Abstract
Purpose
We report survival and recurrence outcomes in all patients undergoing radical or partial nephrectomy for renal cell carcinoma, as related to surgical waiting time.
Materials and Methods
We retrospectively reviewed the records of 722 patients who underwent surgical resection for renal cell carcinoma. Patients were subdivided by waiting time from the initial urology visit until surgery. Surgical waiting time was evaluated as a continuous variable and by monthly subgroups. Univariate and multivariate analyses were performed to evaluate factors associated with overall, disease specific and recurrence-free survival.
Results
Mean time from the first visit to surgery was 1.2 months with 64.1% and 94.3% of patients undergoing surgery within 30 days and within 3 months, respectively. Overall and disease specific survival was not affected by surgical waiting time regardless of how time was analyzed. On univariate analysis 5-year recurrence-free survival was poorer in patients undergoing surgery within 1 month vs more than 1 month (75.7% vs 88.4%, p = 0.02). On multivariate analysis T stage (p <0.0001), grade (p = 0.009), lymph node involvement (p = 0.0001) and histology (p = 0.006) were independent predictors of recurrence-free survival, while surgical waiting time was not (p = 0.18). Surgical waiting time less than 1 month was associated with higher stage and higher grade tumors (p <0.0001 and 0.0006, respectively).
Conclusions
Surgical waiting time from initial urological consultation to operative intervention does not adversely affect the outcome of renal cell carcinoma within the time frames analyzed in this study, in which 94% of cases occurred within 3 months. Individual urologist judgment remains a critical factor in the appropriate and timely care of the patient with a suspicious renal mass.
Keywords: kidney, carcinoma, renal cell, waiting lists, nephrectomy, outcome assessment (health care)
Surgical removal of the tumor or kidney continues to be the primary treatment modality for all grades and types of renal malignancies. Therefore, the time from initial presentation or diagnosis of a renal tumor until the time that it is resected can potentially affect patient outcomes. The impact of surgical delays has been explored for bladder and prostate cancer but to our knowledge not for RCC. For invasive bladder cancer several reports suggest that a surgical delay of longer than 3 months may adversely impact patient outcomes.1 For prostate cancer the results are more controversial.2
Currently no guidelines exist regarding the appropriate interval from diagnosis to surgical intervention for kidney cancer. Recommendations for timely referral and treatment have been suggested but no clinical data substantiate a specific time frame. Currently 2 national organizations, the Canadian Society of Surgical Oncology and United Kingdom Health Service, recommend no more than 4 weeks of waiting time from diagnosis to surgical treatment for any malignancy.3,4 The Fraser Institute in Canada performed a nationwide survey of specialists and the reported median reasonable waiting time for genitourinary malignancies was 3.3 weeks.5
Only 3 studies in the urological literature have addressed waiting time for renal cancer surgery. All 3 series assessed only the mean waiting time for surgery in different settings but none looked at the impact of waiting time on patient outcome or tumor pathology.6 Several groups have performed focused analyses in groups of patients on active surveillance who underwent delayed intervention for renal masses.7,9 After an average delay of 12, 15 and 10 months groups of 13, 50 and 27 patients with a median tumor size of 3.1, 2.6 and 2.0 cm, respectively, underwent intervention. All studies showed favorable outcomes with no tumor upstaging in these predominantly T1 lesions.
To our knowledge we report the first study in the peer reviewed literature specifically evaluating outcomes in all patients with RCC undergoing radical or partial nephrectomy, as related to the full spectrum of surgical waiting times. We evaluated differences in the recurrence rate or survival based on waiting time from the initial patient visit to a urologist until the date of tumor resection.
MATERIALS AND METHODS
We examined data on 722 patients during a continuous 18-year period from 1988 to 2006 at a single institution who underwent partial or radical nephrectomy for RCC. Data were obtained from an institutional review board approved, prospective nephrectomy database and the Vanderbilt Cancer Registry database, supplemented by a retrospective review of the patient medical records. Demographic data and patient characteristics were captured along with the date of initial imaging when available and the date of the patient first presentation to a urologist for evaluation of a kidney mass. Surgical waiting time was defined as the period from the date of this initial visit to the date of surgical resection. Only patients with pathologically confirmed RCC were included. All histological variants of RCC were included. The followup data obtained included pathological stage, histological tumor characteristics, followup, RCC recurrence, death and cause of death. Patients were followed at 3 and 6 months, every 6 months for at least 2 years and annually thereafter.
The primary end points of our study were OS, DSS and RFS. OS and DSS were calculated in the entire cohort. RFS was calculated after excluding patients with metastases at presentation, VHL disease or incomplete data. Time to recurrence or death was defined from the date of the patient operation. Patients who were recurrence-free or alive at last followup were censored. Survival was estimated using the Kaplan-Meier method and univariate analysis was performed using the log rank test. Surgical waiting times were analyzed as categorical variables by grouping less than 1 month vs longer, less than 2 months vs longer, less than 3 months vs longer and less than 6 months vs longer as well as by comparing patients in 4 time blocks, including less than 1, 1 to 3, 3 to 6 and more than 6 months. A continuous variable with a Cox proportional hazard model was also used. Multivariate analysis using a Cox proportional hazard model was subsequently performed to evaluate independent parameters associated with RFS. The specific variables evaluated were T stage, AJCC stage, histology pattern, tumor grade, tumor size, sarcomatoid features and lymph node status. All p values were 2-sided and p <0.05 was considered statistically significant. Subsequent chi-square analysis was performed to compare tumor characteristics between the less vs greater than 1-month subgroups. Data were analyzed using StatView®.
RESULTS
This review included 722 patients who underwent surgical resection for RCC. Of the patients 67 were excluded due to incomplete records, leaving a cohort of 655 with a mean ± SD age of 60.5 ± 12.7 years. Radical nephrectomy was performed in 474 patients (72.4%), 178 underwent partial nephrectomy (27.2%) and 3 underwent a combined procedure. Mean followup in the entire group was 31 months (range 0 to 214) and mean followup in patients alive at last followup was 35 months (range 0 to 214). Mean surgical waiting time from the initial visit to resection was 1.2 months (range 0 to 30). Of the patients 64.1% underwent surgery within 30 days of the initial visit and 94.3% underwent surgery within 3 months of the initial consultation. Table 1 lists patient characteristics.
Table 1.
Patient characteristics in entire cohort
| Demographic | |
|---|---|
| No. pts | 655 |
| No. men (%) | 435 (66.4) |
| No. women (%) | 220 (33.6) |
| No. race (%): | |
| White | 592 (90.4) |
| Black | 55 (8.4) |
| Other | 8 (1.2) |
| No. ASA class (%): | |
| 1 | 4 (0.6) |
| 2 | 185 (28.2) |
| 3 | 308 (47.0) |
| 4 | 30 (4.6) |
| Unknown | 128 (19.6) |
| No. confirmed VHL (%) | 3 (0.5) |
| Mean age at surgery (range) | 60.5 (18–87) |
| No. nephrectomy (%): | |
| Partial | 178 (27.2) |
| Radical | 474 (72.4) |
| Partial + radical | 3 (0.4) |
| No. operative approach (%): | |
| Open | 525 (80.2) |
| Hand assisted laparoscopic | 92 (14.0) |
| Pure laparoscopic | 38 (5.8) |
| Time from initial visit to surgery: | |
| Overall mean mos (range) | 1.2 (0–30) |
| No. less than 1 mo (%) | 420 (64.1) |
| No. 1–3 mos (%) | 198 (30.2) |
| No. 3–6 mos (%) | 17 (2.6) |
| No. greater than 6 mos (%) | 20 (3.1) |
| Mean mos followup (range): | |
| Overall | 31 (0–214) |
| Survivors | 35 (0–214) |
Table 2 lists RCC pathology and tumor characteristics. Conventional clear cell histology was the most prevalent tumor type (71.6%). Mean tumor size was 6.4 ± 4.4 cm. Of the tumors 49.0% were pathological stage T2 or higher and 50.2% were AJCC stage 2 or greater.
Table 2.
Tumor pathology and characteristics in entire cohort
| Variable | No. Pts (%) |
|---|---|
| Mean ± SD cm tumor size (range) | 6.4 ± 4.4 (0.2–35) |
| T stage: | |
| T1a | 231 (35.3) |
| T1b | 103 (15.7) |
| T2 | 81 (12.4) |
| T3a | 94 (14.3) |
| T3b | 119 (18.2) |
| T3c | 5 (0.8) |
| T4 | 22 (3.3) |
| AJCC stage: | |
| 1 | 326 (49.8) |
| 2 | 75 (11.5) |
| 3 | 181 (27.6) |
| 4 | 73 (11.1) |
| Histology: | |
| Conventional clear cell | 469 (71.6) |
| Papillary | 95 (14.5) |
| Chromophobe | 40 (6.1) |
| Collecting duct Ca | 5 (0.8) |
| Unclassified RCC | 12 (1.8) |
| Unknown | 34 (5.2) |
| Grade: | |
| 1 | 69 (10.5) |
| 2 | 315 (48.1) |
| 3 | 156 (23.8) |
| 4 | 81 (12.4) |
| Unknown | 34 (5.2) |
| Sarcomatoid features | 39 (5.5) |
| Ca in lymph nodes | 48 (7.3) |
| Metastases at presentation: | |
| Present | 88 (13.4) |
| Absent | 567 (86.6) |
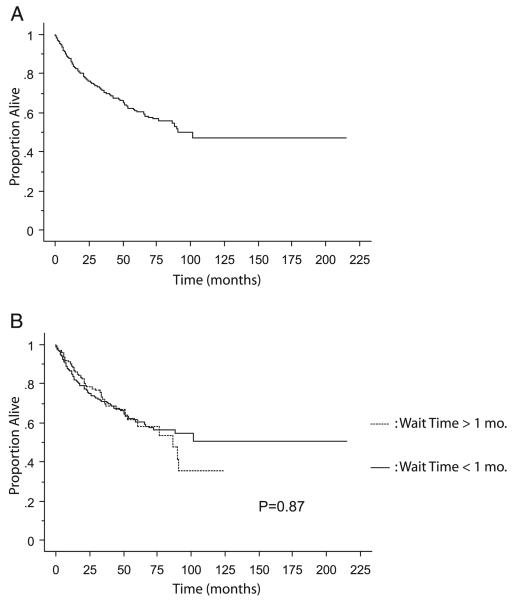
Median OS calculated in the entire cohort of 655 patients was 90.5 months. The end point of death was attained in 180 patients. Actuarial estimated 5-year OS in the whole cohort was 61.0% (fig. 1, A). When the cohort was subdivided into surgical waiting times of less and more than 1-month groups, 5-year OS was 60.7% and 61.4%, respectively (p = 0.87, fig. 1, B). Univariate analyses were performed with OS as the end point, specifically grouping patients into surgical delays of less than 1 month vs longer (p = 0.87), less than 2 months vs longer (p = 0.46), less than 3 months vs longer (p = 0.71) and less than 6 months vs longer (p = 0.75) with no differences demonstrated when waiting time was dichotomized at any of these time points. Surgical waiting time was grouped into 4 categories, including less than 1, 1 to 3, 3 to 6 and greater than 6 months. Again, there was no association with OS (p = 0.98). A Cox proportional hazard model using time as a continuous variable revealed no discernible relationship between waiting time and change in OS (p = 0.35). Statistically significant predictors of worse OS were T stage, AJCC stage, histology, tumor grade, sarcomatoid features, lymph node involvement and metastases at presentation (each p <0.0001, table 3).
Fig. 1.
Kaplan-Meier curve of OS in entire cohort (A) with break-down comparing patients with less vs more than 1-month waiting time for surgery (B).
Table 3.
Univariate analysis of OS, DSS and RFS
| Variable Compared | p Value |
|---|---|
| OS | |
| Race | 0.17 |
| Sex | 0.20 |
| ASA class | 0.06 |
| T stage | <0.0001 |
| AJCC stage | <0.0001 |
| Tumor histology | <0.0001 |
| Tumor grade | <0.0001 |
| Sarcomatoid features | <0.0001 |
| Lymph node involvement | <0.0001 |
| Metastases at presentation | <0.0001 |
| Mos from presentation to surgery: | |
| Less than 1 | 0.87 |
| Less than 2 | 0.46 |
| Less than 3 | 0.71 |
| Less than 6 | 0.75 |
| Comparing all 4 groups individually | 0.98 |
| Cox proportional HR using time as continuous variable* | 0.35 |
| DSS | |
| Race | 0.47 |
| Sex | 0.23 |
| ASA class | 0.40 |
| T stage | <0.0001 |
| AJCC stage | <0.0001 |
| Tumor histology | <0.0001 |
| Tumor grade | <0.0001 |
| Sarcomatoid features | <0.0001 |
| Lymph node involvement | <0.0001 |
| Metastases at presentation | <0.0001 |
| Mos from presentation to surgery: | |
| Less than 1 | 0.30 |
| Less than 2 | 0.45 |
| Less than 3 | 0.73 |
| Less than 6 | 0.86 |
| Comparing all 4 groups individually | 0.94 |
| Cox proportional HR using time as continuous variable* | 0.66 |
| RFS | |
| Race | 0.90 |
| Sex | 0.53 |
| ASA class | 0.83 |
| T stage | <0.0001 |
| Tumor histology | <0.0001 |
| Tumor grade | <0.0001 |
| Sarcomatoid features | <0.0001 |
| Lymph node involvement | <0.0001 |
| Mos from presentation to surgery: | |
| Less than 1 | 0.02 |
| Less than 2 | 0.06 |
| Less than 3 | 0.10 |
| Less than 6 | 0.33 |
| Cox proportional HR using time as continuous variable* | 0.14 |
For OS, DSS and RFS HR 1.029 (95% CI 0.969–1.094), 0.976 (95% CI 0.875–1.088) and 0.850 (95% CI 0.690–1.050), respectively.
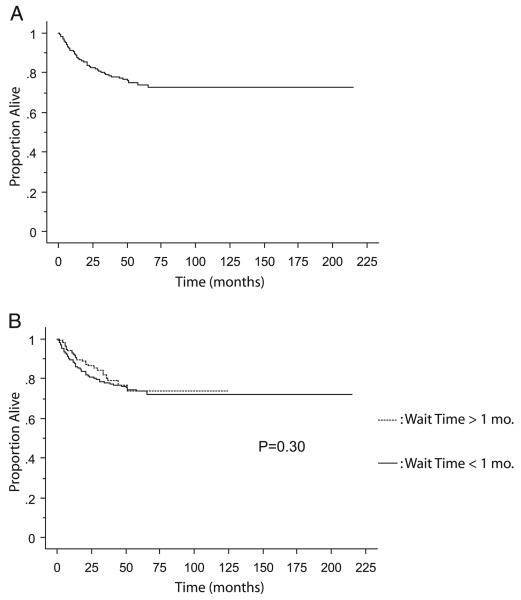
DSS was calculated in the 633 patients in the cohort with complete data available for review. The end point of death from RCC was attained in 111 patients. Actuarial estimated 5-year DSS in the whole cohort was 74.1% (fig. 2, A). When the cohort was subdivided into surgical waiting times of less and more than 1-month groups, 5-year DSS was 73.8% and 73.6%, respectively (p = 0.30, fig. 2, B). Univariate analyses and Cox proportional HRs were calculated as described for OS, and surgical waiting time was not a significant variable associated with worse DSS (table 3). Statistically significant predictors of worse DSS survival were T stage, AJCC stage, histology, tumor grade, sarcomatoid features, lymph node involvement and metastases at presentation (each p <0.0001).
Fig. 2.
Kaplan-Meier curve for DSS in entire cohort (A) with breakdown comparing patients with less vs more than 1-month waiting time for surgery (B).
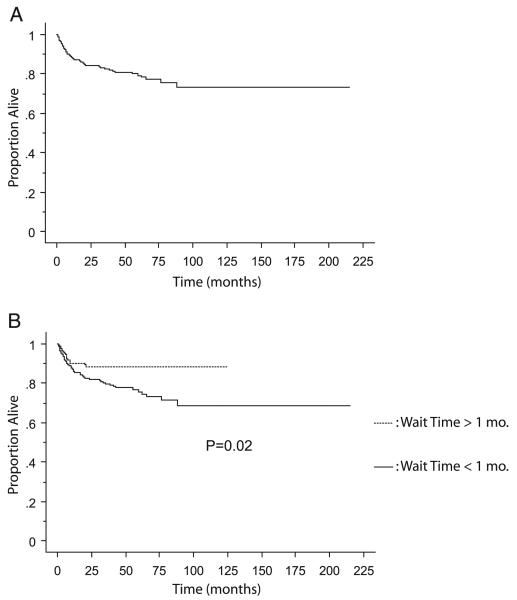
When looking at RFS in the cohort, 88 patients were excluded due to metastases at presentation, 3 were excluded due to VHL and 6 had data missing, leaving a subgroup of 558. Recurrence was noted in 91 patients and actuarial estimated 5-year RFS in the whole cohort was 79.2% (fig. 3, A). Subdividing the cohort into surgical waiting times of less vs more than 1-month groups was associated with significantly worse estimated 5-year RFS (75.7% and 88.4%, respectively, p = 0.02, fig. 2, B). Statistically significant predictors of worse OS were T stage, histology, tumor grade, sarcomatoid features and lymph node involvement (each p <0.0001). On multivariate analysis that included all statistically significant variables from univariate analysis surgical waiting time less than 1 month was not an independent predictor of worse RFS (p = 0.18). Independent predictors of worse RFS were T stage (p <0.0001), lymph node involvement (p = 0.0001), histology (p = 0.006) and grade (p = 0.0009, table 4).
Fig. 3.
Kaplan-Meier curve for RFS in entire cohort (A) with breakdown comparing patients with less vs more than 1-month waiting time for surgery (B).
Table 4.
Multivariate analysis of variables affecting RFS
| Variable Analyzed | p Value | HR (95% CI) |
|---|---|---|
| Mos from initial visit to surgery: | ||
| Less than 1 | 0.18 | 1.515 (0.821–2.794) |
| Greater than 1 | Referent | |
| Histology: | 0.006 | |
| Conventional clear cell | 0.04 | 2.919 (1.029–8.280) |
| Papillary | 0.22 | 2.234 (0.618–8.080) |
| Chromophobe | 0.95 | 0.944 (0.104–8.583) |
| Collecting duct Ca | 0.0004 | 28.870 (4.516–184.540) |
| Unclassified RCC | Referent | |
| Grade: | 0.0009 | |
| 1 | 0.0094 | 0.063 (0.008–0.507) |
| 2 | 0.0003 | 0.242 (0.113–0.517) |
| 3 | 0.0297 | 0.442 (0.212–0.923) |
| 4 | Referent | |
| Sarcomatoid features: | ||
| Absent | 0.18 | 0.554 (0.230–1.332) |
| Present | Referent | |
| Lymph node involvement: | ||
| No | 0.0001 | 0.261 (0.131–0.518) |
| Yes | Referent | |
| T stage: | <0.0001 | |
| T1 | <0.0001 | 0.200 (0.100–0.397) |
| T2 | 0.2794 | 0.327 (0.327–1.381) |
| T3 or Greater | Referent |
Tumor characteristics were compared between the less and more than 1-month waiting time groups (table 5). Patients who underwent surgical resection in the less than 1-month group were more likely to have larger, more aggressive tumors with higher T stage, higher grade and higher AJCC stage, and they were more likely to have lymph node involvement at surgery.
Table 5.
Tumor characteristics in patients with less vs greater than 1-month waiting time for surgical intervention
| Variable Evaluated | No. Less Than 1 Mo (%) |
No. Greater Than 1 Mo (%) |
p Value |
|---|---|---|---|
| T stage: | <0.0001 | ||
| 1 | 182 (41.6) | 152 (69.7) | |
| 2 | 69 (15.8) | 12 (5.5) | |
| 3 or Greater | 186 (42.6) | 54 (24.8) | |
| AJCC stage: | <0.0001 | ||
| 1 | 175 (40.0) | 151 (69.3) | |
| 2 | 63 (14.4) | 12 (5.5) | |
| 3 | 142 (32.5) | 39 (17.9) | |
| 4 | 57 (13.1) | 16 (7.3) | |
| Histology: | 0.22 | ||
| Conventional clear cell | 320 (77.1) | 149 (72.3) | |
| Papillary | 59 (14.2) | 36 (17.5) | |
| Chromophobe | 22 (5.3) | 18 (8.7) | |
| Collecting duct Ca | 4 (1.0) | 1 (0.5) | |
| Unclassified RCC | 10 (2.4) | 2 (1.0) | |
| Grade: | 0.0006 | ||
| 1 | 35 (8.4) | 34 (16.5) | |
| 2 | 204 (49.2) | 111 (53.9) | |
| 3 | 110 (26.5) | 46 (22.3) | |
| 4 | 66 (15.9) | 15 (7.3) | |
| Sarcomatoid features: | 0.08 | ||
| Present | 31 (7.1) | 8 (3.7) | |
| Absent | 406 (92.9) | 210 (96.3) | |
| Lymph node involvement: | 0.01 | ||
| Yes | 40 (9.2) | 8 (3.7) | |
| No | 397 (90.8) | 210 (96.3) | |
| Metastases at surgery: | 0.07 | ||
| Present | 66 (15.1) | 22 (10.1) | |
| Absent | 371 (84.9) | 196 (89.9) |
DISCUSSION
After the referral of a new patient for a renal mass suspicious for RCC there is an inevitable delay between the initial consultation to removal of the mass. The question that is often associated with this surgical delay is what time frame is appropriate and whether a delay of any length has adverse effects on the patient outcome. Our study of individuals who underwent radical or partial nephrectomy for RCC shows that surgical waiting time was not adversely associated with OS, DSS or RFS after accounting for tumor stage, grade and histology. The significantly worse RFS in patients who underwent the operation within a month of the first visit was likely secondary to larger, more sinister-appearing renal masses being pushed up in the operating schedule, while smaller, less indolent-appearing tumors were planned for more elective resection at the next most available date. This explains why operative waiting time ± 1 month was not an independent predictor of RFS when other potential confounding variables were accounted for on multivariate analysis. Specifically as proven in past studies, tumor size, grade and stage significantly correlated with RFS.10 Overall our 5-year RFS from RCC in patients undergoing nephrectomy was 79.2%, consistent with another published rate in the literature of 78% in similar patients.11
There is currently a debate in the surgical oncology literature about what are appropriate waiting periods from diagnosis to operative intervention. There are only 3 studies in the urological literature that have addressed waiting times for renal cancer surgery. Nuttall et al found a mean waiting time from the decision to perform radical nephrectomy to surgery of 23.6 days,12 while Subramonian et al found a mean waiting time of 26 days from the diagnosis of RCC to surgery.13 The third study, which was performed by Simunovic et al, showed a median of 64 days in 58 patients from the time of referral to a specialist to the day of nephrectomy.14 The mean waiting time of 1.2 months in the current study is comparable to the mean time of between 26 and 64 days reported previously.
None of the prior studies addressed the potential impact of waiting time to surgery and the outcome of renal malignancies. To our knowledge our study is the first to address this topic in all patients undergoing operative intervention. It suggests that the time between the first visit to a urologist and subsequent nephrectomy does not adversely affect patient outcomes with respect to OS, DSS or RFS. More critical to patient outcome are other predictors, such as tumor size, grade and pathological characteristics.
For other urological malignancies the topic of surgical waiting time is currently being addressed. With muscle invasive urothelial carcinoma a delay of more than 3 months from pathological diagnosis to cystectomy has been shown to have poorer progression-free survival.1 Patients undergoing definitive therapy for prostate cancer are at an equivalent risk for biochemical RFS even with a delay in therapy of more than 3 months.2 The delay in treatment for prostate cancer and urothelial carcinoma is easier to track because each depends on the pathological diagnosis, whereas renal masses are not typically biopsied preoperatively. The decision to operate is primarily based on imaging and the pathological diagnosis comes postoperatively. With the most powerful predictors being tumor size, stage and grade the only preoperative data available to the urologist is tumor size. Combined with the knowledge that RCC grows at an average rate of 0.49 to 0.86 cm per year our study reinforces that larger tumors that are more predisposed to adverse pathological results should be removed promptly and smaller, less indolent tumors may be removed electively with no significant effect on the likelihood that a patient would experience recurrence in the future.15,16
This study has several limitations. This was in part a retrospective review of the data, which may introduce the inherent bias found in any retrospective study. We attempted to analyze time from the first documented x-ray to nephrectomy as well as time from the first clinic visit to surgery. However, it proved unreliable to determine the exact date of the first radiographic study showing a renal mass, so that this analysis was omitted. This may have resulted in understating the true amount of the delay from diagnosis to operative intervention, although the time frame from initial urological consultation to nephrectomy is the variable most at the control of the treating surgeon. This study included only patients who underwent surgery and it did not include those with a renal mass who were under surveillance or who underwent an energy ablative approach. Therefore, it cannot be directly compared to other important studies in the literature that address that patient population.
Finally, it is important to recognize that the process of having a patient see a urologist and then go on to surgery during a certain time frame is a dynamic one that involves many variables, of which some are hard to quantify in any meaningful way. This also leads to an important caveat with the findings of this study. This study is not an endorsement of long waiting times from diagnosis to surgery for renal masses. Patients with more worrisome-appearing masses appear to have undergone surgery more rapidly, which may well account at least in part for the results presented. Therefore, it remains critical for the urologist to assess the individual circumstances in each instance to determine the optimal time to move ahead with surgery in any given patient.
CONCLUSIONS
The strongest predictors of OS, DSS and RFS in patients undergoing radical or partial nephrectomy for RCC are T stage, AJCC stage, tumor grade, histology and lymph node involvement at surgical resection. Surgical waiting time from the first visit with a urologist to operative intervention does not adversely affect the outcome of RCC within the time frames analyzed in this study, in which 94% of the cases occurred within 3 months. Individual urologist judgment remains a critical factor in the appropriate and timely care of the patient with a suspicious renal mass.
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- ASA
American Society of Anesthesiologists
- DSS
disease specific survival
- OS
overall survival
- RCC
renal cell carcinoma
- RFS
recurrence-free survival
- VHL
von Hippel-Lindau
REFERENCES
- 1.May M, Nitzke T, Helke C, Vogler H, Hoschke B. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. 2004;38:231. doi: 10.1080/00365590410029141. [DOI] [PubMed] [Google Scholar]
- 2.Phillips JJ, Hall MC, Lee WR, Clark PE. Does a delay in initiating definitive therapy affect biochemical recurrence rates in men with clinically localized prostate cancer? Urol Oncol. 2007;25:196. doi: 10.1016/j.urolonc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Position Statement Canadian Society for Surgical Oncology. 2005 Available at http://www.cos.ca/csso. Accessed June 30, 2007.
- 4.Cancer Waiting Targets: A Guide. Department of Health, National Health Service; 2005. Welcome to the Department of Health. Available at http://www.dh.gov.uk. Accessed June 30, 2007. [Google Scholar]
- 5.Esmail N, Walker M. Waiting Your Turn: Hospital Waiting Lists in Canada. 15th ed. The Fraser Institute; Tampa: 2005. [Google Scholar]
- 6.Jewett M, Rendon R, Dranitsaris G, Drachenberg D, Tanguay S, Donnelly B, et al. Does prolonging the time to renal cancer surgery affect long-term cancer control: a systematic review of the literature. Can J Urol. 2006;3(suppl.):54. [PubMed] [Google Scholar]
- 7.Kouba E, Smith A, McRackan D, Wallen EM, Pruthi RS. Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol. 2007;177:466. doi: 10.1016/j.juro.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 8.Viterbo R, Crispen PL, Greenberg RE, Chen DY, Uzzo RG. Delayed management of high grade renal tumors. J Urol. 2006;175(suppl.):350. abstract 1089. [Google Scholar]
- 9.Viterbo R, Chawla SN, Crispen PL, Greenberg RE, Chen DY, Uzzo RG. Delayed management of incidentally detected renal masses does not limit or complicate treatment options. J Urol. 2005;173(suppl.):23. abstract 82. [Google Scholar]
- 10.Park WH, Eisen T. Prognostic factors in renal cell cancer. BJU Int. 2007;99:1277. doi: 10.1111/j.1464-410X.2007.06828.x. [DOI] [PubMed] [Google Scholar]
- 11.Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer. 2005;104:1362. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 12.Nuttall M, Cathcart P, van der Meulen J, Gillatt D, McIntosh G, Emberton M. A description of radical nephrectomy practice and outcomes in England: 1995–2002. BJU Int. 2005;96:58. doi: 10.1111/j.1464-410X.2005.05567.x. [DOI] [PubMed] [Google Scholar]
- 13.Subramonian KR, Puranik S, Mufti GR. How will the two-weeks-wait rule affect delays in management of uro-logical cancers? J R Soc Med. 2003;96:398. doi: 10.1258/jrsm.96.8.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165:421. [PMC free article] [PubMed] [Google Scholar]
- 15.Kassouf W, Aprikian AG, Laplante M, Tanguay S. Natural history of renal masses followed expectantly. J Urol. 2004;171:111. doi: 10.1097/01.ju.0000102409.69570.f5. [DOI] [PubMed] [Google Scholar]
- 16.Sowery RD, Siemens DR. Growth characteristics of renal cortical tumors in patients managed by watchful waiting. Can J Urol. 2004;11:2407. [PubMed] [Google Scholar]