Abstract
Background
While a large body of literature exists on cognitive functioning in alcohol-exposed children, it is unclear if there is a signature neuropsychological profile in children with Fetal Alcohol Spectrum Disorders (FASD). This study assesses cognitive functioning in children with FASD from several American Indian reservations in the Northern Plains States, and it applies a hierarchical model of simple versus complex information processing to further examine cognitive function. We hypothesized that complex tests would discriminate between children with FASD and culturally similar controls, while children with FASD would perform similar to controls on relatively simple tests.
Methods
Our sample includes 32 control children and 24 children with a form of FASD [fetal alcohol syndrome (FAS) = 10, partial fetal alcohol syndrome (PFAS) = 14]. The test battery measures general cognitive ability, verbal fluency, executive functioning, memory, and fine motor skills.
Results
Many of the neuropsychological tests produced results consistent with a hierarchical model of simple versus complex processing. The complexity of the tests was determined “a priori” based on the number of cognitive processes involved in them. Multidimensional scaling was used to statistically analyze the accuracy of classifying the neurocognitive tests into a simple versus complex dichotomy. Hierarchical logistic regression models were then used to define the contribution made by complex versus simple tests in predicting the significant differences between children with FASD and controls. Complex test items discriminated better than simple test items. The tests that conformed well to the model were the Verbal Fluency, Progressive Planning Test (PPT), the Lhermitte memory tasks and the Grooved Pegboard Test (GPT). The FASD-grouped children, when compared to controls, demonstrated impaired performance on letter fluency, while their performance was similar on category fluency. On the more complex PPT trials (problems 5–8), as well as the Lhermitte logical tasks, the FASD group performed the worst.
Conclusions
The differential performance between children with FASD and controls was evident across various neuropsychological measures. The children with FASD performed significantly more poorly on the complex tasks than did the controls. The identification of a neurobehavioral profile in children with prenatal alcohol exposure will help clinicians identify and diagnose children with FASD.
Keywords: fetal alcohol syndrome, fetal alcohol spectrum disorders, hierarchical model, neuropsychological characteristics, information processing, American Indian
Although there exists a large body of literature on the cognitive functioning in children with prenatal alcohol exposure, a neurobehavioral profile that is unique to alcohol-exposed children has eluded researchers to date. Identification of a signature profile of a disorder is important for improving diagnosis and planning interventions.
Neurocognitive functioning in children with prenatal alcohol exposure
Intellectual functioning
Researchers have consistently found intellectual deficits in children with fetal alcohol syndrome (FAS). The average IQs of these children fall in the mild retardation to borderline ranges (Mattson et al., 1997; Streissguth, Randels, & Smith, 1991). Given that subtests provide useful information pertinent to defining behavioral phenotypes, researchers have conducted “profile analyses” of subtest scores from standardized test batteries (Adnams et al., 2001; Mattson et al., 1997). Results from these analyses show that children with FAS earn lower scores than controls on all subtests. Mattson and colleagues (1998) also concluded that there was no significant discrepancy between verbal and non-verbal abilities.
Attention and concentration
Although there is some consensus among researchers that attention and executive control functioning are ‘core deficits’ in children with a fetal alcohol spectrum disorder (FASD), there is less agreement as to what skills are more impaired. Carmichael Olson and colleagues (1998) found that adolescents with prenatal alcohol exposure were more impaired on tests assessing attention and memory tasks in the visual-spatial domain and short-term auditory memory. Using Mirsky’s model, Coles and colleagues (1997) found that children with prenatal alcohol exposure were impaired only in shift and encode elements. In a subsequent study of sustained attention in alcohol-affected children, Coles, Platzman, Lynch, & Freides, (2002) found a significant modality by group interaction. That is, the alcohol-exposed group had more difficulty sustaining attention when processing visual stimuli than when processing auditory stimuli.
Executive functioning
Subsumed under the umbrella term of executive functioning (EF) are a number of abilities that are involved in achieving a goal in an efficient manner. Abilities include planning, verbal fluency, set shifting, and response inhibition. Evidence from both animal and human research points to two domains of EF: cognition-based and emotion-related (Dias, Robbins, & Roberts, 1996). Commonly used tests of EF, such as cognitive planning, conceptual set shifting, and rapid generation of verbal or non-verbal responses, assess cognition-based EF.
Look-ahead puzzles are often used to measure cognitive planning abilities. Kodituwakku and colleagues (Kodituwakku et al., 1995; Kodituwakku, May, Clericuzio, & Weers, 2001) reported that alcohol-exposed children were markedly impaired with planning ability, as assessed by the Progressive Planning Test (PPT), a look-ahead puzzle. However, using the California Tower Test, Mattson and colleagues (1999) obtained less pronounced deficits in planning with alcohol-affected children. Many researchers have utilized the Wisconsin Card Sorting Test to assess the shifting aspect of attention (Carmichael Olson, et al., (1998); Kodituwakku, (1995); Lezak, 1995). Researchers have found variable levels of impairments in the shift element of attention in alcohol-exposed children. However, on most laboratory tasks that measure response inhibition, children with prenatal alcohol exposure do not evidence impulsivity (Kodituwakku et al., 2001; Coles et al., 1997).
Impaired verbal fluency has also been found in children with a FASD (Kodituwakku et al., 2006; Mattson & Riley, 1999). Letter fluency is a complex task that involves a number of operations simultaneously, including rapid generation of words by phonemic similarity, checking responses to ensure that they meet the test constraints, and keeping a record of responses already produced in working memory. Letter fluency can be contrasted with category fluency, in which subjects are required to rapidly generate exemplars from a semantic category (e.g., animals, fruit and vegetables). Replicating previous findings, Kodituwakku and colleagues (2006) reported that children with FAS displayed greater difficulty in letter fluency than in category fluency.
Learning and memory
Children with prenatal alcohol exposure perform less competently than controls on learning and memory tasks that involve conscious effort of encoding and retrieval. Mattson and colleagues (Mattson et al., 1996; Mattson & Roebuck, 2002) have reported that children with heavy prenatal alcohol exposure obtained lower scores than controls on verbal and non-verbal learning tasks such as the California Verbal Learning Test for Children. Verbal memory deficits in children with prenatal alcohol exposure have also been documented with other methods of testing such as story recall (Streissguth, Barr, & Sampson, 1989).
There exist numerous reports in the literature on spatial learning, specifically on place learning, in alcohol-exposed children. Uecker and Nadel (1996) found a general spatial memory deficit in alcohol-exposed children. Streissguth and colleagues (1994) have found that prenatal alcohol exposure was associated with deficient performance on a visually-guided maze-learning test. Place learning difficulties in alcohol-exposed children have also been demonstrated with a virtual Morris water task (Hamilton, Kodituwakku, Sutherland, & Savage, 2003).
There is also accumulating evidence that alcohol exposure does not produce impairments in recognition memory and procedural memory (Carmichael Olson et al., 1998; Mattson and Riley, 1999). On tests of memory and learning, children with prenatal alcohol exposure demonstrate normal rates of acquisition and retention of relatively simple information, but they evidence difficulty in learning relatively complex information.
Motor
A few researchers have failed to find an association between prenatal alcohol exposure and motor development (Chandler, Richardson, Gallegher, & Day, 1996; Fried & Watkinson, 1990). However, there are studies of motor performance of alcohol-exposed children showing deficient manual dexterity and disturbance of balance (Roebuck, Simmons, Mattson, & Riley, 1998; Mattson et al., 1998).
Kalberg, et al, (2006) reported that children with FAS exhibited significantly discrepant development between their fine-motor and gross-motor development as measured by the Vineland Adaptive Behavior Scales (VABS), with fine-motor scores significantly lower. Because the children were matched on several factors, including communication age equivalent scores, this finding suggests that the fine-motor delays in children with FAS do not necessarily parallel the cognitive/language delays, but may be related to specific neurobehavioral deficits that affect fine motor skills.
A hierarchical model of ability organization and fetal alcohol spectrum disorders
Many theorists have proposed a hierarchical model of ability organization (Cattell, Seitz, & Rausche, 1971; Snow, 1978). Using a non-metric multidimensional scaling method, Guttman (1954) demonstrated that a test could be represented as a point in a two dimensional space defined by complexity and content (e.g., verbal, spatial, numerical). Thus, tests of varying complexity from different content areas can be represented in a circle called radex. Complex tests fall in the middle of the circle, while simple tests scale in the periphery. Moreover, Marshalek and colleagues (1983) concluded that complex tasks require more involvement of executive functioning.
Despite some inconsistencies, there is evidence that children with prenatal alcohol exposure are markedly impaired in relatively complex tasks, irrespective of the domain of functioning, whereas these children perform in the normal range on relatively simple tests. Tests of executive control functioning typify complex tasks that require greater cognitive effort. Alcohol-exposed children, however, are able to solve relatively simple look-ahead puzzles as competently as controls. Moreover, researchers have obtained evidence that alcohol-exposed children are deficient in letter fluency as indexed by difficulty in generating words beginning with given letters under specific constraints imposed by the examiner (Schonfeld et al., 2001).
As noted above, the letter fluency task is more demanding than the category fluency task in that it requires more executive control. However, whether the letter fluency task is more sensitive to the effect of prenatal alcohol exposure has yet to be addressed. Moreover, children with prenatal alcohol exposure demonstrate deficient free recall of information, although their recognition memory is relatively intact.
Despite inconsistencies in the findings on neurocognitive functioning of children with FASD, there is an emerging pattern of results. Children with FASD tend to perform worse than controls on relatively complex tasks, regardless of the domain of functioning. This pattern of results conforms to a hierarchical model of cognitive abilities. Examining the topography of cognitive abilities in alcohol-exposed children is an essential step toward finding the neurobiological basis of cognitive dysfunction of these children. An hypothesis of cognitive functioning in children with prenatal alcohol exposure was recently proposed by Kodituwakku (2007). Accordingly, the essence of the cognitive-behavioral phenotype1 associated with FAS is a generalized deficit in processing complex information.
A goal of the current research is to examine the neurocognitive profiles of Native American children exposed to alcohol prenatally. Specifically, we administered a neurobehavioral test battery to children with a formal diagnosis of FAS or partial fetal alcohol syndromes (PFAS) from six Northern Plains Indian reservations and one urban site. This study tested the conceptual model proposed by Kodituwakku (2007) by examining performance on simple versus complex information processing. We investigated the hypothesis that children with FAS and PFAS, compared to culturally similar controls, would display deficits on relatively complex tasks, but not on simple ones drawn from different domains of functioning. Furthermore, the continuum of disability will be evident when comparing the control group to children with a diagnosis of PFAS and FAS.
Materials and Methods
Sample
Fifty-six children, 24 with FAS or PFAS and 32 control grouped children, participated in this study. The FASD group was comprised of 15 males and 9 females diagnosed with either Partial FAS (n = 14) or FAS (n = 10). The children ranged in age from 7 to 17 years with a mean age of 12 years. The FASD sample consisted of 19 children with confirmed prenatal alcohol exposure, while 5 children met the diagnosis without evidence of confirmed prenatal alcohol exposure. The control group was comprised of 14 males and 18 females, and ranged in age from 7 to 17 years with a mean age of 11 years. Because there is so much debate regarding the effects of prenatal versus postnatal environment, we selected children of similar backgrounds to control for possible postnatal effects (e.g., SES, and cultural). As a general control for postnatal effects, our sample included only Native American children from highly similar communities with similar traditional tribal cultures and history. Although there were substantial missing data, comparisons between the groups with a FASD and control groups revealed no statistically significant differences with mother’s education, marital status, mother’s and father’s Hollingshead SES index, and mother’s age at birth when compared using independent sample t-tests.
The current study utilized a sub-sample of children first diagnosed in a larger FAS epidemiological study of The University of New Mexico, Center on Alcohol, Substance Abuse and Addictions. The original epidemiology study included children recruited through population-based, active case ascertainment methods by experienced clinician researchers trained and capable of recognizing and diagnosing the full range of FASD. While participants in the current research were recruited from the existing epidemiology pool of American Indian children diagnosed as having PFAS or FAS, case controls were selected from the same communities and matched for age and ethnicity.
Recruitment
Participants with FASD, who had previously been diagnosed by our research team, were contacted through a letter that explained our study. The control group was recruited through advertisements and announcements in tribal centers and community gatherings. The following exclusionary criteria were utilized in the recruitment of the potential control participants: moderate or severe mental retardation, head trauma, co-morbid genetic syndromes, severe psychiatric illness (psychosis), substance abuse, and neurological problems such as seizure disorder.
Data Collection
Research site coordinators contacted parents through a letter or phone call and explained our study. We obtained informed consent from the parents/legal guardians and the assent from the children. The proposed research protocol and consent/assent forms were reviewed by the Human Research Review Committees at The University of New Mexico. Approval was obtained from the tribal councils of the participating American Indian sites and the U.S. Indian Health Service IRB's. Upon completion of the tests, incentive fees of fifty dollars were paid to each child.
Dysmorphological exam
Issues related to reliability and validity of diagnosis can be resolved by having experienced dsymorphologists systematically evaluate affected children (May et al., 2000). Three pediatric dysmorphologists with extensive experience in diagnosing FASD and related disorders performed the diagnostic evaluations using the revised Institute of Medicine (IOM) criteria (Hoyme, et al, 2005; Stratton, et al, 1996). Maternal consumption of alcohol during pregnancy, growth deficiency, reported neurocognitive problems, and structural defects, especially involving the craniofacial regions, were used by the dysmorphologists to make diagnoses (Hoyme, et al., 2005). The maternal drinking information, the medical morphology information and the testing results were all then used to make a final diagnosis in an interdisciplinary case conference of each child enrolled in the study.
Within the IOM-endorsed criteria adapted for practical applications in a clinical setting, a child meeting the following criteria received a diagnosis within the FASD continuum. To receive a diagnosis of FAS a child must have two or more of the cardinal facial anomalies of FAS (short palpebral fissures, thin vermilion border, and/or smooth philtrum), prenatal and/or postnatal growth retardation (≤ 10th percentile) and small head circumference (≤ 10th percentile) or other evidence of structural brain abnormalities with or without confirmation of maternal drinking. For partial fetal alcohol syndrome (PFAS) a child must have two or more typical facial features, and one or more of the following characteristics: prenatal and/or postnatal growth retardation (≤ 10th percentile), evidence of abnormal brain growth or structure (e.g., microcephaly ≤ 10 percentile), or evidence of characteristic behavioral or cognitive abnormalities, with or without evidence of maternal drinking during pregnancy (for complete diagnostic criteria see Hoyme et al., 2005).
Procedure
In the Plains, as in other active case ascertainment studies in the United States, clinical evaluations of the children were completed in a two-step process (May, Hymbaugh, Aase, & Samet, 1983; May & Hymbaugh, 1982; May & Gossage, 2002). First, children with a suspected history of prenatal alcohol exposure were referred by full time field staff at each reservation and examined by one of three experienced clinical geneticists/dysmorphologists. Next, a neuropsychological test battery aimed at assessing intellectual, verbal, planning, memory, and motor skills was administered to participants. All available birth mothers and/or adoptive parents were interviewed to determine a variety of maternal risk factors in the prenatal period of the study child, including extensive questioning on alcohol exposure by quantity, frequency, and general timing during the gestation (May et al., 2005).
Approximately 20% of the control mothers reported some prenatal alcohol exposure, but the use was minimal and infrequent. Furthermore, no evidence of binge drinking was found in the control group. It is likely that a number of control children had some exposure; however, every control child was examined by dsymorphologists and did not meet the criteria for any diagnosis within the continuum of FASD.
Instruments used
A core test battery used in this study is a subset of a larger battery designed by the NIAAA-supported Collaborative Initiative on FASD (CIFASD). We tested the above hypotheses by using a carefully designed battery consisting of ‘complex’ and ‘simple’ tests. Only subtests that allowed a ‘simple’ versus ‘complex’ dichotomy were included in the hierarchical model analyses. The complexity of the tests was determined “a priori” based on the number of cognitive processes involved in them. The select instruments used were: the Leiter International Performance Scale –Revised, Delis-Kaplan Executive Function System (Verbal Fluency subtest), Progressive Planning Test (PPT), and the Grooved Pegboard Test (GPT). The Lhermitte Spatial/Logical Test of Memory; Delayed Recognition Span Test of Memory (DRST); Wechsler Intelligence Scale for Children –IV (Digit Span Subtest) were additional memory tests which were added to the CIFASD core battery and only admininistered to this cohort in this study.
The following tests were selected to test the hierarchical model: Verbal Fluency (Letter/Category); PPT, Lhermitte Spatial/Logical Test of Memory; DRST; Digit Span; and the GPT. The ‘simple’ and ‘complex’ hypothesis was guided by the work of Passingham et al., 1985, Rushworth et al., 1997, and Rolls et al., 1998 where executive functioning was explored through planning performance on novel tasks among subjects with frontal lobe damage. Central to the function of planning a novel task are working memory and response inhibition. The tests in our battery attempted to tap into the working memory and response inhibition abilities of the subject. This required increasingly more complex mental operations (short-term memory, working memory, encoding ability, sequencing ability, and the ability to self-monitor). In other words, the more complex tasks required the subject to hold information “on-line” in increasing amounts and durations of time. In the delayed response condition, the complexity is increased by the need for the subject to hold incidental learning in memory for a prescribed amount of time. The specific cognitive processing involved with each subtest is described under the individual tests.
Leiter International Performance Scale- Revised (Stoelting, 2001)
This measure provides an estimation of intellectual functioning through a Brief IQ Screener. The Leiter-R Brief IQ Screener is highly correlated (.85) with other frequently used measures of ability, including the WISC-III Full Scale IQ (Roid & Miller, 1997). The collection of 4 subtests in the Brief IQ Screener was used to estimate global intellectual functioning.
D-KEFS Verbal Fluency (Delis, Kaplan, & Kramer, 2001)
This test, which measures letter fluency in an oral form, has proven to be one of the most useful neuropsychological instruments in both research and clinical practice (Delis, Kaplan, & Kramer, 2001). It is thought to be sensitive to frontal lobe involvement in general and left-frontal lobe damage in particular (Benton, 1968). The D-KEFS Verbal Fluency Test is comprised of three conditions: Letter Fluency, Category Fluency, and Category Switching. For the Letter Fluency condition, the child is asked to verbally list words that begin with a specified letter as quickly as possible in three one-minute trials while also following a variety of rules and limitations regarding their responses. These rules specify that the child must not repeat responses, utilize proper nouns, or produce variations of responses. Thus, the child must retain the rules in memory while also tracking his/her responses and producing the maximum number of words in the time allotted. In the Category Fluency condition, the child is asked to name words that belong to a designated semantic category as quickly as possible in two trials of one minute each. In a normally functioning individual it is typically easier to retrieve information from a semantic category than from a lexical category (as in the Letter Fluency condition) because such retrieval is more familiar and often more rehearsed. Therefore the Category Fluency condition is intended to be the simpler of the two tasks. The third condition, Category Switching, evaluates the child’s ability to alternate between listing words from two different semantic categories as quickly as possible. This condition combines the practiced retrieval from two semantic categories with the novel concept of switching between these two categories. Overall, the D-KEFS Verbal Fluency measures an individual’s ability to generate words in an effortful, phonemic format as well as from over-learned concepts.
Progressive Planning Test (Kodituwakku, 1993)
A look-ahead puzzle called the Progressive Planning Test (PPT) has been used to assess cognitive planning in children with prenatal alcohol exposure (Kodituwakku et al., 1995). The test consists of 12 problems of increasing difficulty. The number of mental manipulations (mental reversals) one has to carry out to solve a problem determines its complexity. Thus, the problems on the test can be categorized into 3 sets, each containing 4 problems of comparable complexity. The participants are required to move 1 to 5 beads from an initial position to a goal position while following specific rules. Problems 1 through 4 are simple in that they do not involve the manipulation of information in working memory. In contrast, problems 5 through 8 involve mental manipulation and hence, are complex. Two scores calculating the number of cards solved are computed to reflect differential performance on simple and complex problems.
Logical and spatial memory tests (Lhermitte & Sigonoret, 1972)
Our study contrasted the performance of children with FASD and controls using a modified version of the Lhermitte-Signoret memory test, which is comprised of two parts: spatial and logical. The spatial memory test consists of a 3 × 3 grid and nine line-drawn pictures of common objects. The object of the test is to learn the location of each drawing on the grid. The examiner places a drawing on its pre-specified location and displays it for 5 seconds. Then the examiner removes it and places another drawing on its prespecified location. This procedure continues until all the drawings have been displayed. Then the examiner presents the drawings to the subject in a random order asking him or her to point to the locations. An incorrect response is immediately corrected by displaying the drawing on its correct location. The number of correct responses over 3 learning trials is the spatial memory score. Increasing numbers of pictures are shown across trials to determine the spatial memory span.
In the logical memory condition, the subject is required to learn a logical pattern on the 3 × 3 grid. The logical pattern is made up of geometric shapes that vary by color and shape. The top row of shapes are triangles, the middle row are circles, and the bottom row are squares. The shapes in the three columns from left to right are colored in blue, red, and yellow respectively. Given that this pattern is broken into 9 pieces and each piece is removed after displaying for 5 seconds, the subject has to mentally create the pattern. As in the spatial memory test, the number of shapes correctly placed over 3 learning trials, and a 15-minute delay, are computed. Given the task demands, the logical memory is presumed to be greater in difficulty than the spatial memory task.
Delayed Recognition Span Test (DRST) (Moss, Albert, Butters, & Payne, 1986)
This test measures spatial recognition memory and is thought to involve parietal lobe functioning. Our modified version of this test utilizes 15 disks that contain various stimuli based on the subtest being performed. In the current study the pictorial and spatial subtests were administered. For the pictorial memory test, the disks contained pictures of various common objects (e.g., ball, mop, house, etc.). A board with thirty small dots is placed in front of the child. One-by-one the disks are placed on the board on a predetermined position. The researcher covers the board while placing each new disk, then rearranges existing disks, uncovers the board, and asks the child to point out the new picture. The child is allowed to review the new disk along with the previously placed disks for ten seconds before the process is repeated. For successful completion of this task, the child must keep track of an increasingly long series of disks. After all disks are placed, the board is cleared and the child is asked to recall as many of the pictures as possible.
For the spatial portion of this test, all disks were solid pink. Again, the researcher covers the board, places the new disk, uncovers the board, and asks the child to identify the new disk. Unlike the pictorial task, the disks are not rearranged from their original position, as the goal is to test spatial memory. Again, the child is given ten seconds to review the entire board after each new disk is placed before moving on to the next disk. Once all of the disks are placed they are removed from the board and the child is asked to place each disk on its original positions. Given the demand characteristics, it is thought that the spatial task is more complex than the pictorial.
Wechsler Intelligence Scale for Children –IV (WISC-IV) (Digit Span Subtest)
(Wechsler, 2003). The Digit Span Subtest requires the child to orally repeat a series of numbers both forward and backward. The total score is the maximum span of digits that a child can retain and recall correctly. Although it can be seen as a measure of working memory (or short-term memory), other factors such as attention and comprehension also contribute to the performance on this test.
Grooved Pegboard Test (Matthews & Klove, 1964)
The Grooved Pegboard Test (GPT) consists of a pegboard with 25 grooved slots. The child is required to place a grooved peg into each of the slots first with the right hand and then the left hand. The time to complete the task with each hand is recorded. Normative data are available for children ages five to sixteen. This test is thought to be a sensitive measure of lateralized cerebral dysfunction (Mitrushina, Boone, & D’Elia, 1999). Overall, the GPT measures psychomotor speed, fine motor control, and rapid visual-motor coordination.
Data analysis
A measure of nonverbal intelligence, the Leiter International Performance Scale –Revised is part of the CIFASD methodological requirement and reported here as a broad indicator of intellectual differences across the study groups. The Brief IQ Screener used in this study is based on factor analytic models of intelligence proposed by Carroll and Gustafsson (Carroll, 1997; Gustafsson, 1984). For this study, we did not correlate IQ scores with other study measures. All data were analyzed via SPSS (Version 14.0; SPSS for Windows, SPSS Inc., Chicago, IL). Initial demographic analyses between control and FASD groups were conducted using independent sample t-tests, whereas subsequent analyses of dysmorphology results, as well as the neuropsychological results, were analyzed using multivariate analysis of variance (MANOVA). Because the control, PFAS, and FAS groups had unequal sample sizes, post hoc pairwise comparisons were adjusted using Scheffe, which corrects for multiple comparisons within the same sample.
Results
Our primary objective was to determine what tests consistently discriminate between children with FAS or PFAS and controls. We performed a series of t-tests in the initial stage to determine whether the two groups were comparable with respect to demographic variables, height, weight, head circumference, multiple facial features, and total dysmorphology score. Our findings (Table 1) revealed that the groups were similar with respect to age, and gender. Thus, age, and gender were not included as covariates in subsequent analyses.
Table 1.
Child Demographics, Dysmorphology Mean Scores and Standard Deviations Between Control Group and FASD-Grouped Children.
| Control Group (n = 32) | FASD-Grouped (n = 24) | t-test | p | |||
|---|---|---|---|---|---|---|
| Child Age (years) | 11.92 | (3.23) | 12.15 | (3.15) | t(54) = −.26 | .796 |
| Child Gender (%) Males Female |
n = 14 n = 18 |
43.8% 56.3% |
n = 15 n = 9 |
62.5% 37.5% |
t(54) = 1.38 | .171 |
| DYSMORPHOLOGY | ||||||
| Child Height (centile) | 70.91 | (24.73) | 20.95 | (22.57) | t(45) = 7.23 | < .001 |
| Child Weight (centile) | 78.73 | (28.87) | 35.95 | (26.64) | t(45) = 5.08 | < .001 |
| Child Head Circumference (centile) | 81.39 | (18.61) | 28.70 | (34.54) | t(35.62) = 6.54 3 | < .001 |
| Palpebral Fissure Length (centile) | 62.69 | (23.69) | 12.50 | (13.67) | t(34.89) = 8.84 3 | < .001 |
| Lip Philtrum Guide (Philtrum) 1 | 3.21 | (.90) | 3.91 | (.71) | t(45) = −2.94 | .005 |
| Lip Philtrum Guide (Vermilion) 1 | 3.04 | (.76) | 4.25 | (.53) | t(45) = −6.28 | < .001 |
| Total Child Dysmorphology Score 2 | 4.26 | (2.56) | 12.91 | (3.39) | t(45) = −9.82 | < .001 |
The lip-philtrum guide. Developed by researchers at the University of Washington in Seattle, this tool pictorially displays 5 levels of philtral flatness, (1 being normal, 5 being totally flat), and 5 levels of upper lip thinness, (1 being normal, 5 being very thin). This guide helps clinicians recognize the thin upper lip and flat philtrum in children with FAS. It can be obtained through: http://depts.washington.edu/fasdpn/pdfs/guideorder.pdf
Total Dysmorphology score is a composite score derived from a weighted scoring system with the most salient FAS diagnostic features (e.g., head circumference, palpebral fissure length and smoothness of philtrum) scored a three, followed by other features (e.g., weight, and specific facial features) scored with a two, and other physical features related to FASD scored with a one.
Leven’s Test for Equality of Variance was significant, used equal variances not assumed and adjusted degrees of freedom in analysis.
Dysmorphology
Table 1 also presents a summary of both child demographic characteristics and child dysmorphology data. As expected, the two groups were significantly different with respect to all of the dysmorphological variables.
Hierarchical model of ability organization
Two hierarchical (sequential) models were run to test the hypotheses that complex tasks would discriminate between control and FASD-grouped children, and that these children would perform similar to one another on relatively simple tasks. The small samples did not allow analysis of individual tests, so means of simple and complex test scores were calculated, forming two predictor variables: simple (average of scores on category fluency, PPT trials 1–4, Lhermitte spatial, Lhermitte spatial 15-min delay, DRST pictorial, DRST pictorial delay, digit span forward, and pegboard dominant hand) and complex (average of scores on letter fluency, PPT trials 5–8, Lhermitte logical, Lhermitte logical 15-min delay, DRST spatial, DRST spatial delay, digit span backward, and pegboard non-dominant hand). The first model compared control with FASD-grouped children after adjustment for age and gender; the second model compared FAS with PFAS children after adjustment for age and gender. Interactions between age, gender, and score averages did not approach statistical significance and were omitted from the models.
Table 2 shows results of the two models. Age- and gender-adjusted average of complex scores discriminate most highly between control and FASD children, p < .001, Nagelkerke R2 = .37, with 75.5% correct classifications. The average of simple scores did not add significantly to discrimination of these two groups, p = .594, after adjustment for age, gender, and average of complex scores. With simple tasks included in the model, Nagelkerke R2 increases to .375, and correct classifications increase to 77.6%
Table 2.
Hierarchical Logistic Regression Models of FASD.
| Step | Variable(s) entered | Step χ2 |
df | p |
R2 (cumulative) |
Correct responses |
|---|---|---|---|---|---|---|
| Predicting Control versus FASD | ||||||
| 1 | Age and gender | 3.12 | 2 | .21 | .083 | 63.3% |
| 2 | Average of complex scores | 12.56 | 1 | <.001 | .370 | 75.5% |
| 3 | Average of simple scores | .284 | 1 | .594 | .375 | 77.6% |
| Predicting PFAS versus FAS | ||||||
| 1 | Age and gender | .400 | 2 | .819 | .026 | 60.0% |
| 2 | Average of complex scores | .320 | 1 | .571 | .047 | 65.0% |
| 3 | Average of simple scores | .722 | 1 | .395 | .093 | 60.0% |
The bottom half of Table 2 indicates that the age- and gender-adjusted average of complex scores did not discriminate between FAS and PFAS, p = .571, Nagelkerke R2 = .047, with 65.0% . The addition of the average of simple scores did not add significantly to the prediction, p = .395
Multidimensional scaling
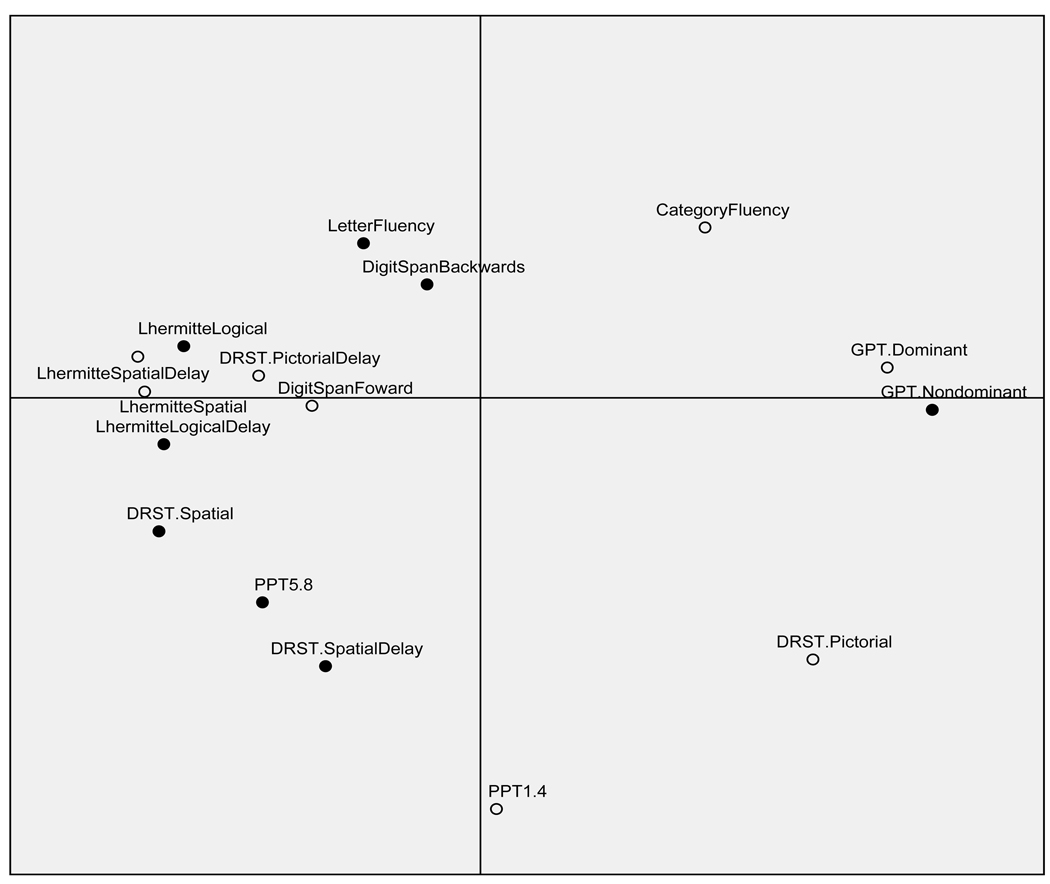
An attempt was made to determine a statistical rationale for the delineation of simple and complex tasks. The small sample precluded confirmatory factor analysis. Instead a highly exploratory multidimensional scaling (MDS) technique was implemented, guided by Guttman’s (1954) Radex theory (Kruskal & Wish, 1978). This allowed for a visual representation of the pattern of proximities (i.e., distances) among the set of various tasks to be viewed on a scatter plot. The resulting matrix of similarities between various neuropsychological tasks is presented in Figure 1. Effectively, MDS plots the neuropsychological tasks on a perceptual map such that those tests which are perceived to be very similar are placed near each other on the map, and those tests that are perceived to be very different are placed far away from each other. This metric technique applies an algorithm and maps the tests in the space of the smallest dimensionality capable of reflecting pairwise distances (here, Pearson correlations converted to Euclidean distances) between them. Thus, by representing the pattern of proximities in a two-dimensional plot, one can visually examine the hidden structure underlying the data. Radex theory of complexity proposes that the first two dimensions for mental tests reflect both kind of complexity and degree of complexity, with simple tasks falling to the periphery and complex tasks falling toward the radex (center) of the scatter plot. An examination of Figure 1 shows that the simple and complex tasks selected fall within these guidelines for the most part. Only three of the 15 tasks seriously depart from this pattern; Digit Span Forward and DRST Pictorial Delay, deemed simple, fall within the radex, whereas GTP Non-Dominant Hand, deemed complex, falls toward the periphery. Moreover, the tasks are relatively dispersed based on their respective domains of functioning (e.g., memory, planning, verbal, and motor). R2, a measure of proportion of variance of the scaled data accounted for by their corresponding distances, was found to be .82. However, the finding that stress (departure from fit) was found to be .24, indicating that the result of the MDS analysis can be viewed only tentatively.
Figure 1.
Euclidean distance model of complexity. Filled circles represent tests selected as complex, empty circles represent tests selected as simple.
To further examine cognitive ability in children with FASD, the results were also analyzed by domains of: intellectual functioning, verbal fluency, planning ability, memory ability, and motor ability (see Table 3). Analyses were tested at α = .0025 to compensate for inflated Type 1 error due to multiple testing.
Table 3.
Neuropsychological Mean Scores and Standard Deviations Between Control Group and FASD-Grouped Children.
| Control Group (n = 32) | FASD-Grouped (n = 24) | t-test | p | |||
|---|---|---|---|---|---|---|
| Intellectual Functioning | ||||||
| Leiter Brief IQ 1 | 101.96 | (16.12) | 84.16 | (15.20) | t(54) = 4.18 | < .001** |
| Verbal Fluency | ||||||
| D-KEFS Letter Fluency 2 | 9.72 | (2.24) | 7.61 | (3.01) | t(53) = 2.97 | .004 |
| D-KEFS Category Fluency 2 | 9.34 | (2.92) | 8.57 | (2.84) | t(53) = .98 | .329 |
| D-KEFS Category Switching 2 | 9.22 | (2.41) | 6.30 | (2.65) | t(53) = 4.23 | < .001** |
| Set Loss Errors 2 | 10.31 | (3.41) | 6.26 | (4.13) | t(41.70) = 3.85* | < .001** |
| Planning Ability | ||||||
| PPT Total Score 3 | 42.09 | (15.73) | 27.39 | (12.35) | t(53) = 3.72 | < .001** |
| # of Cards Solved PPT Trials 1 – 4 3 | 3.96 | (.17) | 3.82 | (.65) | t(24.35) = 1.02 | .315 |
| # of Cards Solved PPT Trials 5 – 8 3 | 2.68 | (1.11) | 1.30 | (1.42) | t(53) = 4.02 | < .001** |
| Memory Functions | ||||||
| Lhermitte Spatial Score 3 | .96 | (.07) | .87 | (.13) | t(31.10) = 2.58 * | .015 |
| Lhermitte Spatial 15 Minute Delay 3 | 8.61 | (.80) | 8.18 | (1.36) | t(31.23) = 1.32 * | .195 |
| Lhermitte Logical Score 3 | .84 | (.17) | .59 | (.25) | t(36.23) = 4.10 * | < .001** |
| Lhermitte Logical 15 Minute Delay 3 | 8.03 | (1.76) | 5.45 | (2.70) | t(33.42) = 3.92 * | < .001** |
| DRST Pictorial Total Correct 3 | 14.26 | (1.92) | 14.54 | (1.18) | t(50) = -.59 | .552 |
| DRST Pictorial Immediate Delay 3 | 8.89 | (2.20) | 8.40 | (2.38) | t(49) = .75 | .454 |
| DRST Spatial Total Correct 3 | 13.89 | (1.39) | 13.04 | (1.75) | t(49) = 1.92 | .060 |
| DRST Spatial Immediate Delay 3 | 10.72 | (2.58) | 10.31 | (2.58) | t(49) = .55 | .582 |
| WISC-IV Forward Digit Span 3 | 8.25 | (2.39) | 6.29 | (1.70) | t(54) = 3.40 | .001** |
| WISC-IV Backward Digit Span 3 | 7.15 | (1.96) | 5.58 | (1.81) | t(54) = 3.05 | .003 |
| WISC-IV Digit Span Total Score 2 | 9.50 | (2.60) | 6.79 | (2.79) | t(54) = 3.73 | < .001** |
| Motor Functioning | ||||||
| GPT Dominant Hand 4 | .15 | (1.07) | .38 | (1.20) | t(54) = -.77 | .442 |
| GPT Nondominant Hand 4 | .07 | (.84) | .52 | (.77) | t(54) = −2.06 | .044 |
Standard Score
Scaled Score
Raw Score
Z-score (for GPT score only, lower score indicates better performance).
Leven’s Test for Equality of Variance was significant, used equal variances not assumed and adjusted degrees of freedom in analysis.
Significant using adjusted alpha level = .0025 to compensate for inflated Type 1 error.
Intellectual functioning
Overall there was a significant difference in intellectual ability (as measured by the Leiter) between the estimated IQ of the FASD-grouped children as compared to the control grouped children, p < .001. The mean IQ was 101.96 compared to 84.16 for the children with FASD. A summary of mean ratings and standard deviations between control group and FASD-grouped children on various neuropsychological domains are listed in Table 3.
Verbal fluency
When comparing the groups on a test of verbal fluency, the alcohol-affected children were impaired in letter fluency, p = .004, but unimpaired in category fluency, when compared to controls. The category switching subtest discriminated highly between the two groups, p < .001, as was the number of set loss errors, p < .001.
Planning ability
Overall, we found that children with FASD demonstrated significant planning difficulty on the PPT when compared to controls, as reflected in their total score, p < .001. In follow-up analyses, we found that only complex planning problems that involve mental manipulation discriminated the two groups. As shown in Table 3, the FASD group performed equal to the control group in the number of cards solved on PPT trials 1–4, but the former performed markedly worse in the number of cards solved on more complex PPT trials 5–8 planning problems 5–8.
Memory ability
In the Lhermitte spatial condition, the FASD group performed significantly poorer than the control group, p = .015, but they performed as competently in the spatial 15 minute delay. In the logical memory condition, the groups were significantly different in both the overall score, p < .001, as well as the 15 minute delay, p < .001. On the DRST memory test, the FASD group displayed intact recognition memory, as well as intact immediate recall of information. Moreover, the two groups were not significantly different in terms of their spatial memory and delay recall. Effectively, the Lhermitte Spatial and Logical memory tests were able to discriminate between the two groups, whereas the DRST Pictorial and Spatial memory tests were not sensitive to the impairments of FASD.
On the Digit Span Subtest of the WISC-IV, the FASD-group had significantly fewer correct recall of digits forward, p = .001, and digits backward, p = .003, when compared to control children. When comparing Digit Span scaled scores, the group differences were highly significant, p < .001 indicating impairments in working memory among children with FASD.
Motoric ability
When comparing fine motor control, the groups did not differ using their dominant hand when required to place pegs into slotted holes, but were different while using their nondominant hand, p = .044, although this finding was not significant using the adjusted alpha level.
Additional analysis comparing control, PFAS, and FAS groups
To further examine whether there is a FASD continuum of disability, additional analyses were conducted comparing the control group of children to the children with a diagnosis of PFAS and FAS. Table 4 presents a summary of both child demographic characteristics as well as child dysmorphology data comparisons among control, PFAS, and FAS groups. The demographic characteristics of the children did not differ significantly in terms of age or gender. The three groups were significantly different with respect to all of the dysmorphological variables. In all comparisons, the dysmorphology scores were worse in the FAS group than the PFAS group.
Table 4.
Child Demographics, Dysmorphology Mean Scores and Standard Deviations Between Control, PFAS and FAS Groups.
| Control (n = 32) | PFAS (n = 14) | FAS (n = 10) | F-test | p | ||||
|---|---|---|---|---|---|---|---|---|
| Child Age (years) | 12.19 | (3.53) | 11.62 | (3.40) | 12.89 | (2.76) | F (2, 47) = .418 | .661 |
| Child Gender (%) Males Female |
n = 14 n = 18 |
43.8% 56.3% |
n = 8 n = 6 |
57.1% 42.9% |
n = 7 n = 3 |
70% 30% |
F (2, 47) = .682 | .511 |
| Dysmorphology | ||||||||
| Child Height (centile) | 70.91 bc | (24.73) | 31.21 ac | (24.83) | 6.60 ab | (4.35) | F (2, 47) = 33.37 | < .001 |
| Child Weight (centile) | 78.73 bc | (28.87) | 48.71 ac | (26.64) | 18.10 ab | (21.94) | F (2, 47) = 18.58 | < .001 |
| Child Head Circumference (centile) | 81.39 bc | (18.61) | 39.14 a | (38.83) | 14.10 a | (21.54) | F (2, 47) = 25.39 | < .001 |
| Palpebral Fissure Length (centile) | 62.69 bc | (23.69) | 13.00 a | (16.36) | 11.80 a | (9.51) | F (2, 47) = 39.12 | < .001 |
| Lip Philtrum Guide (Philtrum)1 | 3.21 c | (.90) | 3.71 | (.61) | 4.20 a | (.78) | F (2, 47) = 5.52 | .007 |
| Lip Philtrum Guide (Vermilion)1 | 3.04 bc | (.76) | 4.21 a | (.42) | 4.30 a | (.67) | F (2, 47) = 19.42 | < .001 |
| Total Child Dysmorphology Score 2 | 4.20 bc | (2.56) | 11.14 ac | (3.13) | 15.40 ab | (1.89) | F (2, 47) = 71.20 | < .001 |
indicate significant post hoc pairwise comparision between groups adjusted using Scheffe (a = control, b = PFAS, c = FAS groups).
The lip-philtrum guide. Developed by researchers at the University of Washington in Seattle, this tool pictorially displays 5 levels of philtral flatness, (1 being normal, 5 being totally flat), and 5 levels of upper lip thinness, (1 being normal, 5 being very thin). This tool helps clinicians recognize the thin upper lip and flat philtrum in children with FAS. It can be ordered through: http://depts.washington.edu/fasdpn/pdfs/guideorder.pdf
Total Dysmorphology score is a composite score derived from a weighted scoring system with the most salient FAS diagnostic features (e.g., head circumference, palpebral fissure length and smoothness of philtrum) scored a three, followed by other features (e.g., weight, and specific facial features) scored with a two, and other physical features related to FASD scored with a one.
Post-hoc tests, adjusted for multiple pairwise comparisons between the groups, revealed significant differences. Also, there were some non-significant differences between the PFAS and FAS groups on child head circumference, palpebral fissure length, philtrum, and vermilion mean scores. Child height, head circumference, and total dysmorphology score were the variables most discriminating among all three groups.
Intellectual functioning
Overall there was a statistically significant mean score difference in the estimated IQ between the three groups, p < .001. A summary of mean ratings and standard deviations on various neuropsychological domains are listed in Table 5. These were tested at α = .0025 to compensate for inflated Type 1 error due to multiple testing.
Table 5.
Neuropsychological Mean Scores and Standard Deviations Between Control, PFAS, and FAS Grouped children.
| Control (n = 32) | PFAS (n = 14) | FAS (n = 10) | F-test | p | ||||
|---|---|---|---|---|---|---|---|---|
| Intellectual Functioning | ||||||||
| Leiter Brief IQ 1 | 101.96bc | (16.12) | 84.07a | (17.02) | 82.40a | (12.71) | F (2, 55) = 9.31 | < .001** |
| Verbal Fluency | ||||||||
| D-KEFS Letter Fluency 2 | 9.72 b | (2.24) | 6.85a | (2.44) | 6.60 | (3.50) | F (2, 54) = 5.91 | .005** |
| D-KEFS Category Fluency 2 | 9.34 | (2.92) | 7.46 | (2.50) | 10.00 | (2.70) | F (2, 54) = 2.84 | .067 |
| D-KEFS Category Switching 2 | 9.22 bc | (2.41) | 6.15 a | (3.28) | 6.50a | (1.65) | F (2, 54) = 8.85 | < .001** |
| Set Loss Errors 2 | 10.31 bc | (3.41) | 6.46a | (4.46) | 6.00a | (3.88) | F (2, 54) = 7.80 | .001** |
| Planning Ability | ||||||||
| PPT Total Score 3 | 42.09 bc | (15.73) | 26.46a | (9.83) | 28.60a | (15.52) | F (2, 55) = 6.89 | .002** |
| PPT Trials 1 – 4 3 | 3.96 | (.17) | 4.00 | (.00) | 3.60 | (.96) | F (2, 55) = 3.26 | .046 |
| PPT Trials 5 – 8 3 | 2.68 bc | (1.11) | 1.23a | (1.42) | 1.40 a | (1.50) | F (2, 55) = 8.01 | .001** |
| Memory Functions | ||||||||
| Lhermitte Spatial Score 3 | .96 b | (.07) | .89a | (.15) | .90 | (.10) | F (2, 53) = 3.65 | .033 |
| Lhermitte Spatial 15 Minute Delay 3 | 8.61 | (.80) | 7.83 | (1.58) | 8.60 | (.96) | F (2, 53) = 2.52 | .090 |
| Lhermitte Logical Score 3 | .84 b | (.17) | .52a | (.25) | .69 | (.24) | F (2, 53) = 10.53 | < .001** |
| Lhermitte Logical 15 Minute Delay 3 | 8.03 bc | (1.76) | 5.33a | (2.74) | 5.60a | (2.79) | F (2, 53) = 8.72 | .001** |
| DRST Pictorial Total Correct 3 | 14.44 | (1.68) | 14.75 | (.86) | 14.30 | (1.49) | F (2, 51) = .273 | .762 |
| DRST Pictorial Immediate Delay 3 | 8.89 | (2.20) | 8.33 | (2.53) | 8.50 | (2.32) | F (2, 51) = .293 | .747 |
| DRST Spatial Total Correct 3 | 13.89 | (1.39) | 13.33 | (1.72) | 12.70 | (1.82) | F (2, 51) = 2.29 | .111 |
| DRST Spatial Immediate Delay 3 | 10.72 | (2.58) | 10.75 c | (2.86) | 9.80 b | (2.25) | F (2, 51) = .518 | .599 |
| WISC IV Digit Span Forward 3 | 8.25 b | (2.39) | 6.21a | (1.84) | 6.40 | (1.57) | F (2, 56) = 5.71 | .006** |
| WISC IV Digit Span Backward 3 | 7.15b | (1.96) | 4.92a | (1.59) | 6.50 | (1.77) | F (2, 56) = 7.05 | .002** |
| WISC IV Digit Span Scaled Score 2 | 9.50b | (2.60) | 6.28a | (2.49) | 7.50 | (3.17) | F (2, 56) = 7.58 | .001** |
| Motor Functioning | ||||||||
| GPT Dominant Hand 4 | .15 | (1.07) | .35 | (1.30) | .43 | (1.11) | F (2, 56) = .307 | .737 |
| GPT Nondominant Hand 4 | .07 | (.84) | .52 | (.88) | .54 | (.64) | F (2, 56) = 2.09 | .133 |
indicate significant post hoc pairwise comparison between groups adjusted using Scheffe (a = control, b = PFAS, c = FAS groups).
Standard Score
Scaled Score
Raw Score
Z-score (for GPT score only, lower score indicates better performance).
Significant using adjusted alpha level = .0025 to compensate for inflated Type 1 error.
Verbal fluency
No statistically significant differences between the groups were revealed when comparing letter fluency or category fluency. The category switching however was found to discriminate between the groups, p < .001, as well as the set loss errors, p = .001.
Post hoc between group comparisons, revealed significant differences on category switching between the control and the PFAS p = .003, and the control and the FAS p = .018; differences in the set loss errors (control versus PFAS, p = .012; control versus FAS, p = .010) were also significant.
Planning ability
Overall, the three groups of children demonstrated significant differences in planning ability as reflected in their overall total score on the PPT, p = .002. We found that only complex planning problems that involve mental manipulation discriminated between the groups. There were no differences between the groups in the number of cards solved on easy PPT trials 1–4, but significant differences on the number of cards solved on complex PPT trials 5–8, p = .001.
Memory ability
The results showed that there were no overall significant differences between the groups on spatial memory, or on spatial memory 15 minute delay. However, in the logical memory condition the groups were significantly different in both the overall score, p < .001, and on the 15 minute delay, p = .001.
On the DRST pictorial memory, the groups displayed comparable recognition memory, as well as on the DRST pictorial immediate delay. Also the groups were not significantly different in terms of their spatial memory, or on their immediate delay. This test discriminated less than the other tests of memory.
On the Digit Span Subtest, there was not an overall significant group difference on forward but there was a significant group difference on the backward recall of digits, p = .002. On the Digit Span scaled score there were overall significant group differences, p = .001. Post-hoc tests revealed that the control and the PFAS groups were significantly different from one another p = .002.
Motoric ability
When comparing the groups on fine motor control, the groups did not differ using their dominant hand or while using their nondominant hand.
Discussion
The aims of this study were two-fold. The first was to administer a neurocognitive test battery to children with prenatal alcohol exposure from similar American Indian communities in the Northern Plains. By involving only children from similar cultures with similar family structure and socio-economic backgrounds, an element of control is added for a variety of post-natal influences in the children’s learning and intelligence. Therefore, the differences recorded here may well be differences arising from prenatal alcohol exposure and its effects rather than from differential environments and early childhood experiences. We feel that these results and analyses describe well the continuum pattern of damage from FAS to PFAS to controls that are found among affected children in Plains Indian communities. Other cultures and social settings may well produce different patterns, but a common set of effects may also emerge as reported here. Furthermore, our data show a continuum of effects on many of the tests that vary by severity of diagnosis, and by implication, severity of dysmorphology. Overall, the children with FAS consistently perform more poorly on complex tests than do those with PFAS, while the controls perform the best. Thus the spectrum of deficits and its associations with severity of dysmorphology is demonstrated.
The second goal was to examine the effects of simple versus complex processing, in hopes of further examining cognitive dysfunction in alcohol-affected children. In the comparisons where the most difficult aspects of several tests were separated from the less difficult, the hierarchical model was supported. The more severe the FASD diagnosis, the more difficult the task was for the child. In other words, the graduated nature of difficulty within several tests discriminated between the levels of FASD with the PFAS children doing worse than the controls, and the FAS doing worse yet than the PFAS. This does not mean that dysmorphology severity alone predicts neurocognitive functioning in alcohol-exposed children, but it does indicate that the overall severity of dysmorphology is commonly associated with poor performance on these tests.
In terms of the identification of a behavioral phenotype within our sample, our results identified several neuropsychological tests that distinguished, in various capacities, between children diagnosed with PFAS, FAS and controls. These results are promising because they identify appropriate tests that can be used in combination with one another to successfully distinguish between controls and children with varying levels of alcohol exposure. While further research is needed, these results may provide stronger evidence of a behavioral phenotype of FASD and provide a starting point for delineating a battery of tests to reveal unique features seen in children diagnosed with FAS or PFAS.
As expected, many of the tests (i.e., Verbal Fluency, PPT, and the Lhermitte memory tasks) produced results consistent with a hierarchical model as demonstrated by multi-dimensional scaling, sequential logistic regression, and individual comparisons. Our verbal fluency findings were consistent with the expectation that alcohol-exposed children would perform equally well as controls on simple tasks (i.e., Category Fluency), but would show impaired performance on the more complex tasks (i.e., Letter Fluency, and Category Switching). Additionally, children with FAS or PFAS were more likely to experience difficulty in remembering and conforming to pre-specified rules. This resulted in a significant difference between groups with regard to the number of set loss errors.
Performance on the PPT also demonstrated no significant differences between control and alcohol-exposed children on the simple trials (PPT 1–4), while highly significant results were found between groups on complex trials (PPT 5–8). The total PPT score also discriminated between the groups.
The Lhermitte Spatial versus the Lhermitte Logical is another example of how such memory tests can be used to help better understand the neurobehavioral characteristics of children diagnosed with either FAS or PFAS. The Lhermitte Spatial memory task is the simpler of the two tasks and resulted in controls and affected groups performing similarly, whereas the Lhermitte Logical memory task represents the complex and discerning portion of this measure. As expected, significant differences were found between controls and affected children on the logical task.
While the majority of measures utilized in our test battery provided substantial evidence of neuropsychological deficits in children with FAS or PFAS, not all measures did so. The DRST Spatial and Pictorial memory tasks did not distinguish between the groups. It is possible that lack of significance on these measures could be due to the small sample size. Further analyses using these measures with a larger sample size may be of benefit.
Unlike the previous research (Mattson et al., 1997; and Streissguth et al., 1991), reporting the intellectual level of FAS-diagnosed children to be in the mild retardation to borderline range, our data suggest that while the mean IQ scores of the FASD-grouped children were significantly lower than that of the control group; their overall mean IQ score fell well within the low average range, at least as measured by the Leiter.
Limitations
Although obvious confounding factors (e.g., head trauma, seizure disorder, drug and alcohol abuse, and group disparities in age, gender, and ethnicity) were controlled through the study design, there can be other potential sources of variability. For example, differing postnatal environments (e.g., family and household quality) may have complicated the interpretation of our data. Another potential source of error concerns the cross-cultural variability in test-taking attitudes. Even though we used extended practice to ensure that the child would understand test instructions, as Nell (2000) points out, children growing up in impoverished conditions may not be “test-wise”. Another limitation may include our choice of the modified IOM diagnostic system for the evaluation of FASD. Also noted, is our small, biased sample, large age range, and the limitation of generalizability of our results to other groups of individuals with FASD. And lastly, our selected tests may limit the generalizability of our hierarchical model of ability organization with FASD. Other diagnostic systems, larger samples, and different tests could be used to address the questions examined in this paper.
Future directions
The merits of the current study include; 1) access to a population with a relatively high prevalence of FASD which allowed us to test a substantial number of children with the most severe diagnoses within the FASD continuum (FAS and PFAS), and 2) the fact that extensive interdisciplinary studies are underway that will help with the meaningfulness of the results. Additionally, a large dataset obtained in cross-cultural settings will eventually allow researchers to test hypotheses pertaining to cultural effects on test performance in FASD.
A delineation of the neurocognitive phenotype in children with substantial prenatal alcohol exposure will allow clinicians to accurately diagnose children within the full range of FASD. A key recommendation of the Institute of Medicine committee for improving diagnosis was, “investigation of the differences in expression and specificity of behavioral deficits in FAS and ARND” (Stratton et al., 1996, p. 6). It is now documented that those children who do not receive services because of a lack of diagnosis are more likely to develop secondary disabilities during their adolescence and adulthood (Striessguth, Barr, Kogan, & Bookstein, 1996). Therefore, the identification of a profile of strengths and weaknesses in alcohol-exposed children is a critical step toward developing evidence-based interventions for them. Educators and neuropsychologists have long recognized that how a child attains a solution to a problem is as informative as the solution itself. In other words, both a response to a test item and the processes leading to the response provide useful clues to brain organization. Therefore, a process analysis of test performance would provide useful information for defining behavioral phenotypes. Future research incorporating a process analysis of test performance on FASD is warranted. It is hoped, that the knowledge gained through this research will be useful for improving the diagnosis of children with various levels of FASD.
Acknowledgments
This project was funded in part by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD)–U01-AA01786 and UO1-AA11685 which has funded FASD research in the Northern Plains. All research methods, procedures, and consent forms were approved by the Human Research Review Committee (HRRC) of The University of New Mexico Health Sciences Center, approval # 03-168.
We gratefully acknowledge the support and assistance of some of the many people who have made this research possible. Drs. Edward Riley and Sarah Mattson have been leading collaborators in the CIFASD Consortium project, and have been particularly strong advocates for using neuropsychological testing to better define a behavioral phenotype for FASD. In addition, Drs. Faye Calhoun, Kenneth Warren, and Ting Kai Li of NIAAA were instrumental in bringing the CIFASD research team together. The authors would like to acknowledge the expertise of the dsymorphologists who participated on this project, Eugene Hoyme, M.D., Luther Robinson, M.D., and Melanie Manning, M.D. Also, special acknowledgement is given to Piyadassa Kodituwakku, Ph.D., for his assistance in the conceptualization of this project. The authors also acknowledge the expert assistance of Carol Keaster, Craig Sivak, Renee Parker, Irene Lake, and Lauren Bryce.
Footnotes
Formally defined by O’Brien and Yule (1995), a behavioral phenotype is a characteristic pattern of motor, cognitive, linguistic, and social abnormalities which is consistently associated with a biological disorder.
References
- Adnams CM, Kodituwakku PW, Hay A, Molteno CD, Viljoen D, May PA. Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcoh Clin Exp Res. 2001;25:557–562. [PubMed] [Google Scholar]
- Benton AL. Differential behavior effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FD. Deficits in adolescents with fetal alcohol syndrome: Clinical finding. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Carroll MB. The three-stratum theory of cognitive abilities. In: Flanagan DP, Genshaft JL, Harrison PL, editors. In Contemporary Intellectual Assessment: Theories, Tests and Issues. New York: Guilford; 1997. pp. 122–130. [Google Scholar]
- Cattell RB, Seitz W, Rausche A. Stability of the personality structure in children based on questionnaire (Q) data measurements. Z Exp Angew Psychol. 1971;18:513–524. [PubMed] [Google Scholar]
- Chandler LS, Richardson GA, Gallegher JD, Day N. Prenatal exposure to alcohol and marijuana: Effects on motor development of preschool children. Alcohol Clin Exp Res. 1996;20:455–461. doi: 10.1111/j.1530-0277.1996.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactive disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26:263–271. [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delise-Kaplan Executive Function System: Examiner’s Manual. Texas: The Psychological Corporation San Antonio; 2001. [Google Scholar]
- Fried PA, Watkinson B. 36- and 48- month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Devel Behav Pediatrics. 1990;11:49–58. [PubMed] [Google Scholar]
- Gustafsson JE. A unique model for the structure of intellectual abilities. Intelligence. 1984;8:179–203. [Google Scholar]
- Guttman L. A new approach to factor analysis: The radex. In: Lazerfield PF, editor. In Mathematical thinking in social sciences. Glencoe, Ill: Free Press; 1954. [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage D. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation In virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller J, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A Practical Clinical Approach to Diagnosis of Fetal Alcohol Spectrum Disorders: clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Hoyme HE, Trujillo PM, Buckley D, Aragon AS, May PA. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2037–2045. doi: 10.1111/j.1530-0277.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Progressive Planning Test: Manual. Albuquerque, NM: University of New Mexico Department of Psychology; 1993. [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neurosci Behav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Colleen AM, Hay A, Kitching AE, Burger E, Kalberg WO, Viljoen DL, May PA. Letter and category fluency in children with fetal alcohol syndrome from a community in South Africa. J Stud Alcohol. 2006;67:502–509. doi: 10.15288/jsa.2006.67.502. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Handmaker NS, Cutler SA, Weathersby EK, Handmaker SD. Specific impairments of self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19:1558–1564. doi: 10.1111/j.1530-0277.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, May PA, Clericuzio CL, Weers D. Emotion-related learning in individuals prenatally exposed to alcohol: An investigation of the relation between set shifting, extinction of responses, and behavior. Neuropsychologica. 2001;39:699–708. doi: 10.1016/s0028-3932(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kruskal JB, Wish M. Multidimensional Scaling. Beverly Hills, CA: Sage University Press; 1978. [Google Scholar]
- Lezak MD. Neuropsychological Assessment Third Edition. New York: Oxford University Press Inc; 1995. [Google Scholar]
- Lhermitte F, Signoret JL. Analyze neuropsychologique et différenciation des syndromes amnésiques. Revue Neurologique. 1972;126:164–178. [PubMed] [Google Scholar]
- Marshalek B, Lohman DF, Snow RE. The complexity continuum in the Radex and hierarchical models of intelligence. Intelligence. 1983;7:107–127. [Google Scholar]
- Matthews CG, Klove H. Instruction manual for the adult neuropsychological test battery. Madison, WI: University Wisconsin Medical School; 1964. [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Grambling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Implicity and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc. 1999;5:462–471. doi: 10.1017/s1355617799555082. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and non-verbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Pub Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hymbaugh KJ. A pilot project on fetal alcohol syndrome among American Indians. Alcohol Health Res World. 1982;7:3–9. [PubMed] [Google Scholar]
- May PA, Gossage JP. The prevalence of fetal alcohol syndrome within three New Mexico Indian communities. Albuquerque, NM: The University of New Mexico Center on Alcoholism, Substance Abuse, and Addictions; 2002. [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais A-S, Hendricks LS, Croxford JA, Viljoen DL. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health. 2005;95:1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hymbaugh KJ, Aase JM, Samet JM. Epidemiology of fetal alcohol syndrome among American Indians of the Southwest. Soc Biol. 1983;30:374–387. doi: 10.1080/19485565.1983.9988551. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Boone K, D’Elia L. Handbook of Normative Data for Neuropsychological Assessment. New York: Oxford University Press; 1999. [Google Scholar]
- Moss MB, Albert MS, Butters N, Payne M. Differential patterns of memory loss among patients with Alzheimer’s disease, Huntington’s disease, and Korsakoff’s syndrome. Arch Neurol. 1986;43:239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- O’Brien G, Yule W. In: Why behavioral phenotypes? In Behavioral phenotypes: Clinics in developmental medicine No. 138. O’ Brien G, Yule W, editors. Cambridge, UK: Cambridge University Press; 1995. pp. 1–23. [Google Scholar]
- Passingham RE. Memory of monkeys (Macaca Mulatta) with lesions in prefrontal cortex. Behav Neuroscience. 1985;99(1):3–21. doi: 10.1037//0735-7044.99.1.3. [DOI] [PubMed] [Google Scholar]
- Roebuck MR, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998;22:252–258. [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale- Revised. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Rolls ET, Treves A. Neural Networks and Brain Function. Oxford: Oxford; 1998. [Google Scholar]
- Rushworth MF, Nixon PD, Eacot MJ, Passingham RE. Ventral prefrontal cortex is not essential for working memory. J of Neurscience. 1997;17(12):4829–4838. doi: 10.1523/JNEUROSCI.17-12-04829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and non-verbal fluency in children with heavy prenatal alcohol exposure. J Stud Alcohol. 2001;62:239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Snow RE. Theory and method for research on aptitude processes. Intelligence. 1978;2:225–278. [Google Scholar]
- SPSS Inc. SPSS Base 14.0 for Windows User's Guide. Chicago IL: SPSS Inc; 1999. [Google Scholar]
- Stoelting Company. Leiter International Performance Scale (LIPS) Wood Dale, IL: Stoetling Co; 2001. [Google Scholar]
- Stratton K, Howe C, Battaglia F, editors. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment (Institute of Medicine) Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Streissguth AP, Randels SP, Smith DF. A test-retest study of intelligence in patients with prenatal alcohol syndrome: Implication for care. J Am Acad Child Adol Psychiat. 1991;30:584–587. doi: 10.1097/00004583-199107000-00009. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Carmichael Olson H, Bookstein FL, Barr HM, Scott M, Feldman J, Mirsky AF. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring- a longitudinal prospective study. Alcohol Clin Exp Res. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Neurobehavioral effects of prenatal alcohol: I. Research strategy. Neurotox Teratol. 1989;11:461–476. doi: 10.1016/0892-0362(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE). Final Report. Seattle, WA: University of Washington School of Medicine; 1996. [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34:209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC–IV technical and interpretive manual. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]