Nature has seen the evolution of extremely intelligent and complex adaptive systems to drive the biological processes found in everyday life. For example, a cell can fuse information-rich genetic processes with nanometer-scale sensors and actuators, becoming one of the most efficient autonomous molecular systems. These basic processes that occur at the molecular level lead us toward a compelling engineering approach: the fusion of biotechnology, nanotechnology, and information science.
Nanotechnology has enabled the production of new materials and molecular-scale devices. Biotechnological advancements have allowed scientists to physically manipulate genetic pathways or engineering strains of proteins to possess novel functionalities. Informatics has served as the catalyst for organizing and understanding vast knowledge from a system point of view.
The fusion of biotechnology, nanotechnology, and information science will culminate in system architectures that can rival those that have taken millions of years to come to fruition. With this comes the hope of achieving a fundamental comprehension of how to manipulate and control cells on the molecular level. It will also enable us to question just how much further we can push the envelope of human engineering [1].
The Institute for Cell Mimetic Space Exploration (CMISE) is one of four NASA University Research, Engineering and Technology Institutes for developing technologies [2] on the nanometer scale for the study of biological phenomena. With these unique nano modalities, the Center for Cell Control (CCC), a National Institute of Health Nanomedicine Development Center, will apply engineering feedback control schemes to direct information-rich biological cells towards therapeutic use.
Nature's Model for Bio, Nano, and Information Fusion: the Living Cell
The cell is the most fundamental biological unit, a magnificent, self-organized system that performs the complex processes of life. A cell consists of a large number of functional macromolecules, such as the millions of proteins with sizes ranging from one to tens of nanometers. Self organization of these nanometer-scale machineries confined within a fluidic capsule forms a live cell at a size scale of only a few micrometers.
Cellular activities are manifestations of the intra- and intermolecular transports and motions of cellular molecules. These activities result in a comprehensive set of functionalities: to sense (monitor its biological surroundings and responses), to decide (evaluate incoming signals and trigger an optimal response through information analysis), and to actuate (modify its nanometer-scale surrounding to make it more suitable for survival). The cell's responses to the internal and external stimulations through organized molecular activities, governed by a complex information processing network, render it an ideal model for a bio, nano, and information fusion system.
Core Technologies for Studying Bio, Nano, and Information Fusion Systems
In order to understand and control the functions of micron-size cells consisting of nanoscale biomolecules, we need to develop devices that match the length scale necessary for efficient interface. Micro-electromechanical systems technology (MEMS) enables us to design and fabricate miniature sensors and actuators that can be used to directly manipulate microscale subjects. Many recently developed transducers further extend the range of applications to nanoscales. With these micro/nano modalities (MEMS/ NEMS), we can interrogate and wield the cells and bio-molecules with unprecedented abilities.
Micro/Nano Fluidics
Cells live in liquid. The handling of the fluid is a backbone technology for biotechnical research. Due to the complex interactions of cells and macro bio-molecules with the solid boundary, the moving, stopping, mixing, and separation of fluids and particles in microscale reactors present challenges that were not experienced in macroscale fluid mechanics. Starting in the early 1990s, we have explored the scientific issues and developed manufacturing techniques for the micro/nano fluidic field [3].
Force fields generating fluid motion in micro/nano channels include hydrodynamic pressure, electrokinetic force, surface tension, etc. Fluid of either high or low ionic concentration can be driven by pressure gradient. Tai's group has developed the complete set of pumps, valves, and flow sensors for measuring shear stress, pressure, flow rate, etc. [11], [12] Their unique parylene manufacturing technique can produce a fully integrated microfluidic system consisting of all necessary devices (Figure 1). Experience tells us that the alternative method of assembling devices one by one into a hydride platform will be extremely inefficient for assay analysis.
FIGURE 1.
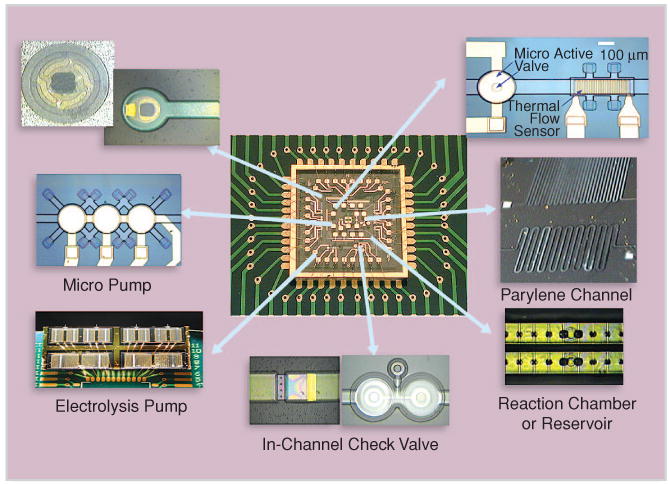
An integrated pressure-driven microfluidic circuitry (figure courtesy of Prof. Y.C. Tai, Caltech).
A cell can fuse information-rich genetic processes with nanometer-scale sensors and actuators, becoming one of the most efficient autonomous molecular systems.
Surface tension becomes a dominating force in small droplets. Instead of driving fluid in a continuous stream as we always do, Kim's group [4] pioneered a digital fluidics that uses surface tension to move liquid droplets (Figure 2) for almost all forms of microfluidic operations. Surface tension is a function of surface electrical potential. Imbalanced surface tension force acting on the droplets moves the fluid along the designed potential gradient. Ways of splitting and merging droplets have been developed to meet the needs in sample preparations.
FIGURE 2.
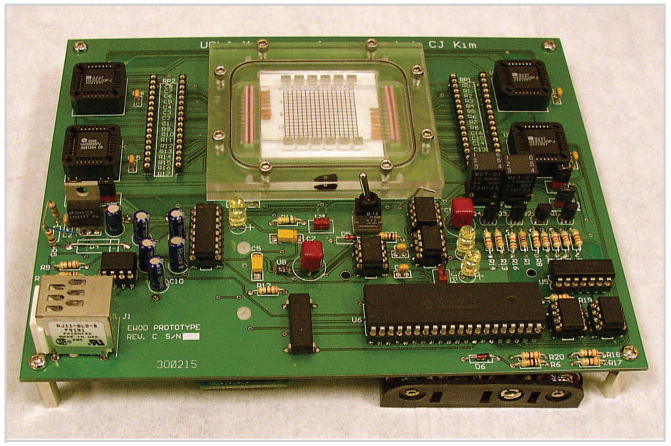
Digital fluidic system, complete except for battery (figure reprinted with permission from [5]).
Opto-Electronic Tweezers
For biomedical sample preparations, moving particles—e.g., cells—inside a fluid is a common procedure. Optoelectronic tweezers (OETs) constitute an optical-image-driven dielectrophoresis technique that permits high-resolution patterning of nonuniform electric fields on a photoconductive surface for manipulating microscale particles, such as individual live cells [6]. The optical power used in this method is 100,000 times less intense than laser optical tweezers and, therefore, causes almost no measurable effect on biological functions. Combining a light source and a digital micromirror spatial light modulator to pattern the optical images in real time, a sequence of filter images—i.e., a movie—projected at different locations from left to right (Figure 3) over the fluid domain containing particles of different sizes can let smaller particles pass the filter and large particles move to the right side. Many other types of OET-based optical micro machineries can be designed and applied to control particle positions by simply modifying the images of the OET movie algorithm.
FIGURE 3.
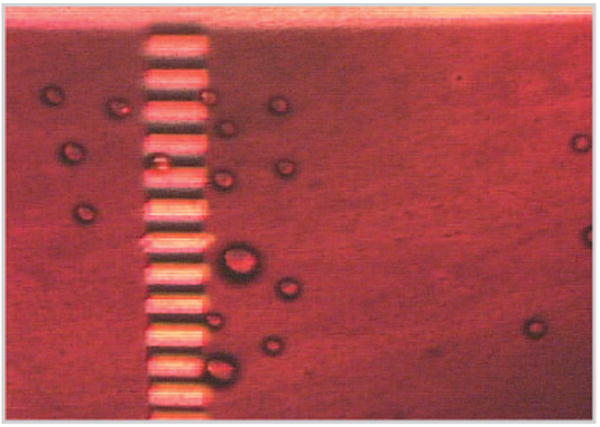
An OET-based filter for selecting micro particles by size (figure courtesy of Prof. Ming C. Wu and Aaron Ohta, UC Berkeley).
Superlens-Based Nanoscope
An electronic microscope enables us to visualize subjects down to the sub-nanometer range, but not in a living cell. An optical microscope can image particles in a living cell for sizes above the diffraction limit, about 200 nm. Zhang's group developed a superlens-based nanoscope for moving toward the bio-nano world. Superlens is a nanometer thin slab of material that utilizes plasmonic resonances to enhance and compensate the evanescent loss of the imaging electromagnetic wave [7]. This superlens-based optical technique can image with spatial resolution below 60 nm, more than six times below the optical diffraction limit of the 365-nm light source (Figure 4).
FIGURE 4.
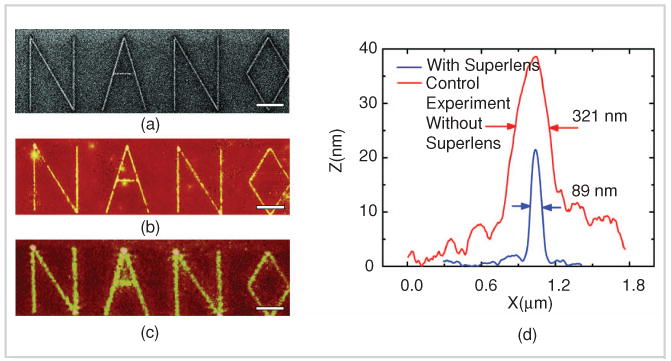
Testing the silver superlens (figure reprinted with permission from [7], AAAS).
Applications of Atomic Force Microscope for Investigating Bio Systems
The atomic force microscope (AFM) is also used by researchers to quantitatively profile the global gene expression from very small samples [8]. The detection of differences and modulation in global expression patterns yields a deeper understanding of the interconnected circuitries of normal and diseased tissues. This is common practice in biomedical research and drug discovery, and it is gaining acceptance in a clinical setting for disease prediction, diagnostics, and treatment. The AFM technique allows the profiling of small samples (having single-molecule sensitivity) obtained in typical clinical and experimental settings. Figure 4 shows a measurement that captures the specific enzyme cleavages on a DNA molecule fixed on a surface, providing a partial nucleotide sequence fingerprint that can be used to identify the molecule.
When placing an AFM tip on top of a cell membrane, almost monotone frequency waves were detected [9], [10]. The detected vibrations represent response of a cell to intrinsic or external perturbations which excite a thin wall structure containing millions of macro molecules suspended in liquid and supported by a microtubular network. These signals are similar to the sound from a stethoscope and indicate the overall physiological state of a cellular system.
Interdisciplinary Research and Education
The genetic code provides the blueprint for the biomolecules and the network of pathways that form an information-rich cellular system. In order to understand and to direct the cells for diagnostic and therapeutic purposes, the CMISE and CCC have developed a suite of unique nanotechnology-based tools for exploration at both the molecular and system levels. These teams of faculty and student/postdoctorate members include specialists from engineering and the physical and biomedical sciences. The path of interdisciplinary research and education is both challenging and exciting. After overcoming the hurdles in understanding the language and appreciating the cultures of other disciplines, the path leads towards unprecedented accomplishments that would never be expected from a narrowly focused field. Only a cohesive interdisciplinary effort can enable us to move toward the goal of fusing bio, nano, and information technologies to further the endeavor of improving human health.
Biotechnological advancements have allowed scientists to physically manipulate genetic pathways or engineering strains of proteins to possess novel functionalities.
FIGURE 5.
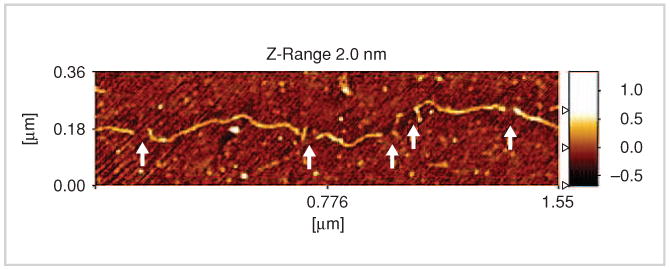
An AFM image of a fixed DNA molecule with identifiable enzymed-induced cleavages (figure reprinted with permission from [8], Institute of Physics Publishing Ltd.).
Acknowledgments
The authors would like to express their gratitude to the students/postdoctorates and faculty members of the CMISE and CCC. Their ingenious ideas, hard work, and congenial working demeanors make the challenging research a fun experience. This work is supported by a NASA URETI program (contract NCC-2-1364) and the NIH Roadmap for Medical Research NDC program (PN2 EY018228).
References
- 1.Ho D, Garcia D, Ho CM. Nanomanufacturing and characterization modalities for bio-nano-informatics systems. J Nanoscience Nanotechnology. 2006;6(4):875–891. doi: 10.1166/jnn.2006.173. [DOI] [PubMed] [Google Scholar]
- 2.Chen JM, Ho CM. Path to bio-nano-information fusion. Progress in Convergence, Annals New York Acad Sciences. 2007;1093:123–142. doi: 10.1196/annals.1382.009. [DOI] [PubMed] [Google Scholar]
- 3.Ho CM, Tai YC. Micro-electro-mechanical-systems and fluid flows. Ann Rev Fluid Mech. 1998;30:579–612. [Google Scholar]
- 4.Cho SK, Moon H, Kim CJ. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J Microelectromech Systems. 2003;12(1):70–80. [Google Scholar]
- 5.Gong J, Fan SK, Kim CJ. Portable digital microfluidics platform with active but disposable lab-on-chip. IEEE MEMS-2004 Proc. 2004:355–358. [Google Scholar]
- 6.Chiou PY, Ohta AT, Wu MC. Massively parallel manipulation of single cells and microparticles using optical images. Nature. 2003;436(7049):370–372. doi: 10.1038/nature03831. [DOI] [PubMed] [Google Scholar]
- 7.Fang N, Lee H, Sun C, Zhang X. Sub-diffraction-limited optical imaging with a silver superlens. Science. 2005;308(5721):534–537. doi: 10.1126/science.1108759. [DOI] [PubMed] [Google Scholar]
- 8.Reed J, Mishra B, Pittenger B, Magonov S, Troke JJ, Teitell MA, Gimzewski JK. Single molecule transcription profiling with AFM. Nanotechnol. 2007;18(4) doi: 10.1088/0957-4484/18/4/044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelling A, Sehati S, Gralla EB, Valentine JS, Gimzewski JK. Local nanomechanical motion of the cell wall of saccharomyces cerevisiae. Science. 2004;305(5687):1147–1150. doi: 10.1126/science.1097640. [DOI] [PubMed] [Google Scholar]
- 10.Gimzewski JK, Reed J, Teitell MA, Malan PG. Immunological biosensors. In: Wild D, editor. The Immunoassay Handbook. London: Elsevier; 2005. pp. 265–280. [Google Scholar]
- 11.Xie J, Shih J, Tai YC. Integrated surface-micromachined mass flow controller. IEEE MEMS-2003 Proc. 2003:20–23. [Google Scholar]
- 12.Xie J, Shih J, Lin Q, Yang B, Tai YC. Surface micromachined electrostatically actuated micro peristaltic pump. Lab Chip. 2004;4(5):495–501. doi: 10.1039/b403906h. [DOI] [PubMed] [Google Scholar]


