine (Table 1) (Hukkanen et al. 2005c). Total clearance of nicotine averages about 1200 ml min−1. Nonrenal clearance represents about 70% of liver blood flow. Assuming most nicotine is metabolized by the liver, this means that about 70% of the drug is extracted from blood in each pass through the liver.
Table 1.
Nicotine absorption pharmacokinetics of different forms of nicotine administration in single doses (modified from Hukkanen et al. 2005c)
| Type of nicotine administrationa | Cmaxb ng ml−1 | Tmaxb,c min | Bioavailability % |
|---|---|---|---|
| Smoking (one cigarette, 5 min) (~2 mg/cigaretted) | 15–30 (venous) | 5–8 (venous) | 80–90 (of inhaled nicotine) |
| 20–60 (arterial) | 3–5 (arterial) | ||
| Intravenous ~5.1 mg (60 μg/kg, 30 min) | 30 (venous) | 30 (venous) | 100 |
| 50 (arterial) | 30 (arterial) | ||
| Nasal spray 1 mg | 5–8 (venous) | 11–18 (venous) | 60–80 |
| 10–15 (arterial) | 4–6 (arterial) | ||
| Gum (30 min, total dose in gum) | |||
| 2 mg | 6–9 | 30 | 78 |
| 4 mg | 10–17 | 30 | 55 |
| Inhaler 4 mg released (one 10 mg cartridge, 20 min) | 8.1 | 30 | 51–56 |
| Lozenge (20–30 min) | |||
| 2 mg | 4.4 | 60 | 50 |
| 4 mg | 10.8 | 66 | 79 |
| Sublingual tablet 2 mg (20–30 min) | 3.8 | ~60 | 65 |
| Tooth patch 2 mg | ~3.2 | ~120 | |
| Transdermal patch (labeled dose) | |||
| 15 mg/16 h (Nicotrol) | 11–14 | 6–9 h | 75–100 |
| 14 mg/24 h (Nicoderm) | 11–16 | 4–7 h | |
| 21 mg/24 h (Nicoderm) | 18–23 | 3–7 h | 68 |
| 21 mg/24 h (Habitrol) | 12–21 | 9–12 h | 82 |
| Subcutaneous injection 2.4 mg | 15 | 25 | 100 |
| Oral capsule 3–4 mg | 6–8 | 90 | 44 |
| Oral slow-release capsule (colonic absorption) 6 mg | 2.2 | 7.5 h | |
| Oral solution | |||
| 2 mg | 4.7 | 51 | |
| ~3.0 mg (45 μg/kg) | 2.9 | 66 | 20 |
| Enema | |||
| ~3.5 mg (45 μg/kg) | 2.3–3.1 | 20–80 | 15–25 |
| 6 mg | 6–9 | 45 | |
Products in italics are currently marketed in the United States
Cmax and Tmax values are for peripheral venous blood unless otherwise indicated
Tmax values are measured from the start of the administration
Estimated dose of 2 mg of nicotine per cigarette is higher than the usual 1–1.5 mg per cigarette since nicotine absorption from smoking a single cigarette was studied after at least overnight abstinence from smoking in these studies
The metabolism of cotinine is much slower than that of nicotine. Cotinine clearance averages about 45 ml min−1. Clearance of (3′ R, 5′ S)-trans-3′-hydroxycotinine is also quite slow – about 82 ml min−1.
5.3 Use of the Nicotine Metabolite Ratio
The 3′-hydroxycotinine/cotinine ratio (3HC/cotinine) in plasma and saliva has been evaluated as a non-invasive probe for CYP2A6 activity (Dempsey et al. 2004). The ratio was highly correlated with oral clearance of nicotine and the oral clearance and half-life of cotinine. Correlation coefficients of oral nicotine and cotinine clearances with plasma 3′-hydroxycotinine/cotinine ratios were 0.78 and 0.63, respectively, at 6 h after oral nicotine dosing.
The availability of a phenotypic marker of CYP2A6 activity is important because there is wide variability in nicotine clearance among people with wild-type CYP2A6 genes and only a small proportion of the genetic variability in nicotine clearance can be explained by known CYP2A6 gene variants, at least in whites (Swan et al. 2005). The 3′-hydroxycotinine/cotinine ratio can be used to phenotype nicotine metabolism and CYP2A6 enzyme in smokers while smoking their usual cigarettes (Johnstone et al. 2006; Kandel et al. 2007; Lerman et al. 2006; Patterson et al. 2008). The 3HC/cotinine ratio has been studied as predictor of response to pharmacotherapy.
In one trial, where transdermal nicotine and nicotine nasal spray were compared, the nicotine metabolite ratio (derived from nicotine taken in from tobacco) was shown to be a strong predictor of smoking cessation, both at the end of treatment and in 6 months, in people treated with transdermal nicotine but not nicotine nasal spray (Lerman et al. 2006). In patients treated with transdermal nicotine, slow metabolizers had better cessation response and higher plasma nicotine concentration while using the patch than faster metabolizers, suggesting that higher nicotine levels might be responsible for a better cessation outcome. In contrast, smokers treated with nicotine nasal spray showed no difference in plasma nicotine concentration as a function of the rate of nicotine metabolism, consistent with the idea that nicotine taken in from the spray is titrated by the smoker to the desired effect. However, another recent trial examined the association between the nicotine metabolite ratio and response to bupropion therapy (Patterson et al. 2008). Faster metabolism of nicotine was associated with lower success rate in quitting in a placebo-treated group; but among smokers receiving bupropion, the rate of nicotine metabolism had no differential effect. Bupropion is not metabolized by CYP2A6. Therefore, the findings of the Patterson study are consistent with the idea that rapid metabolizers of nicotine are generally more dependent and have a harder time quitting than do slow metabolizers. The mechanisms of such a relationship have not been proven, but may include more severe withdrawal symptoms and/or a different type of nicotine reinforcement related to more rapid loss of tolerance in fast metabolizers.
Various enzymes involved in nicotine metabolism and their genetics are described in detail in the chapter by Mwenifumbo and Tyndale in this volume.
6 Factors Influencing Nicotine Metabolism
There is considerable inter individual variability in the rate of elimination of nicotine and cotinine in people (Swan et al. 2005). Besides genetic variations discussed by Mwenifumbo and Tyndale, a number of factors that might explain individual variability have been studied.
6.1 Physiological Influences
6.1.1 Diet and Meals
An implication of the high degree of hepatic extraction is that clearance of nicotine should be dependent on liver blood flow. Thus, physiological events, such as meals, posture, exercise, or drugs perturbing hepatic blood flow, are predicted to affect the rate of nicotine metabolism. Meals consumed during a steady state infusion of nicotine result in a consistent decline in nicotine concentrations, the maximal effect seen 30–60 min after the end of a meal (Gries et al. 1996; Lee et al. 1989). Hepatic blood flow increases about 30% and nicotine clearance increases about 40% after a meal.
Menthol is widely used as a flavorant in foods, mouthwash, toothpaste, and cigarettes. A moderate inhibition of CYP2A6-mediated nicotine metabolism in human liver microsomes by menthol and various related compounds has been reported (MacDougall et al. 2003). This is supported by a crossover study in people, showing that mentholated cigarette smoking significantly inhibits metabolism of nicotine to cotinine and nicotine glucuronidation when compared to smoking nonmentholated cigarettes (Benowitz et al. 2004).
Grapefruit juice inhibits CYP2A6, as evidenced by inhibition of coumarin metabolism in people (Runkel et al. 1997). Grapefruit juice has been shown to inhibit the metabolism of nicotine to cotinine in nonsmokers who were given nicotine orally, with evidence of a greater effect with larger doses of grapefruit juice (Hukkanen et al. 2006). Grapefruit juice also increased renal clearance of nicotine and cotinine by an unknown mechanism. Grapefruit juice had no significant effect on overall exposure to nicotine (area under the plasma concentration–time curve) because the effects of slowed metabolism were offset by the effects on increased renal clearance. Whether the effects of grapefruit juice on nicotine levels in users of tobacco are significant has not been investigated. Consumption of watercress enhances the formation of nicotine glucuronide, cotinine glucuronide, and 3′-hydroxycotinine glucuronide in smokers (Hecht et al. 1999b). Watercress has no effect on the excretion of nicotine, cotinine, and 3′-hydroxycotinine in smokers. Thus, watercress may induce some UGT enzymes involved in nicotine metabolism, but has no effect on CYP2A6-mediated nicotine metabolism.
6.1.2 Age
Clearance of nicotine is decreased in the elderly (age >65) compared to adults (Molander et al. 2001). Total clearance was lower by 23%, and renal clearance lower by 49% in the elderly compared to young adults. Lower nicotine metabolism in the elderly may be contributed to by reduced liver blood flow, since no decrease in CYP2A6 protein levels or nicotine metabolism in liver microsomes due to age has been detected (Messina et al. 1997). No differences in steady-state nicotine plasma levels or estimated plasma clearance values were detected in three age groups (18–39, 40–59, and 60–69 years) using patches with the same nicotine content (Gourlay and Benowitz 1996). The volume of distribution of nicotine is lower in older subjects due to a decrease in lean body mass (Molander et al. 2001).
Neonates have diminished nicotine metabolism, as demonstrated by a nicotine half-life of three to four times longer in newborns exposed to tobacco smoke than in adults (Dempsey et al. 2000). Cotinine half-life is reported to be similar in neonates, older children, and adults in two studies (Dempsey et al. 2000; Leong et al. 1998). Other studies found that the half-life of urine cotinine was about three times longer in children less than one year old than to the cotinine half-life in adults (Collier et al. 1994). Urine cotinine half-life can be influenced by variations in urine volume and excretion of creatinine. The study by Dempsey et al. was the only one in which the half-life of cotinine was calculated based on both the blood and urine cotinine concentrations (Dempsey et al. 2000). In that study, both the blood and urine half-lives were similar to adult values, supporting the notion that neonates have the same cotinine half-life as older children and adults.
Why nicotine has a much longer half-life in neonates than in adults, whereas the cotinine half-life is essentially the same in newborns and adults, might partially be explained by differing sensitivities of nicotine and cotinine clearances to changes in hepatic blood flow. As a drug with a high extraction ratio, the clearance of nicotine is influenced by changes in hepatic blood flow, whereas clearance of cotinine with low extraction ratio is more dependent on changes in intrinsic clearance, i.e., amount and activity of metabolic enzymes. Studies in newborn animals, mainly sheep, have shown that hepatic blood flow is low immediately after delivery because of the loss of the umbilical venous blood supply and the patency of ductus venosus (Gow et al. 2001). Hepatic blood flow (ml−1 min−1 mg of liver) rises to adult levels within the first week, due to increased blood flow in the portal vein and gradual closure of ductus venosus, which is complete by the eighteenth day in human neonates. This would mean that nicotine clearance should rise and the nicotine half-life shorten within the first couple of weeks as hepatic blood flow increases. Another explanation could be that nicotine and cotinine are metabolized mainly by enzymes other than CYP2A6 in neonates. However, neonates have only slightly lower amounts of CYP2A6, CYP2D6, and CYP2E1 protein in liver microsomes, whereas the CYP2B6 amount is clearly diminished in neonates compared to adults and older children (Tateishi et al. 1997).
6.1.3 Chronopharmacokinetics of Nicotine
During sleep, hepatic blood flow declines and nicotine clearance falls correspondingly. Blood nicotine levels rise during constant infusion at night. Nicotine clearance varies by approximately 17% (from peak to trough) with a minimum between 6 p.m. and 3 a.m. Thus, the day/night variation and meal effects of nicotine clearance result in circadian variations in plasma concentrations during constant dosing of nicotine (Gries et al. 1996).
6.1.4 Gender Related Differences in Nicotine Metabolism
Differences between Men and Women
A twin study with intravenous infusions of both nicotine and cotinine clearly shows that nicotine and cotinine clearances are higher in women than in men; oral contraceptive use further accelerates nicotine and cotinine clearances in women (Benowitz et al. 2006). Nicotine clearance and cotinine clearance were 13 and 24% higher, respectively, in women not using oral contraceptives than in men. Oral contraceptive use induced increases in nicotine and cotinine clearance by 28 and 30%, respectively, compared to women not using oral contraceptives. The gender difference was also detected in recent studies on smokers, showing that the ratio of 3HC/cotinine in blood or urine is significantly higher in women indicating faster metabolism in women than men (Johnstone et al. 2006; Kandel et al. 2007).
Pregnancy and Menstrual Cycle
Pregnancy has a marked inducing effect in nicotine and especially cotinine clearance. Clearance is increased by 60 and 140% for nicotine and cotinine, respectively, in pregnancy compared to postpartum (Dempsey et al. 2002). Nicotine is a rapidly cleared drug with a high affinity for CYP2A6 and its rate of clearance is primarily controlled by hepatic blood flow, while the rate of cotinine clearance is primarily determined by the activity of metabolizing enzymes in the liver. The finding that in pregnancy cotinine clearance is increased more than nicotine clearance indicates that the increase in clearance is most likely caused by induction of CYP2A6, and not by an increase in hepatic blood flow. A study comparing women during pregnancy and again postpartum, found that the mean salivary cotinine concentration per cigarette was higher when not pregnant (3.5 ng ml−1 vs. 9.9 ng ml−1), consistent with higher cotinine clearance during pregnancy (Rebagliato et al. 1998). Pregnant smokers had substantially lower levels of serum nicotine than expected when standardized for their nicotine intake compared to population-based values (Selby et al. 2001). Nicotine and cotinine glucuronidation is induced by pregnancy, while 3′-hydroxycotinine glucuronidation is not (Dempsey et al. 2002). Menstrual cycle (follicular phase vs. luteal phase) has no effect on nicotine and cotinine pharmacokinetics in healthy nonsmoking women (Hukkanen et al. 2005b). Pregnancy also increases the rate of formation of nicotine N′-oxide, indicating induction of the enzyme, flavin-containing monooxygenase 3 (Hukkanen et al. 2005a).
The above-mentioned results show that gender has substantial effects on nicotine and cotinine metabolism. Higher metabolism of nicotine and cotinine is detected in women than in men, in users of oral contraceptives than in women not using oral contraceptives, and in pregnant women than in the same subjects postpartum. Furthermore, the inducing effect has a dose–response relationship; gender differences are relatively small, oral contraceptive use further induces metabolism in women, and pregnancy shows the most striking induction compared to postpartum. Changes in clearance appear to be related to the amount of sex hormones present; women have higher concentrations of estrogens and progesterone than men do, oral contraceptive users have higher concentrations of these hormones than women not using oral contraceptives, and pregnancy results in the highest concentrations of circulating sex hormones. These results suggest that CYP2A6 activity is induced by sex hormones and there is recent in-vitro evidence for the induction of human CYP2A6 by estrogen acting on the estrogen receptor (Higashi et al. 2007).
Kidney Disease
Kidney failure not only decreases renal clearance of nicotine and cotinine, but also metabolic clearance of nicotine (Molander et al. 2000). Metabolic clearance of nicotine is reduced by 50% in subjects with severe renal impairment compared to healthy subjects. It is speculated that accumulation of uremic toxins may inhibit CYP2A6 activity or downregulate CYP2A6 expression in liver. Hepatic metabolism of several drugs is reduced in kidney failure, mainly via downregulation of CYP enzymes and/or inhibition of transporters (Nolin et al. 2003).
6.2 Medications
6.2.1 Inducers
A few drugs have been shown to induce CYP2A6 in human primary hepatocyte culture. These include prototypical inducers rifampicin, dexamethasone, and phenobarbital, although there is wide interindividual variability in response (Madan et al. 2003; Meunier et al. 2000; Rae et al. 2001). Rifampicin was also shown to inhibit CYP2A6 activity as measured by coumarin 7-hydroxylase (Xia et al. 2002). Thus the presence of rifampin may inhibit while chronic administration of rifampin may induce CYP2A6. That might explain the highly variable effects on CYP2A6 induction seen in studies with rifampicin.
There is evidence for the induction of CYP2A6 in vivo by phenobarbital and other anticonvulsant drugs. Two-day treatment with phenobarbital (100 mg per day p.o.) prior to a liver biopsy resulted in induction of metabolism of nicotine to cotinine in hepatocytes (Kyerematen et al. 1990). Liver microsomes from phenobarbital-treated patients have higher amounts of CYP2A6 protein than microsomes from untreated patients (Cashman et al. 1992). A recent study showed that the antimalarial drug artemisinin significantly altered the pharmacokinetics of both nicotine and coumarin, suggesting induction of CYP2A6. (Asimus et al. 2008).
As mentioned earlier, nicotine and cotinine clearances are higher in women using oral contraceptives than in women not using oral contraceptives (Benowitz et al. 2006). Oral contraceptive use induced nicotine and cotinine clearances by 28 and 30%, respectively. A previous small-scale study with caffeine phenotyping of CYP2A6 activity showed a 22% increase in CYP2A6 activity in oral contraceptive users compared to women not using contraceptives (Krul and Hageman 1998).
6.2.2 Inhibitors
Several compounds are inhibitors of CYP2A6-mediated nicotine metabolism in vitro, including methoxsalen (8-methoxypsoralen), tranylcypromine, tryptamine and coumarin (Le Gal et al. 2003; MacDougall et al. 2003; Nakajima et al. 1996; Zhang et al. 2001). Raloxifene is a potent inhibitor of aldehyde oxidase and it has been shown to inhibit the formation of cotinine from nicotine-Δ1′ (5′)-iminium ion in human liver cytosol (Obach 2004).
Only methoxsalen (used in the photochemotherapy of psoriasis) and tranyl-cypromine (a monoamine oxidase inhibitor) have been demonstrated to inhibit nicotine metabolism in people (Sellers et al. 2000, 2003). These compounds are only moderately specific for CYP2A6; methoxsalen is also a potent inhibitor of CYP1A2, and tranylcypromine inhibits CYP2B6 and CYP2E1 (Taavitsainen et al. 2001; Zhang et al. 2001). Methoxsalen reduces first-pass metabolism of oral nicotine, decreases clearance of subcutaneously administered nicotine, and decreases urinary levels of 3′-hydroxycotinine in smokers (Sellers et al. 2000, 2003). Tranyl-cypromine has been shown to reduce first-pass metabolism of oral nicotine (Tyndale and Sellers 2001). As smokers smoke at least in part to maintain desired levels of nicotine in the brain, decreased metabolism and higher concentration of nicotine result in a reduction in the number of cigarettes smoked (Sellers et al. 2000). Also, as CYP2A6 is involved in the activation of carcinogenic NNK, inhibition of CYP2A6 routes the metabolism of NNK towards the inactive NNAL-glucuronide (Sellers et al. 2003). Thus, CYP2A6 inhibitors might be of use in reduction of smoking, thereby decreasing the exposure to carcinogenic metabolites, possibly reducing the risk of cancer, and enhancing the efficacy of nicotine replacement therapies.
6.3 Smoking
6.3.1 Inhibiting Effect of Smoking on Nicotine Clearance
Cigarette smoking itself influences the rate of metabolism of nicotine. Cigarette smoking is known to accelerate the metabolism of some drugs, especially the ones primarily metabolized by CYP1A2 (Zevin and Benowitz 1999). However, we found that the clearance of nicotine was significantly slower in cigarette smokers than in nonsmokers (Benowitz and Jacob 1993). In support of this observation are crossover studies comparing the clearance of nicotine in the same subjects when smoking compared to when not smoking. After 4 days of smoking abstinence, nicotine clearance was increased by 14% (Benowitz and Jacob 2000), and after 7 days of abstinence, nicotine clearance was 36% higher (Lee et al. 1987), when compared to overnight abstinence from cigarettes.
These studies suggest that there are substance(s) in tobacco smoke, as yet unidentified, that inhibit the metabolism of nicotine. Because nicotine and cotinine are metabolized by the same enzyme, the possibility that cotinine might be responsible for the slowed metabolism of nicotine in smokers was examined. In a study in which nonsmokers received an intravenous infusion of nicotine with and without pretreatment with high doses of cotinine, there was no effect of cotinine on the clearance of nicotine (Zevin et al. 1997). Also, carbon monoxide at levels and in patterns similar to those experienced during smoking had no effect on nicotine and cotinine clearance (Benowitz and Jacob 2000).
Recently, β-nicotyrine, a minor tobacco alkaloid, was shown to effectively inhibit CYP2A6 in vitro (Denton et al. 2004). Thus, β-nicotyrine is one candidate in the search for the inhibiting compound in tobacco smoke. Another possibility is that reduced nicotine clearance is due to downregulation of CYP2A6 expression, and not due to inhibition. Tyndale and coworkers have demonstrated that administration of nicotine for 21 days to monkeys in vivo decreases CYP2A6 activity (nicotine metabolism) by downregulating CYP2A6 mRNA and protein in liver (Schoedel et al. 2003). Interestingly, expression of both CYP2A and CYP3A5 mRNAs are markedly reduced in human pulmonary tissues in smokers compared to nonsmokers (Crawford et al. 1998; Hukkanen et al. 2003). The mechanisms of the downregulation are currently unknown.
6.3.2 Inducing Effect of Smoking on Glucuronidation
The excretion of 3′-hydroxycotinine O-glucuronide is induced by smoking, when compared to not smoking studied with a crossover design (Benowitz and Jacob 2000). The extent of nicotine and cotinine N-glucuronidation was not significantly affected by smoking. Smoking is known to induce glucuronidation of some drugs, such as propranolol and oxazepam (Liston et al. 2001). Urinary excretion of 3′-hydroxycotinine O-glucuronide is correlated with the excretion of NNAL-O-glucuronide (Hecht et al. 1999b), which is formed by UGT1A9 and UGT2B7 (Ren et al. 2000). TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), an AHR (arylhydrocarbon receptor) agonist, induces UGT1A9 but does not induce UGT2B7 in human Caco-2 cells (Munzel et al. 1999). Thus, UGT1A9 could be the inducible component of 3′-hydroxycotinine O-glucuronidation.
6.4 Racial and Ethnic Differences
Racial differences in nicotine and cotinine metabolism have been observed. We compared nicotine and cotinine metabolism in blacks and whites (Benowitz et al. 1999; Perez-Stable et al. 1998). The total and nonrenal clearance of cotinine was significantly lower in blacks than in whites (total clearance 0.57 vs. 0.76 ml min−1 kg−1). Also, the fractional clearance of nicotine to cotinine, and the metabolic clearance of nicotine to cotinine were lower in blacks. The clearance of nicotine tended to be lower in blacks than in whites (18.1 vs. 20.5 ml min−1 kg−1), but this difference was not significant. Excretion of nicotine and cotinine glucuronides was lower in blacks, while excretion of 3′-hydroxycotinine glucuronide was similar in both groups. Nicotine and cotinine glucuronidation appeared to be polymorphic in blacks, with evidence of slow and fast N-glucuronide formers. The distribution of glucuronidation was unimodal in whites. Polymorphic patterns of cotinine glucuronidation in blacks has been detected in other studies (de Leon et al. 2002). Slower metabolism of cotinine explains in part the higher cotinine levels per cigarette detected in blacks than in whites (Caraballo et al. 1998; English et al. 1994; Wagenknecht et al. 1990). One possible explanation for the slower cotinine metabolism in blacks is the significantly higher proportion of menthol cigarette smokers in blacks than in whites (69% vs. 22% in the general US population, 76% vs. 9% in our study) (Benowitz et al. 1999; Giovino et al. 2004). As discussed earlier, menthol cigarette smoking inhibits nicotine oxidation and glucuronidation (Benowitz et al. 2004).
Nicotine and cotinine metabolism among Chinese–Americans, Latinos, and whites has been compared (Benowitz et al. 2002b). Chinese–Americans had the lowest total and nonrenal clearance of nicotine and cotinine, and lowest metabolic clearance of nicotine via the cotinine pathway. Also, nicotine intake per cigarette was lower in Chinese–Americans than in Latinos and whites. No significant differences in nicotine and cotinine metabolism or nicotine intake were detected between Latinos and whites. Glucuronidation of nicotine and metabolites did not differ between the groups. Consistent with the findings in experimental studies, Kandel et al. found in an epidemiologic study that the 3HC/cotinine ratio in the urine of young adult smokers, reflecting CYP2A6 activity, was higher in whites and Hispanics than in blacks and Asians (Kandel et al. 2007).
7 Renal Excretion
Nicotine is excreted by glomerular filtration and tubular secretion, with variable reabsorption depending on urinary pH. With uncontrolled urine pH, renal clearance averages about 35–90 ml min−1, accounting for the elimination of about 5% of total clearance. In acid urine, nicotine is mostly ionized and tubular reabsorption is minimized; renal clearance may be as high as 600 ml min−1 (urinary pH 4.4), depending on urinary flow rate (Benowitz and Jacob 1985). In alkaline urine, a larger fraction of nicotine is unionized, allowing net tubular reabsorption with a renal clearance as low as 17 ml min−1 (urine pH 7.0).
In vitro studies have shown that there are distinct transport systems for both basolateral and apical uptake of nicotine (Takami et al. 1998). Nicotine has been shown to be actively transported by kidney cells, most likely by the organic ion transporter OCT2 (Zevin et al. 1998; Urakami et al. 1998). Cimetidine decreases renal clearance of nicotine by 47% in nonsmoking volunteers (Bendayan et al. 1990). This is consistent with the inhibition of basolateral uptake by cimetidine detected in vitro. Mecamylamine reduces renal clearance of nicotine in smokers dosed with intravenous nicotine when urine is alkalinized, but not when urine is acidified (Zevin et al. 2000).
Renal clearance of cotinine is much less than the glomerular filtration rate (Benowitz et al. 2008b). Since cotinine is not appreciably protein bound, this indicates extensive tubular reabsorption. Renal clearance of cotinine can be enhanced by up to 50% with extreme urinary acidification. Cotinine excretion is less influenced by urinary pH than nicotine because it is less basic and, therefore, is primarily in the unionized form within the physiological pH range. As is the case for nicotine, the rate of excretion of cotinine is influenced by urinary flow rate. Renal excretion of cotinine is a minor route of elimination, averaging about 12% of total clearance. In contrast, 100% of nicotine N′-oxide and 63% of 3′-hydroxycotinine are excreted unchanged in the urine (Benowitz and Jacob 2001; Park et al. 1993).
The genetic contributions to nicotine and cotinine renal clearances have been estimated in a twin study (Benowitz et al. 2008b). This study found a substantial contribution of genetic factors to the net secretory/reabsorbtive clearances of nicotine and cotinine. These findings suggest either that the reabsorption of nicotine and cotinine are active processes and are influenced by the genetics of reabsorptive transporters, or that the active secretory component of renal clearance exerts a substantial effect on the clearance, even in the presence of net reabsorption. It is plausible that the genetic component of the variation in the reabsorbtive clearance of nicotine is determined by the corresponding variation in reabsorptive transporters.
As mentioned previously, renal failure markedly reduces total renal clearance, as well as metabolic clearance of nicotine and cotinine (Molander et al. 2000). Reduction of renal clearance is correlated with the severity of kidney failure; renal clearance is reduced by half in mild renal failure, and by 94% in severe renal impairment. Markedly elevated levels of serum nicotine have been detected in smoking patients with end-stage renal disease undergoing hemodialysis (Perry et al. 1984). This is explained not only by reduced renal clearance, but also by lower metabolic clearance of nicotine in renal disease. It is speculated that accumulation of uremic toxins inhibits CYP2A6 activity or downregulates CYP2A6 expression in liver.
8 Nicotine and Cotinine Blood Levels During Tobacco Use and Nicotine Replacement Therapy
Blood or plasma nicotine concentrations sampled in the afternoon in smokers generally range from 10 to 50 ng ml−1. Typical trough concentrations during daily smoking range between 10 and 37 ng ml−1 and typical peak concentrations range between 19 and 50 ng ml−1. The increment in venous blood nicotine concentration after smoking a single cigarette varies from 5 to 30 ng ml−1, depending on how a cigarette is smoked. In a recent study, the mean nicotine boost after smoking a cigarette was 10.9 ng ml−1 in smokers with no smoking abstinence on the study day (Patterson et al. 2003).
Blood levels peak at the end of smoking a cigarette and decline rapidly over the next 20 min due to tissue distribution. The distribution half-life averages about 8 min. Although the rate of rise of nicotine is slower for cigar smokers and users of snuff and chewing tobacco than for cigarette smokers, peak venous blood levels of nicotine are similar (Benowitz et al. 1988). Pipe smokers, particularly those who have previously smoked cigarettes, may have blood and urine levels of nicotine and cotinine as high as cigarette smokers (McCusker et al. 1982; Wald et al. 1981). Primary pipe smokers who have not previously smoked cigarettes tend to have lower nicotine levels. Likewise, cigar smokers who have previously smoked cigarettes may inhale more deeply and achieve higher blood levels of nicotine than primary cigar smokers, although on average, based on urinary cotinine levels, daily nicotine intake appears to be less for cigar smokers compared with cigarette or pipe smokers (Wald et al. 1984).
The plasma half-life of nicotine after intravenous infusion or cigarette smoking averages about 2 h. However, when half-life is determined using the time course of urinary excretion of nicotine, which is more sensitive in detecting lower levels of nicotine in the body, the terminal half-life averages 11 h (Jacob et al. 1999). The longer half-life detected at lower concentrations of nicotine is most likely a consequence of slow release of nicotine from body tissues. Based on a half-life of 2 h for nicotine, one would predict accumulation over 6–8 h (3–4 half-lives) of regular smoking and persistence of significant levels for 6–8 h after cessation of smoking. If a smoker smokes until bedtime, significant levels should persist all night. Studies of blood levels in regular cigarette smokers confirm these predictions (Benowitz et al. 1982b). Peak and trough levels follow each cigarette, but as the day progresses, trough levels rise and the influence of peak levels become less important. Thus, nicotine is not a drug to which smokers are exposed intermittently and which is eliminated rapidly from the body. On the contrary, smoking represents a multiple dosing situation with considerable accumulation while smoking and persistent levels for 24 h of each day.
Plasma levels of nicotine from nicotine replacement therapies tend to be in the range of low-level cigarette smokers. Thus, typical steady-state plasma nicotine concentrations with nicotine patches range from 10 to 20 ng ml−1, and for nicotine gum, inhaler, sublingual tablet, and nasal spray from 5 to 15 ng ml−1 (Benowitz et al. 1987; Schneider et al. 2001). Usually ad libitum use of NRTs results in one-third to two-thirds the concentration of nicotine that is achieved by cigarette smoking (Schneider et al. 2001). However, users of 4-mg nicotine gum may sometimes reach or even exceed the nicotine levels associated with smoking (McNabb 1984; McNabb et al. 1982). For the sake of comparison, systemic doses from various nicotine delivery systems are as follows: cigarette smoking, 1–1.5 mg per cigarette (Benowitz and Jacob 1984; Jarvis et al. 2001); nicotine gum, 2 mg for a 4-mg gum (Benowitz et al. 1988); transdermal nicotine, 5–21 mg per day, depending on the patch; nicotine nasal spray, 0.7 mg per 1-mg dose of one spray in each nostril (Gourlay and Benowitz 1997; Johansson et al. 1991); nicotine inhaler, 2 mg for a 4-mg dose released from the 10-mg inhaler (Molander et al. 1996); nicotine lozenge, 1 mg for a 2-mg lozenge (Choi et al. 2003); oral snuff, 3.6 mg for 2.5 g held in the mouth for 30 min (Benowitz et al. 1988); and chewing tobacco, 4.5 mg for 7.9 g chewed for 30 min (Benowitz et al. 1988).
Cotinine is present in the blood of smokers in much higher concentrations than those of nicotine. Cotinine blood concentrations average about 250–300 ng ml−1 in groups of cigarette smokers. We have seen levels in tobacco users ranging up to 900 ng ml−1. After stopping smoking, levels decline in a log linear fashion with an average half-life of about 16 h. The half-life of cotinine derived from nicotine is longer than the half-life of cotinine administered as cotinine (Zevin et al. 1997). This is caused by slow release of nicotine from tissues. Because of the long half-life there is much less fluctuation in cotinine concentrations throughout the day than in nicotine concentrations. As expected, there is a gradual rise in cotinine levels throughout the day, peaking at the end of smoking and persisting at high concentrations overnight. Cotinine levels produced by NRTs are usually 30–70% of the levels detected while smoking (Hurt et al. 1994; Schneider et al. 1995).
9 Biomarkers of Nicotine Exposure
Biomarkers are desirable for quantifying the systemic exposure of smokers to toxic constituents of smoke derived from tobacco use or from potential reduced harm products. Measures such as cigarettes per day are imprecise indicators of tobacco smoke exposure because of variability in how smokers smoke their cigarettes. There is considerable individual variability in smoke intake, even by people smoking the same brand of cigarettes (USDHHS 2001). Cigarette design and how the cigarette is smoked influence toxic exposures. For example, light cigarettes are smoked on average more intensely than are regular cigarettes. The optimal assessment of exposure to tobacco smoke would be the analysis of concentrations of chemicals of pathogenetic concern in body fluids of the exposed individual – termed a biological marker or biomarker. A variety of biomarkers of tobacco smoke exposure have been proposed, as summarized in Table 2 and reviewed in detail previously (Hatsukami et al. 2003).
Table 2.
Biomarkers of tobacco exposure
| Biomarker | Precursor | Specimen | t½ | Tobacco specific | Other sources |
|---|---|---|---|---|---|
| Nicotine* | Nicotine | Blood, urine, saliva, hair | 1–2 h | Yes | Nicotine replacement products |
| Cotinine* | Nicotine | Blood, urine, saliva, hair | 16–18 h | Yes | Nicotine replacement products |
| Anatabine* | Anatabine | Urine | 10–16 h | Yes | None |
| NNAL, NNAL-glucuronides | NNK (TSNA) | Blood, urine | 6 week | Yes | None |
| Exhaled CO | Carbon monoxide | Exhaled air | 2–6 h | No | Traffic, body formation |
| Carboxyhemoglobin | carbon monoxide | Blood | 4–6 h | No | Traffic, body formation |
| 1-Hydroxypyrene and other polycyclic aromatic hydrocarbon (PAH) metabolites | PAHs | Urine | 20 h | No | Traffic, grilled meat, occupation, biomass combustion in homes |
| Mercopturic acid metabolites | 1,3-Butadiene | Urine | – | No | Traffic, combustion products |
| Mercopturic acid metabolites | Acrolein | Urine | – | No | Traffic, combustion products |
| Acetonitrile | Acetonitrile | Urine, blood, exhaled air | 32 h | No | None |
| S-Phenyl-mercapturic acid | Benzene | Urine | 9 h | No | Traffic, combustion products |
| Thiocyanate | Hydrogen cyanide | Serum, saliva, urine | 7–14 days | No | Diet |
NNAL 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNAL-gluc 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronide; NNK 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; TSNA tobacco-specific nitrosamines; PAH polycylcic aromatic hydrocarbons
In studies of smoking cessation, anatabine is recommended as nicotine replacement therapies will lead to the presence of nicotine and cotinine without any tobacco exposure
This section focuses on the use of nicotine and cotinine and other tobacco alkaloids as biomarkers of tobacco exposure. Other potential biomarkers of exposure to the particulate or gas phase of tobacco smoke are described in the review papers cited above.
Nicotine measurement is highly specific for tobacco use or exposure (in the absence of nicotine medication use), but because of nicotine’s short half-life (2 h) the method is not recommended for general use. Cotinine is a highly specific and sensitive marker for tobacco use (in the absence nicotine medication use) and has the advantages of a fairly long half-life (16 h). When NRT is not being used, cotinine appears to be the best biomarker for tobacco use. When NRT is used, the minor tobacco alkaloids are useful biomarkers, as described below. A limitation of using cotinine is that it indicates ongoing exposure but not long-term exposure to tobacco smoke. Approaches to longer term monitoring include measurement of nicotine in hair or nails, as discussed below, or measurement of the tobacco-specific nitrosamine 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanol (NNAL) in urine, as described by (Hecht 2003).
9.1 Cotinine as a Biomarker for Intake of Nicotine
The presence of cotinine in biological fluids indicates exposure to nicotine. Because of the long half-life of cotinine it has been used as a biomarker for daily intake, both in cigarette smokers and in those exposed to secondhand tobacco smoke (Benowitz 1996). There is a high correlation among cotinine concentrations measured in plasma, saliva, and urine, and measurements in any one of these fluids can be used as a marker of nicotine intake. There is, however, individual variability in the quantitative relationship between steady state cotinine levels and intake of nicotine. This is because different people convert different percentages of nicotine to cotinine (usual range 50–90%), and because different people metabolize cotinine differently at different rates (usual clearance range 20–75 ml min−1) (Benowitz 1996).
The relationship between nicotine intake and steady state cotinine blood levels can be expressed in the following way, based on steady state exposure conditions: Dnic = CLCOT × CCOT ÷ f, where Dnic is the intake (dose) of nicotine, CLCOT is the clearance of cotinine, CCOT is the steady state blood concentration of cotinine and f is the fraction of nicotine converted to cotinine. On rearranging the equation, Dnic = (CLCOT ÷ f) × CCOT = K × CCOT where K is a constant that converts a given blood level of cotinine to nicotine intake. On average, K = 0.08 mg 24 h−1 ng−1 ml−1 (range 0.05–1.1, CV = 21.9%). Thus, a cotinine level of 30 ng ml−1 in blood corresponds on average to a nicotine intake of 24 mg per day.
While cotinine functions fairly well as a marker of nicotine intake, it is not perfect due to individual variation in metabolism as discussed previously. As described earlier in this chapter, cotinine metabolism is affected by factors such as race, gender, age, genetic variation in the liver enzyme CYP2A6, and/or by the presence of pregnancy, liver or kidney disease. Another limitation to the use of cotinine is that, given an average half-life of 16 h, cotinine levels reflect relatively short-term exposure to tobacco (that is, over the past 3–4 days).
9.2 Nicotine and Cotinine in Hair and Nails
The use of hair as a material in which to measure nicotine and cotinine has been proposed as a way to assess long-term exposure to nicotine from tobacco products. Nicotine and cotinine are incorporated into hair as it grows over time. The average rate of hair growth is 1 cm per month. Thus, measurements of levels of nicotine may provide a way of assessing exposure of a person to nicotine over several months (Al-Delaimy et al. 2002; Florescu et al. 2007).
Potentials problems with the use of hair include a strong influence of hair pigmentation on nicotine and cotinine binding and uptake (Dehn et al. 2001). Nicotine and cotinine are bound to melanin. As a result, dark hair binds much more nicotine than does blond or white hair. This makes comparison across individuals difficult. Also, hair is exposed to nicotine and cotinine from sweat and from sebaceous gland secretions, and to nicotine from environmental tobacco smoke exposure. Washing the hair before analysis may reduce this problem of environmental contamination, but it is not likely to remove all environmental nicotine and cotinine.
Nicotine and cotinine, as well as NNAL, can be measured in nail clippings (Stepanov et al. 2007). Toenail clippings are easy to collect and store and represent cumulative exposure as nails grow at a rate of about 0.1 cm per month. In a group of smokers, the average toenail biomarker concentrations were 5.4 ng nicotine and 0.67 ng cotinine per mg toenail. Plasma levels of nicotine and cotinine were significantly but moderately correlated with toenail levels. Thus, hair or toenail measurements of nicotine or cotinine (or NNAL) are promising biomarkers of long-term tobacco exposure.
9.3 Dietary Sources
Dietary sources of nicotine have been alleged to be a potential confounder of cotinine levels used in measurement of secondhand smoke exposure. Several foods contain small amounts of nicotine (Siegmund et al. 1999). However, the levels of nicotine in foods are quite low. Based on nicotine levels in foods and the usual daily consumption of various nicotine-containing foods, it has been determined that the levels of cotinine produced by even a diet high in nicotine-containing foods is lower than that seen in individuals exposed to moderate levels of secondhand smoke (Benowitz 1996).
9.4 Minor Tobacco Alkaloids
The primary alkaloid in tobacco is nicotine, but tobacco also contains small amounts of minor alkaloids such as anabasine, anatabine, myosmine, and others. The minor alkaloids are absorbed systemically and can be measured in the urine of smokers and users of smokeless tobacco (Jacob et al. 1999). The measurement of minor alkaloids is a way to quantitate tobacco use when a person is also taking in pure nicotine from a nicotine medication or a nontobacco nicotine delivery system. This method has been used to assess tobacco abstinence in clinical trials of smoking cessation with treatment by nicotine medications (Jacob et al. 2002).
9.5 Optimal Cotinine Cut-Points to Distinguish Tobacco Use From No Tobacco Use
Based on the work of Jarvis and coworkers, who measured cotinine levels in individuals attending outpatient clinics in the United Kingdom in the early 1980s, an optimal plasma or saliva cotinine cut-point of 15 ng ml−1 or a urine cotinine of 50 ng ml−1 were determined to discriminate smokers from nonsmokers (some of whom are exposed to secondhand smoke) (Benowitz et al. 2002a). The optimal cut-point depends on the smoking behavior of the smokers and the magnitude of exposure to secondhand smoke. Data from the National Health and Nutrition Examination Surveys (NHANES) from 1999 to 2004 were recently analyzed to assess the optimal serum cotinine in the US population at present (Benowitz et al. 2008a). Using receiver operator characteristic curve analysis, the optimal cotinine cut-points were 3.08 ng ml−1 for adults (sensitivity 96.3%, specificity 97.4%) and 2.99 ng ml−1 for adolescents (sensitivity 86.5%, specificity 93.1%). The decline in the optimal cut-point since 1980 is likely due to the marked reduction in secondhand smoke exposure in the general US population. Of note is that the cut-points are much lower for Mexican Americans than for whites or African Americans, most likely due to both more occasional smoking and lower exposure to secondhand smoke.
Acknowledgments
We thank Marc Olmsted for his excellent editorial assistance. Much of the research described in this chapter was supported by US Public Health Service grants DA02277 and DA12393 from the National Institute on Drug Abuse, National Institutes of Health, and carried out at the General Clinical Research Center at San Francisco General Hospital Medical Center with support of the Division of Research Resources, National Institutes of Health (RR-00083).
References
- Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002;56(1):66–71. doi: 10.1136/jech.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage A, Dollery C, Houseman T, Kohner E, Lewis PJ, Turner D. Absorption of nicotine from small cigars. Clin Pharmacol Ther. 1978;23(2):143–151. doi: 10.1002/cpt1978232143. [DOI] [PubMed] [Google Scholar]
- Armstrong DW, Wang X, Ercal N. Enantiomeric composition of nicotine in smokeless tobacco, medicinal products, and commercial reagents. Chirality. 1998;10:587–591. [Google Scholar]
- Asimus S, Hai TN, Van Huong N, Ashton M. Artemisin and CYP2A6 activity in healthy subjects. Eur J Clin Pharmacol. 2008;64:283–292. doi: 10.1007/s00228-007-0406-1. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Sullivan JT, Shaw C, Frecker RC, Sellers EM. Effect of cimetidine and ranitidine on the hepatic and renal elimination of nicotine in humans. Eur J Clin Pharmacol. 1990;38(2):165–169. doi: 10.1007/BF00265978. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. NIDA Res Monogr. 1990;99:12–29. [PubMed] [Google Scholar]
- Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35(4):499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Nicotine renal excretion rate influences nicotine intake during cigarette smoking. J Pharmacol Exp Ther. 1985;234(1):153–155. [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53(3):316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56(5):483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Effects of cigarette smoking and carbon monoxide on nicotine and cotinine metabolism. Clin Pharmacol Ther. 2000;67(6):653–659. doi: 10.1067/mcp.2000.107086. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Trans-3′-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking. Br J Clin Pharmacol. 2001;51(1):53–59. doi: 10.1046/j.1365-2125.2001.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982a;221(2):368–372. [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P., 3rd Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982b;32(6):758–764. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Savanapridi C. Determinants of nicotine intake while chewing nicotine polacrilex gum. Clin Pharmacol Ther. 1987;41(4):467–473. doi: 10.1038/clpt.1987.58. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268(1):296–303. [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291(3):1196–1203. [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, 3rd, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002a;4:149–159. [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002b;94(2):108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal Serum Cotinine Levels to Distinguish Cigarette Smokers and Non-Smokers within Different Racial/Ethnic Groups in the United States Between 1999–2004. Am J Epidemiol. 2008a doi: 10.1093/aje/kwn301. (in press) [DOI] [PubMed] [Google Scholar]
- Benowitz N, Lessov-Schlaggar C, Swan G. Genetic Influences in the Variation in Renal Clearance of Nicotine and Cotinine. Clin Pharmacol Ther. 2008b;84(2):243–247. doi: 10.1038/clpt.2008.54. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282(1):7–13. [PubMed] [Google Scholar]
- Byrd GD, Chang KM, Greene JM, deBethizy JD. Evidence for urinary excretion of glucuronide conjugates of nicotine, cotinine, and trans-3′-hydroxycotinine in smokers. Drug Metab Dispos. 1992;20(2):192–197. [PubMed] [Google Scholar]
- Byrd GD, Uhrig MS, deBethizy JD, Caldwell WS, Crooks PA, Ravard A, Riggs R. Direct determination of cotinine-N-glucuronide in urine using thermospray liquid chromatography/mass spectrometry. Biol Mass Spectrom. 1994;23(2):103–107. doi: 10.1002/bms.1200230210. [DOI] [PubMed] [Google Scholar]
- Caraballo RS, Giovino GA, Pechacek TF, Mowery PD, Richter PA, Strauss WJ, Sharp DJ, Eriksen MP, Pirkle JL, Maurer KR. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA. 1998;280(2):135–139. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Park SB, Yang ZC, Wrighton SA, Jacob P, Benowitz NL. Metabolism of nicotine by human liver microsomes: stereoselective formation of trans-nicotine N′-oxide. Chem Res Toxicol. 1992;5(5):639–646. doi: 10.1021/tx00029a008. [DOI] [PubMed] [Google Scholar]
- Choi JH, Dresler CM, Norton MR, Strahs KR. Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob Res. 2003;5(5):635–644. doi: 10.1080/1462220031000158690. [DOI] [PubMed] [Google Scholar]
- Collier AM, Goldstein GM, Shrewsbury RP, Davis SM, Koch GG, Zhang C-A, Benowitz NL, Lewtas J, Williams RW. Cotinine elimination and its use as a biomarker in young children involuntarily exposed to environmental tobacco smoke. Indoor Environ. 1994;3:353–359. [Google Scholar]
- Crawford EL, Weaver DA, DeMuth JP, Jackson CM, Khuder SA, Frampton MW, Utell MJ, Thilly WG, Willey JC. Measurement of cytochrome P450 2A6 and 2E1 gene expression in primary human bronchial epithelial cells. Carcinogenesis. 1998;19(10):1867–1871. doi: 10.1093/carcin/19.10.1867. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Lundell B, Curvall M, Thapper L. Nicotine and cotinine concentrations in the nursing mother and her infant. Acta Paediatr Scand. 1990;79(2):142–147. doi: 10.1111/j.1651-2227.1990.tb11430.x. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ, Rogers T, Browne D, Dinsmore L, Ghosheh OH, Dwoskin LP, Crooks PA. Total cotinine in plasma: a stable biomarker for exposure to tobacco smoke. J Clin Psychopharmacol. 2002;22(5):496–501. doi: 10.1097/00004714-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Dehn DL, Claffey DJ, Duncan MW, Ruth JA. Nicotine and cotinine adducts of a melanin intermediate demonstrated by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Chem Res Toxicol. 2001;14(3):275–279. doi: 10.1021/tx000205l. [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001;24(4):277–322. doi: 10.2165/00002018-200124040-00005. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67(5):458–465. doi: 10.1067/mcp.2000.106129. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, Allen F, Schoedel K, Tyndale RF, Benowitz NL. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Denton TT, Zhang X, Cashman JR. Nicotine-related alkaloids and metabolites as inhibitors of human cytochrome P-450 2A6. Biochem Pharmacol. 2004;67(4):751–756. doi: 10.1016/j.bcp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- English PB, Eskenazi B, Christianson RE. Black-white differences in serum cotinine levels among pregnant women and subsequent effects on infant birthweight. Am J Public Health. 1994;84(9):1439–1443. doi: 10.2105/ajph.84.9.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP. A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacol Biochem Behav. 2000;67(3):479–482. doi: 10.1016/s0091-3057(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Florescu A, Ferrence R, Einarson TR, Selby P, Kramer M, Woodruff S, Grossman L, Rankin A, Jacqz-Aigrain E, Koren G. Reference values for hair cotinine as a biomarker of active and passive smoking in women of reproductive age, pregnant women, children, and neonates: systematic review and meta-analysis. Ther Drug Monit. 2007;29(4):437–446. doi: 10.1097/FTD.0b013e318074df6e. [DOI] [PubMed] [Google Scholar]
- Giovino G, Sidney S, Gfroerer J, O’Malley P, Allen J, Richter P, Ph DK. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6(Suppl 1):S67–81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Gori GB, Benowitz NL, Lynch CJ. Mouth versus deep airways absorption of nicotine in cigarette smokers. Pharmacol Biochem Behav. 1986;25(6):1181–1184. doi: 10.1016/0091-3057(86)90108-5. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. The benefits of stopping smoking and the role of nicotine replacement therapy in older patients. Drugs Aging. 1996;9(1):8–23. doi: 10.2165/00002512-199609010-00002. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther. 1997;62(4):453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- Gow PJ, Ghabrial H, Smallwood RA, Morgan DJ, Ching MS. Neonatal hepatic drug elimination. Pharmacol Toxicol. 2001;88(1):3–15. doi: 10.1034/j.1600-0773.2001.088001003.x. [DOI] [PubMed] [Google Scholar]
- Gries JM, Benowitz N, Verotta D. Chronopharmacokinetics of nicotine. Clin Pharmacol Ther. 1996;60(4):385–395. doi: 10.1016/S0009-9236(96)90195-2. [DOI] [PubMed] [Google Scholar]
- Guthrie SK, Zubieta JK, Ohl L, Ni L, Koeppe RA, Minoshima S, Domino EF. Arterial/venous plasma nicotine concentrations following nicotine nasal spray. Eur J Clin Pharmacol. 1999;55(9):639–643. doi: 10.1007/s002280050686. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hecht SS, Hennrikus DJ, Joseph AM, Pentel PR. Biomarkers of tobacco exposure or harm: application to clinical and epidemiological studies. 25–26 October 2001, Minneapolis, Minnesota. Nicotine Tob Res. 2003;5(3):387–396. doi: 10.1080/1462220031000094222. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999a;59(3):590–596. [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev. 1999b;8(10):907–913. [PubMed] [Google Scholar]
- Hecht SS, Hochalter JB, Villalta PW, Murphy SE. 2′-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor. Proc Natl Acad Sci U S A. 2000;97(23):12493–12497. doi: 10.1073/pnas.220207697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61(5):743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33(1):23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, Nakajima M. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935–1941. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Vaisanen T, Lassila A, Piipari R, Anttila S, Pelkonen O, Raunio H, Hakkola J. Regulation of CYP3A5 by glucocorticoids and cigarette smoke in human lung-derived cells. J Pharmacol Exp Ther. 2003;304(2):745–752. doi: 10.1124/jpet.102.038208. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Dempsey D, Jacob P, Benowitz NL. Effect of pregnancy on a measure of FMO3 activity. Br J Clin Pharmacol. 2005a;60(2):224–226. doi: 10.1111/j.1365-2125.2005.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Gourlay SG, Kenkare S, Benowitz NL. Influence of menstrual cycle on cytochrome P450 2A6 activity and cardiovascular effects of nicotine. Clin Pharmacol Ther. 2005b;77(3):159–169. doi: 10.1016/j.clpt.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005c;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Effect of grapefruit juice on cytochrome P450 2A6 and nicotine renal clearance. Clin Pharmacol Ther. 2006;80(5):522–530. doi: 10.1016/j.clpt.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, Lauger GG, Marusic Z, Neese LW, Lundberg TG. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994;271(8):595–600. [PubMed] [Google Scholar]
- Jacob P, 3rd, Benowitz NL. Oxidative metabolism of nicotine in vivo. In: Adlkofer F, Thurau K, editors. Effects of nicotine on biological systems. Birkhauser Verlag; Basel: 1991. pp. 35–44. [Google Scholar]
- Jacob P, Shulgin AT, Benowitz NL. Synthesis of (3′R, 5′S)-trans-3′-hydroxycotinine, a major metabolite of nicotine. Metabolic formation of 3′-hydroxycotinine in humans is highly stereoselective. J Med Chem. 1990;33(7):1888–1891. doi: 10.1021/jm00169a009. [DOI] [PubMed] [Google Scholar]
- Jacob P, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999;89(5):731–736. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1668–1673. [PubMed] [Google Scholar]
- Jarvis MJ, Boreham R, Primatesta P, Feyerabend C, Bryant A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: evidence from a representative population survey. J Natl Cancer Inst. 2001;93(2):134–138. doi: 10.1093/jnci/93.2.134. [DOI] [PubMed] [Google Scholar]
- Johansson CJ, Olsson P, Bende M, Carlsson T, Gunnarsson PO. Absolute bioavailability of nicotine applied to different nasal regions. Eur J Clin Pharmacol. 1991;41(6):585–588. doi: 10.1007/BF00314989. [DOI] [PubMed] [Google Scholar]
- Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, Murphy M, Walton R. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80(4):319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Schaffran C, Udry JR, Benowitz NL. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol. 2007;165(8):901–910. doi: 10.1093/aje/kwm010. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Mehta NY, Sweeney CT, Schwartz SS, Vogler GP, Jarvis MJ, West RJ. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7(4):369–375. doi: 10.1136/tc.7.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul C, Hageman G. Analysis of urinary caffeine metabolites to assess biotransformation enzyme activities by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;709(1):27–34. doi: 10.1016/s0378-4347(98)00016-4. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Morgan M, Warner G, Martin LF, Vesell ES. Metabolism of nicotine by hepatocytes. Biochem Pharmacol. 1990;40(8):1747–1756. doi: 10.1016/0006-2952(90)90351-k. [DOI] [PubMed] [Google Scholar]
- Le Gal A, Dreano Y, Lucas D, Berthou F. Diversity of selective environmental substrates for human cytochrome P450 2A6: alkoxyethers, nicotine, coumarin, N-nitrosodiethylamine, and N-nitrosobenzylmethylamine. Toxicol Lett. 2003;144(1):77–91. doi: 10.1016/s0378-4274(03)00229-7. [DOI] [PubMed] [Google Scholar]
- Lee BL, Benowitz NL, Jacob P., 3rd Influence of tobacco abstinence on the disposition kinetics and effects of nicotine. Clin Pharmacol Ther. 1987;41(4):474–479. doi: 10.1038/clpt.1987.59. [DOI] [PubMed] [Google Scholar]
- Lee BL, Jacob P, 3rd, Jarvik ME, Benowitz NL. Food and nicotine metabolism. Pharmacol Biochem Behav. 1989;33(3):621–625. doi: 10.1016/0091-3057(89)90398-5. [DOI] [PubMed] [Google Scholar]
- Leete E. Biosynthesis and metabolism of the tobacco alkaloids. In: Pelletier SW, editor. Alkaloids: chemical and biological perspectives. Wiley; New York: 1983. pp. 85–152. [Google Scholar]
- Leong JW, Dore ND, Shelley K, Holt EJ, Laing IA, Palmer LJ, LeSouef PN. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther. 1998;11(4):287–290. doi: 10.1006/pupt.1998.0153. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Lindell G, Lunell E, Graffner H. Transdermally administered nicotine accumulates in gastric juice. Eur J Clin Pharmacol. 1996;51(3–4):315–318. doi: 10.1007/s002280050204. [DOI] [PubMed] [Google Scholar]
- Liston HL, Markowitz JS, DeVane CL. Drug glucuronidation in clinical psychopharmacology. J Clin Psychopharmacol. 2001;21(5):500–515. doi: 10.1097/00004714-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Lunell E, Molander L, Ekberg K, Wahren J. Site of nicotine absorption from a vapour inhaler–comparison with cigarette smoking. Eur J Clin Pharmacol. 2000;55(10):737–741. doi: 10.1007/s002280050007. [DOI] [PubMed] [Google Scholar]
- MacDougall JM, Fandrick K, Zhang X, Serafin SV, Cashman JR. Inhibition of human liver microsomal (S)-nicotine oxidation by (−)-menthol and analogues. Chem Res Toxicol. 2003;16(8):988–993. doi: 10.1021/tx0340551. [DOI] [PubMed] [Google Scholar]
- Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, Downey AD, Czerwinski M, Forster J, Ribadeneira MD, Gan LS, LeCluyse EL, Zech K, Robertson P, Jr, Koch P, Antonian L, Wagner G, Yu L, Parkinson A. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003;31(4):421–431. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- McBride JS, Altman DG, Klein M, White W. Green tobacco sickness. Tob Control. 1998;7(3):294–298. doi: 10.1136/tc.7.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker K, McNabb E, Bone R. Plasma nicotine levels in pipe smokers. JAMA. 1982;248(5):577–578. [PubMed] [Google Scholar]
- McKennis H, Jr, Turnbull LB, Bowman ER, Tamaki E. The synthesis of hydroxycotinine and studies on its structure. J Org Chem. 1963;28:383–387. [Google Scholar]
- McNabb ME. Chewing nicotine gum for 3 months: what happens to plasma nicotine levels? Can Med Assoc J. 1984;131(6):589–592. [PMC free article] [PubMed] [Google Scholar]
- McNabb ME, Ebert RV, McCusker K. Plasma nicotine levels produced by chewing nicotine gum. JAMA. 1982;248(7):865–868. [PubMed] [Google Scholar]
- Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282(3):1608–1614. [PubMed] [Google Scholar]
- Meunier V, Bourrie M, Julian B, Marti E, Guillou F, Berger Y, Fabre G. Expression and induction of CYP1A1/1A2, CYP2A6 and CYP3A4 in primary cultures of human hepatocytes: a 10-year follow-up. Xenobiotica. 2000;30(6):589–607. doi: 10.1080/004982500406426. [DOI] [PubMed] [Google Scholar]
- Molander L, Lunell E, Andersson SB, Kuylenstierna F. Dose released and absolute bioavailability of nicotine from a nicotine vapor inhaler. Clin Pharmacol Ther. 1996;59(4):394–400. doi: 10.1016/S0009-9236(96)90107-1. [DOI] [PubMed] [Google Scholar]
- Molander L, Hansson A, Lunell E, Alainentalo L, Hoffmann M, Larsson R. Pharmacokinetics of nicotine in kidney failure. Clin Pharmacol Ther. 2000;68(3):250–260. doi: 10.1067/mcp.2000.109006. [DOI] [PubMed] [Google Scholar]
- Molander L, Hansson A, Lunell E. Pharmacokinetics of nicotine in healthy elderly people. Clin Pharmacol Ther. 2001;69(1):57–65. doi: 10.1067/mcp.2001.113181. [DOI] [PubMed] [Google Scholar]
- Munzel PA, Schmohl S, Heel H, Kalberer K, Bock-Hennig BS, Bock KW. Induction of human UDP glucuronosyltransferases (UGT1A6, UGT1A9, and UGT2B7) by t-butylhydroquinone and 2,3,7,8-tetrachlorodibenzo-p-dioxin in Caco-2 cells. Drug Metab Dispos. 1999;27(5):569–573. [PubMed] [Google Scholar]
- Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24(11):1212–1217. [PubMed] [Google Scholar]
- Neurath GB. Aspects of the oxidative metabolism of nicotine. Clin Investig. 1994;72(3):190–195. doi: 10.1007/BF00189309. [DOI] [PubMed] [Google Scholar]
- Neurath GB, Dunger M, Orth D, Pein FG. Trans-3′-hydroxycotinine as a main metabolite in urine of smokers. Int Arch Occup Environ Health. 1987;59(2):199–201. doi: 10.1007/BF00378497. [DOI] [PubMed] [Google Scholar]
- Neurath GB, Orth D, Pein FG. Detection of nornicotine in human urine after infusion of nicotine. In: Adlkofer F, Thurau K, editors. Effects of nicotine on biological systems. Birkhauser Verlag; Basel: 1991. pp. 45–49. [Google Scholar]
- Nolin TD, Frye RF, Matzke GR. Hepatic drug metabolism and transport in patients with kidney disease. Am J Kidney Dis. 2003;42(5):906–925. doi: 10.1016/j.ajkd.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Obach RS. Potent inhibition of human liver aldehyde oxidase by raloxifene. Drug Metab Dispos. 2004;32(1):89–97. doi: 10.1124/dmd.32.1.89. [DOI] [PubMed] [Google Scholar]
- Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14(11):1465–1481. doi: 10.1021/tx0100901. [DOI] [PubMed] [Google Scholar]
- Park SB, Jacob P, Benowitz NL, Cashman JR. Stereoselective metabolism of (S)-(−)-nicotine in humans: formation of trans-(S)-(−)-nicotine N-1′-oxide. Chem Res Toxicol. 1993;6(6):880–888. doi: 10.1021/tx00036a019. [DOI] [PubMed] [Google Scholar]
- Patterson F, Benowitz N, Shields P, Kaufmann V, Jepson C, Wileyto P, Kucharski S, Lerman C. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12(5):468–471. [PubMed] [Google Scholar]
- Patterson F, Schnoll R, Wileyto E, Pinto A, Epstein L, Shields P, Hawk L, Tyndale R, Benowitz N, Lerman C. Toward Personalized Therapy for Smoking Cessation: A Randomized Placebo-controlled Trial of Bupropion. Clin Pharmacol Ther. 2008;84(3):320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Griffiths W, Dextraze P, Solomon RJ, Trebbin WM. Elevated nicotine levels in patients undergoing hemodialysis. A role in cardiovascular mortality and morbidity? Am J Med. 1984;76(2):241–246. doi: 10.1016/0002-9343(84)90780-0. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289(3):1545–1552. [PubMed] [Google Scholar]
- Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299(3):849–857. [PubMed] [Google Scholar]
- Rebagliato M, Bolumar F, Florey Cdu V, Jarvis MJ, Perez-Hoyos S, Hernandez-Aguado I, Avino MJ. Variations in cotinine levels in smokers during and after pregnancy. Am J Obstet Gynecol. 1998;178(3):568–571. doi: 10.1016/s0002-9378(98)70440-5. [DOI] [PubMed] [Google Scholar]
- Ren Q, Murphy SE, Zheng Z, Lazarus P. O-Glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1- (3-pyridyl)-1-butanol (NNAL) by human UDP-glucuronosyltransferases 2B7 and 1A9. Drug Metab Dispos. 2000;28(11):1352–1360. [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend. 1999;56(2):99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Runkel M, Bourian M, Tegtmeier M, Legrum W. The character of inhibition of the metabolism of 1,2-benzopyrone (coumarin) by grapefruit juice in human. Eur J Clin Pharmacol. 1997;53(3–4):265–269. doi: 10.1007/s002280050374. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, Steinberg C. Efficacy of a nicotine nasal spray in smoking cessation: a placebo-controlled, double-blind trial. Addiction. 1995;90(12):1671–1682. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet. 2001;40(9):661–684. doi: 10.2165/00003088-200140090-00003. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Sellers EM, Palmour R, Tyndale RF. Down-regulation of hepatic nicotine metabolism and a CYP2A6-like enzyme in African green monkeys after long-term nicotine administration. Mol Pharmacol. 2003;63(1):96–104. doi: 10.1124/mol.63.1.96. [DOI] [PubMed] [Google Scholar]
- Selby P, Hackman R, Kapur B, Klein J, Koren G. Heavily smoking women who cannot quit in pregnancy: evidence of pharmacokinetic predisposition. Ther Drug Monit. 2001;23(3):189–191. doi: 10.1097/00007691-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Kaplan HL, Tyndale RF. Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clin Pharmacol Ther. 2000;68(1):35–43. doi: 10.1067/mcp.2000.107651. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF. The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine Tob Res. 2003;5(6):891–899. doi: 10.1080/14622200310001615231. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Trevor AJ, Castagnoli N., Jr Metabolism-dependent covalent binding of (S)-[5–3H]nicotine to liver and lung microsomal macromolecules. Drug Metab Dispos. 1988;16(3):397–402. [PubMed] [Google Scholar]
- Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric Food Chem. 1999;47(8):3113–3120. doi: 10.1021/jf990089w. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Hecht SS, Lindgren B, Jacob P, 3rd, Wilson M, Benowitz NL. Relationship of human toenail nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol to levels of these biomarkers in plasma and urine. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1382–1386. doi: 10.1158/1055-9965.EPI-07-0145. [DOI] [PubMed] [Google Scholar]
- Swan GE, Benowitz NL, Lessov CN, Jacob P, Tyndale RF, Wilhelmsen K. Nicotine metabolism: the impact of CYP2A6 on estimates of additive genetic influence. Pharmacogenet Genomics. 2005;15(2):115–125. doi: 10.1097/01213011-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Taavitsainen P, Juvonen R, Pelkonen O. In vitro inhibition of cytochrome P450 enzymes in human liver microsomes by a potent CYP2A6 inhibitor, trans-2-phenylcyclopropylamine (tranylcypromine), and its nonamine analog, cyclopropylbenzene. Drug Metab Dispos. 2001;29(3):217–222. [PubMed] [Google Scholar]
- Takami K, Saito H, Okuda M, Takano M, Inui KI. Distinct characteristics of transcellular transport between nicotine and tetraethylammonium in LLC-PK1 cells. J Pharmacol Exp Ther. 1998;286(2):676–680. [PubMed] [Google Scholar]
- Tateishi T, Nakura H, Asoh M, Watanabe M, Tanaka M, Kumai T, Takashima S, Imaoka S, Funae Y, Yabusaki Y, Kamataki T, Kobayashi S. A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life Sci. 1997;61(26):2567–2574. doi: 10.1016/s0024-3205(97)01011-4. [DOI] [PubMed] [Google Scholar]
- Tyndale RF, Sellers EM. Variable CYP2A6-mediated nicotine metabolism alters smoking behavior and risk. Drug Metab Dispos. 2001;29(4 Pt 2):548–552. [PubMed] [Google Scholar]
- Tyroller S, Zwickenpflug W, Richter E. New sources of dietary myosmine uptake from cereals, fruits, vegetables, and milk. J Agric Food Chem. 2002;50(17):4909–4915. doi: 10.1021/jf020281p. [DOI] [PubMed] [Google Scholar]
- Urakami Y, Okuda M, Maasuda S, Saito H, Inui KI. Functional characteristics and membrane locatlization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Ther. 1998;287:800–805. [PubMed] [Google Scholar]
- USDHHS. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Smoking and Tobacco Control Monographs, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2001. [Google Scholar]
- Voncken P, Rustemeier K, Schepers G. Identification of cis-3′-hydroxycotinine as a urinary nicotine metabolite. Xenobiotica. 1990;20(12):1353–1356. doi: 10.3109/00498259009046633. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, Jacobs DR. Racial differences in serum cotinine levels among smokers in the coronary artery risk development in (young) adults study. Am J Public Health. 1990;80(9):1053–1056. doi: 10.2105/ajph.80.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald NJ, Idle M, Boreham J, Bailey A. Serum cotinine levels in pipe smokers: evidence against nicotine as cause of coronary heart disease. Lancet. 1981;2(8250):775–777. doi: 10.1016/s0140-6736(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Idle M, Boreham J, Bailey A, Van Vunakis H. Urinary nicotine concentrations in cigarette and pipe smokers. Thorax. 1984;39(5):365–368. doi: 10.1136/thx.39.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149(3):198–202. doi: 10.1007/s002130000382. [DOI] [PubMed] [Google Scholar]
- Xia XY, Peng RX, Yu JP, Wang H, Wang J. In vitro metabolic characteristics of cytochrome P-450 2A6 in Chinese liver microsomes. Acta Pharmacol Sin. 2002;23(5):471–476. [PubMed] [Google Scholar]
- Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- Zevin S, Jacob P, 3rd, Benowitz N. Cotinine effects on nicotine metabolism. Clin Pharmacol Ther. 1997;61(6):649–654. doi: 10.1016/S0009-9236(97)90099-0. [DOI] [PubMed] [Google Scholar]
- Zevin S, Schaner ME, Giacomini KM. Nicotine transport in a human choriocarcinoma cell line (JAR) J Pharm Sci. 1998;87:702–706. doi: 10.1021/js970455v. [DOI] [PubMed] [Google Scholar]
- Zevin S, Jacob P, 3rd, Benowitz NL. Nicotine-mecamylamine interactions. Clin Pharmacol Ther. 2000;68(1):58–66. doi: 10.1067/mcp.2000.108066. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kilicarslan T, Tyndale RF, Sellers EM. Evaluation of methoxsalen, tranyl-cypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab Dispos. 2001;29(6):897–902. [PubMed] [Google Scholar]