Abstract
Object
Significant variation exists in the surgical and medical management of CSF shunt infection. The objectives of this study were to determine CSF shunt reinfection rates following initial CSF shunt infection in a large patient cohort and to determine management, patient, hospital, and surgeon factors associated with CSF shunt reinfection.
Methods
This retrospective cohort study included children who were in the Pediatric Health Information System (PHIS) database, who ranged in age from 0 to 18 years, and who underwent uncomplicated initial CSF shunt placement in addition to treatment for initial CSF shunt infection between January 1, 2001, and December 31, 2008. The outcome was CSF shunt reinfection within 6 months. The main predictor variable of interest was surgical approach to treatment of first infection, which was determined for 483 patients. Covariates included patient, hospital, surgeon, and other management factors.
Results
The PHIS database includes 675 children with initial CSF shunt infection. Surgical approach to treatment of the initial CSF shunt infection was determined for 483 children (71.6%). The surgical approach was primarily shunt removal/new shunt placement (in 286 children [59.2%]), but a substantial number underwent externalization (59 children [12.2%]), of whom a subset went on to have the externalized shunt removed and a new shunt placed (17 children [3.5% overall]). Other approaches included nonsurgical management (64 children [13.3%]) and complete shunt removal without shunt replacement (74 children [15.3%]).
The 6-month reinfection rate was 14.8% (100 of 675 patients). The median time from infection to reinfection was 21 days (interquartile range [IQR] 5–58 days). Children with reinfection had less time between shunt placement and initial infection (median 50 vs 79 days, p = 0.06). No differences between those with and without reinfection were seen in patient factors (patient age at either shunt placement or initial infection, sex, race/ethnicity, payer, indication for shunt, number of comorbidities, distal shunt location, and number of shunt revisions at first infection); hospital volume; surgeon volume; or other management factors (for example, duration of intravenous antibiotic use). Nonsurgical management was associated with reinfection, and complete shunt removal was negatively associated with reinfection. However, reinfection rates did not differ between the 2 most common surgical approaches: shunt removal/new shunt placement (44 [15.4%] of 286; 95% CI 11.4%–20.1%) and externalization (total 12 [20.3%] of 59; 95% CI 11.0%–32.8%). Externalization followed by shunt removal/new shunt placement (5 [29.4%] of 17; 95% CI 10.3%–56.0%) and nonsurgical management (15 [23.4%] of 64; 95% CI 13.8%–35.7%) had higher, but nonstatistically significant, reinfection rates. The length of stay was shorter for nonsurgical management.
Conclusions
Surgical approach to treatment of initial CSF shunt infection was not associated with reinfection in this large cohort of patients.
Keywords: cerebrospinal fluid, hydrocephalus, infection, pediatric neurosurgery, epidemiology, reinfection
Cerebrospinal fluid shunt placement is the mainstay of hydrocephalus treatment.17 While allowing children with hydrocephalus to avoid further brain injury, CSF shunts can be associated with new and chronic surgical and medical problems.33 Infections are frequent complications12,23 and have been seen in 11.7% of patients undergoing CSF shunt placement.32
There is significant variation in surgical and medical decision-making in the treatment of CSF shunt infection. 18,19,21,39 Surgical approaches to the treatment of CSF shunt infection include: shunt removal and external ventricular drain insertion followed by new shunt placement once the CSF is sterile (referred to hereafter as shunt removal/new shunt placement), shunt externalization followed by shunt replacement (referred to hereafter as externalization), shunt externalization followed by shunt removal and external ventricular drain insertion followed by new shunt placement (referred to hereafter as failed externalization), or nonsurgical management.10,11,15,25,35,37 The superiority of either surgical approach in preventing CSF shunt reinfection is unclear.20,40 Medical decisions in the treatment of CSF shunt infection include administration of intravenous antibiotics with or without intrathecal antibiotics.5,18 While intravenous antibiotics are a mainstay of clinical practice in conjunction with surgery,5,31,37 duration of intravenous antibiotic use varies widely18 and depends, in part, on the surgical approach used26,36 and pathogen involved.5,41
There have been 2 recent observational studies of treatment of CSF shunt infection of note. Kestle et al.18 observed 70 patients after CSF shunt infection. Surgical approaches to treatment of CSF shunt infection included shunt removal/new shunt placement (71%), externalization (24%), and nonsurgical management (4%). The duration of intravenous antibiotic treatment ranged from 4 to 47 days. Reinfection occurred at a rate of 26% within 12 months. Kulkarni et al.19 observed 51 patients after CSF shunt infection. Surgical approaches to treatment of CSF shunt infection included shunt removal/new shunt placement (55%), externalization (37%), and shunt removal with no shunt replacement (hereafter referred to as complete shunt removal; 8%). The mean duration of intravenous antibiotic treatment was 11.2 days. Reinfection occurred at a rate of 19.6% within 6 months.
Our goal was to study CSF shunt reinfection in a large cohort of patients. The specific objectives were to determine CSF shunt reinfection rates following initial CSF shunt infection and to determine management, patient, hospital, and surgeon factors associated with CSF shunt reinfection. The hypothesis tested was that reinfection rates would be higher when infections were surgically treated with externalization than those treated with shunt removal/new shunt placement.
Methods
Study Design/Data Source
This was a retrospective cohort study using the PHIS database. The PHIS was developed by the Child Health Corporation of America (Shawnee Mission, KS) and contains administrative and limited clinical data on all discharges from member hospitals (41 not-for-profit free-standing children’s hospitals in the US). The data warehouse function for PHIS is managed by Thomson Reuters (Evanston, IL), and data are subjected to several reliability and validity checks before incorporation into the database. Patients can be identified using consistently encrypted medical record numbers, allowing cross-linking of encounters over time. The study was reviewed and exempted from annual review by the institutional review board at the University of Utah.
Study Population
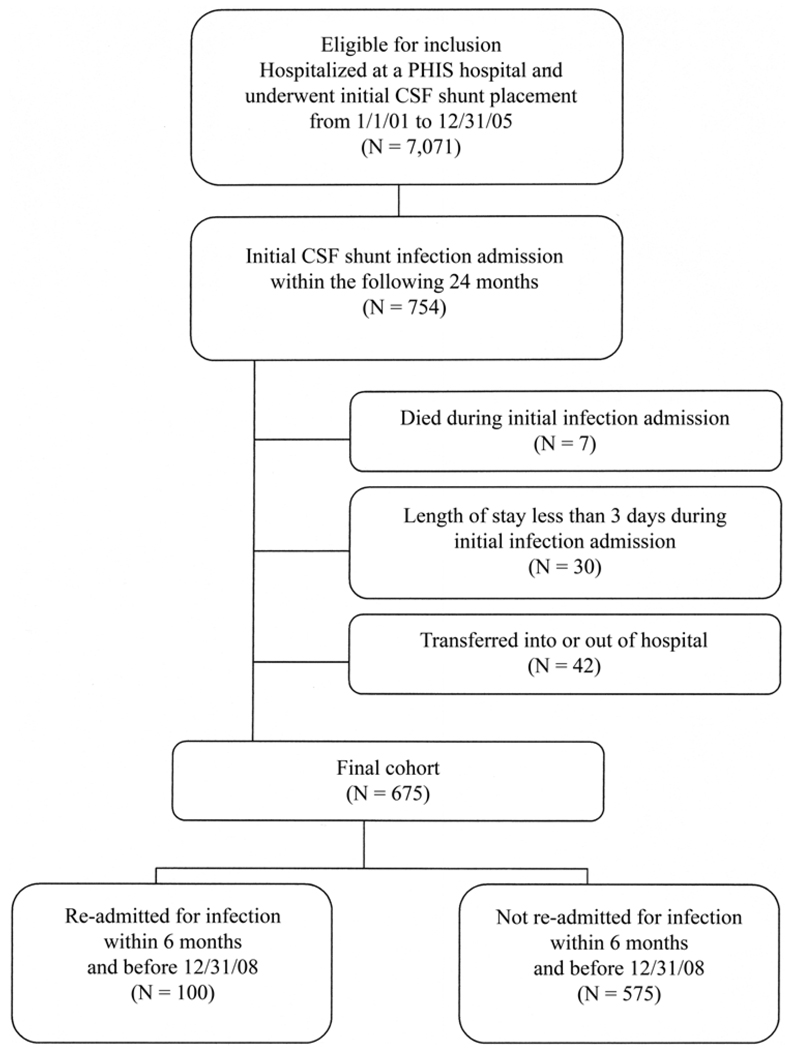
Children between 0 and 18 years of age who underwent initial CSF shunt placement with a discharge date between January 1, 2001, and December 31, 2005 were identified from all hospitals in the PHIS using an iterative query based on ICD-9-CM procedure and diagnosis codes.32 Of 7071 eligible children, 754 (10.7%) had initial CSF shunt infection, defined as an admission within 24 months following initial CSF shunt placement with a primary diagnosis of CSF shunt infection (ICD-9-CM code 996.63) (Fig. 1). We then applied the following exclusion criteria: death during initial infection admission (7 children), LOS less than 3 days during initial infection admission (given that these admissions were probably not truly infections [30 children]), and transfer into or out of the PHIS hospital during the infection admission (42 children). Then final cohort consisted of 675 children, all of whom, regardless of documentation of subsequent care, were included in the study. Most of the cohort (75% [508 of 675]) had subsequent care within PHIS before December 31, 2008.
FIG. 1.
Flow chart showing the study population.
Outcome Variable
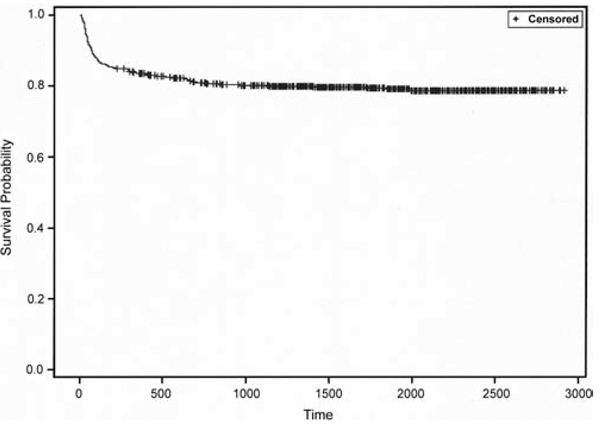
The primary outcome variable was a readmission within 6 months for CSF shunt infection. Readmission was defined as an admission subsequent to initial CSF shunt infection admission and through December 31, 2008, with any ICD-9-CM discharge diagnosis code for shunt infection (996.63). For the final cohort of 675 children, 140 (20.7%) had a readmission for reinfection. Six months was selected for duration of surveillance for readmission after review of survival analysis of time to reinfection (Fig. 2).
FIG. 2.
Plot showing the survival analysis of time to reinfection following initial CSF shunt infection in 675 patients.
Predictor Variable
The predictor variable of interest of CSF shunt reinfection was surgical approach to treatment of infection. Categories included shunt removal/new shunt placement, externalization, nonsurgical management, complete shunt removal, excluded, and unknown.
Validation of Study Population and Outcome Variable
To confirm the selection of patients with initial CSF shunt infection into the cohort, an independent chart review was conducted at a single institution.32 Most (92% [11 of 12]) of that institution’s CSF shunt infection admissions met National Nosocomial Infection Surveillance criteria for CSF shunt infection.1 During 534 (79.1%) of 675 initial CSF shunt infection episodes and 78 (78%) of 100 reinfection episodes, at least one CSF culture was obtained.
To confirm assignment of predictor variable of interest (surgical approach to the treatment of infection), administrative data for each initial infection admission were reviewed extensively. For each initial infection admission, 2 authors, a pediatric hospitalist and pediatric neurosurgeon (T.D.S. and J.R.C.), reviewed the procedure codes, procedure days, and diagnosis codes. Assignments were made by consensus to 1 of 9 original categories of surgical approach, including definite and probable shunt removal/new shunt placement; definite, probable, and failed externalization; nonsurgical management; complete shunt removal; excluded; and unknown. Examples of assignments include removal of ventricular shunt (02.43) and ventriculostomy (02.2) on an early hospital day, followed by extracranial ventricular shunt (0.2.3×) on a later hospital day (definite shunt removal/new shunt placement); and incision of peritoneum (54.95) on an early hospital day, followed by replacement of a ventricular shunt (02.42) (definite externalization). The complete list of the exact combinations and sequences of procedure codes for each surgical approach are available from the authors on request. For the group of children whose surgical assignment was complete shunt removal (80 children), the validity of this assignment was checked by examining for subsequent CSF shunt revisions, which none of the patients had. Of the original 80 patients, 6 had subsequent admissions for CSF shunt infection; these patients were reassigned to the unknown category prior to analyses. When codes indicated the presence of a spinal thecal (rather than ventricular) shunt, the assignment was designated as excluded (2 patients). When consensus between reviewers could not be achieved, the surgical assignment was designated as unknown (190 patients).
Covariate
Covariates available in PHIS include patient, hospital, surgeon, and management factors (Table 1).
TABLE 1.
Characteristics in 675 children with initial CSF shunt infection
| Factor | No. of Patients (%) or Median (IQR) |
|---|---|
| initial CSF shunt placement | |
| age | |
| ≤30 days | 233 (34.5) |
| 1–6 mos | 200 (29.6) |
| 6–48 mos | 152 (22.5) |
| ≥48 mos | 90 (13.3) |
| sex | |
| male | 362 (53.6) |
| female | 313 (46.4) |
| race/ethnicity* | |
| non-Latino black | 160 (25.0) |
| Latino | 78 (12.2) |
| Asian | 15 (2.3) |
| other | 65 (10.2) |
| non-Latino white | 321 (50.2) |
| payer | |
| public | 349 (51.7) |
| private | 188 (27.9) |
| other | 138 (20.4) |
| indication for CSF shunt placement | |
| IVH | 77 (11.4) |
| myelomeningocele | 72 (10.7) |
| CNS tumor | 60 (8.9) |
| trauma | 10 (1.5) |
| meningitis | 18 (2.7) |
| comorbidities† | |
| none | 459 (68.0) |
| 1 | 176 (26.1) |
| ≥2 | 40 (5.9) |
| distal shunt location | |
| VP | 661 (97.9) |
| other | 14 (2.1) |
| initial CSF shunt infection | |
| age | |
| ≤30 days | 23 (3.4) |
| 1–6 mos | 240 (35.6) |
| 6–48 mos | 281 (41.6) |
| ≥48 mos | 131 (19.4) |
| interval CSF shunt revisions | 1 (1, 2) |
| interval time from initial shunt placement (days) | 72 (27–340) |
| interval time from last op (days) | 34 (17–97) |
| hospital vol‡ | |
| 1–20 | 35 (5.2) |
| 21–40 | 186 (27.6) |
| 41–60 | 330 (50.1) |
| ≥61 | 116 (17.2) |
| surgeon vol‡ | |
| ≤10 | 213 (31.6) |
| 11–20 | 265 (39.3) |
| 30 | 108 (16.0) |
| >30 | 89 (13.2) |
| op approach to initial shunt infection (483 patients) § | |
| shunt removal/new shunt placement | 286 (59.2) |
| externalization | 59 (12.2) |
| nonop management | 64 (13.3) |
| complete shunt removal | 74 (15.3) |
| duration of intravenous antibiotic use (days) | 9 (0–16) |
Race/ethnicity data were missing for 36 children.
Chronic medical condition count.
The volume represents the number of initial CSF shunt placements/year.
Those whose surgical assignments could not be determined were excluded.
Patient factors at initial CSF shunt placement included age, sex, race/ethnicity, payer, indication for CSF shunt placement, comorbidities, and distal shunt location.32 Age was categorized a priori in groups relevant to the diagnosis and management of hydrocephalus (≤ 30 days, 1–6 months, 6–48 months, and ≥ 48 months). Race/ethnicity was categorized into 5 mutually exclusive designations (non-Latino black, Latino, Asian, other, and non-Latino white). Payer was categorized by public (Medicare, Medicaid, Title V, or other government), private (Blue Cross, other insurance company, or health maintenance organization), or other (self-pay or no charge). An indication for CSF shunt placement was determined after review of the ICD-9-CM diagnosis codes that occurred in frequency of 1% or more of the study population (that is, the top 200 diagnosis codes). Etiology was determined at the time of initial shunt placement by the concurrent assignment of diagnosis codes for IVH, myelomeningocele, CNS tumor, meningitis, and trauma. The indications were neither mutually exclusive nor did they describe the entire population. Comorbidities were grouped into complex chronic conditions, herein referred to as chronic medical condition count, an ICD-9-CM diagnosis code-based system of classifying pediatric conditions associated with morbidity and mortality.7,8 The neuromuscular and malignancy categories were redefined to exclude indications for CSF shunts as previously described.33 The number of chronic medical conditions were classified into groups (none, 1, and 2 or more). Initial distal shunt location was categorized by the assignment of any ICD-9-CM procedure code into ventriculoperitoneal (02.34) and other (any other 02.3×).
Patient factors at initial CSF shunt infection included age, interval CSF shunt revision procedures (that is, the number of shunt revision procedures performed between initial placement and initial shunt infection), interval time from initial shunt placement in days, and interval time from last surgery (either initial CSF shunt placement or revision) to initial CSF shunt infection. The CSF shunt revision procedures between initial shunt placement and initial shunt infection were identified by subsequent admissions with the ICD-9-CM discharge procedure codes for shunt removal (02.43) and shunt placement (02.3), or shunt replacement (02.42, 54.95), or the ICD-9-CM discharge diagnosis code for shunt malfunction (996.2), without the presence of the ICD-9-CM discharge diagnosis code for shunt malfunction (996.63).
Hospital volume and surgeon volume were determined as previously described.32 Management factors assessed included surgical approach to treatment of initial CSF shunt infection (as described above) and duration of intravenous antibiotic use in days.
Statistical Analysis
Univariate analysis was performed to describe the study population (Table 1 and text). For categorical variables, proportions are reported; for continuous variables, median and IQR are reported. Bivariate analyses using chi-square tests were performed to examine the association of patient, hospital, and surgeon factors with CSF shunt reinfection (Table 2). Because none of the variables demonstrated significant associations in bivariate analyses (p < 0.05), multivariate analyses were not performed. However, further descriptive analyses of outcomes associated with surgical approaches were performed. Reinfection rates and associated 95% CIs were generated for each surgical approach. The median LOS and associated IQRs were also generated for each surgical approach (Table 3). All statistical analyses were performed using SAS (version 9.2, SAS Institute, Inc.), and p values less than 0.05 were considered statistically significant.
TABLE 2.
Characteristics in children with and without CSF shunt reinfection at 6 months
| No. of Patients (%) or Median (IQR) | |||
|---|---|---|---|
| Factor | w/o Reinfection | w/ Reinfection | p Value |
| no. of patients | 575 | 100 | |
| initial CSF shunt placement | |||
| age | 0.37 | ||
| ≤30 days | 197 (34.3) | 36 (36.0) | |
| 1–6 mos | 168 (29.2) | 32 (32.0) | |
| 6–48 mos | 136 (23.7) | 16 (16.0) | |
| ≥48 mos | 74 (12.9) | 16 (16.0) | |
| sex | 0.94 | ||
| male | 308 (53.6) | 54 (54.0) | |
| female | 267 (46.4) | 46 (46.0) | |
| race/ethnicity | 0.78 | ||
| non-Latino white | 274 (50.4) | 47 (49.5) | |
| non-Latino black | 136 (25.0) | 24 (25.3) | |
| Latino | 64 (11.8) | 14 (14.7) | |
| Asian | 12 (2.2) | 3 (3.2) | |
| other | 58 (10.7) | 7 (7.4) | |
| payer | 0.64 | ||
| public | 294 (51.1) | 55 (55.0) | |
| private | 164 (28.5) | 24 (24.0) | |
| other | 117 (20.3) | 21 (21.0) | |
| indication for shunt placement | |||
| IVH | 66 (11.5) | 11 (11.0) | 0.89 |
| myelomeningocele | 66 (11.5) | 6 (6.0) | 0.10 |
| CNS tumor | 50 (8.7) | 10 (10.0) | 0.67 |
| meningitis | 14 (2.4) | 4 (4.0) | 0.37 |
| trauma | 10 (1.7) | 0 (0.0) | 0.18 |
| comorbidities | 0.63 | ||
| none | 392 (68.2) | 67 (67.0) | |
| 1 | 151 (26.3) | 25 (25.0) | |
| ≥2 | 35 (5.6) | 8 (8.0) | |
| distal shunt location | 0.96 | ||
| VP | 563 (97.9) | 98 (98.0) | |
| other | 12 (2.1) | 2 (2.0) | |
| initial CSF shunt infection | |||
| age | 0.67 | ||
| ≤30 days | 19 (3.3) | 4 (4.0) | |
| 1–6 mos | 200 (34.3) | 40 (40.0) | |
| 6–48 mos | 241 (41.9) | 40 (40.0) | |
| ≥48 mos | 115 (20.0) | 16 (16.0) | |
| interval CSF shunt revisions | 1 (1, 2) | 1 (1, 2) | 0.43 |
| interval time from initial shunt placement (days) | 79 (28–360) | 50 (22–229) | 0.06 |
| interval time from last op (days) | 34 (17–100) | 23 (14–50) | 0.03 |
| hospital vol | 0.77 | ||
| 1–20 | 28 (4.9) | 7(7.0) | |
| 21–40 | 155 (27.0) | 31 (31.0) | |
| 41–60 | 292 (50.8) | 46 (46.0) | |
| ≥61 | 100 (17.4) | 16 (16.0) | |
| surgeon vol | 0.50 | ||
| ≤10 | 181 (31.5) | 32 (32.0) | |
| 11–20 | 223 (38.8) | 42 (42.0) | |
| 21–30 | 97 (16.9) | 11 (11.0) | |
| >30 | 74 (12.9) | 15 (15.0) | |
| op approach to initial shunt infection (483 patients) | |||
| shunt removal/new shunt placement | 242 (58.7) | 44 (62.0) | 0.61 |
| externalization | 47 (11.4) | 12 (16.9) | 0.19 |
| nonop management | 49 (11.9) | 15 (21.1) | 0.03 |
| complete shunt removal | 74 (18.0) | 0 (0.0) | <0.001 |
| duration of intravenous antibiotic use (days) | 9 (0–16) | 7.5 (0–17) | 0.98 |
TABLE 3.
Reinfection rates for surgical approaches to treatment of initial CSF shunt infection in 483 patients
| Op Approach to Initial Shunt Infection |
No. of Patients |
6-Mo Reinfection Rate (95% CI) |
Median LOS in Days (IQR) |
|---|---|---|---|
| shunt removal/new shunt placement | 286 | 15.4 (11.4–20.1) | 16 (12–23) |
| externalization | 59 | 20.3 (11.0–32.8) | 18 (12–23) |
| nonop management | 64 | 23.4 (13.8–35.7) | 3.5 (3–7) |
| complete shunt removal | 74 | 0 (0.0–0.0) | 13 (10–18) |
Results
The PHIS includes 675 children who underwent an uncomplicated initial CSF shunt placement, had 24 months of follow-up, and subsequently developed an initial CSF shunt infection for which the treatment could be adequately characterized. Of these 675 children, 14.8% (100 of 675) developed a second CSF shunt infection within 6 months and 16.4% (111 of 675) within 12 months (Fig. 1). Among children who experienced reinfection within 6 months, the median time from infection to reinfection was 21 days (IQR 5–58 days).
Descriptions of the patient, hospital, surgeon, and management factors for the study population are shown in Table 1. At least one revision procedure before initial CSF shunt infection was performed in 36.4% of patients (246 of 675). The median time to initial CSF shunt infection following CSF shunt placement was 72 days and following last surgery was 34 days. The surgical approach to treatment of the initial CSF shunt infection was determined for 483 children (71.6%). The surgical approach was primarily shunt removal/new shunt placement (in 286 patients [59.2%]), but a substantial number of patients underwent externalization (59 [12.2%], which failed in 17 [3.5%] of 483) and nonsurgical management (64 patients [13.3%]). In 74 patients (15.3%), the initial management was shunt removal, but there was no record of subsequent shunt replacement; these were termed “Complete shunt removal.” The mean duration of intravenous antibiotic use was 9 days (IQR 0–16 days).
The same patient, hospital, and surgeon factors were tested for associations with reinfection; none of them demonstrated a statistically significant association with reinfection (p < 0.05) (Table 2). Children with reinfection tended to have an earlier initial CSF shunt infection following initial CSF shunt placement (50 days [IQR 22–229 days] with reinfection vs 79 days [IQR 28–360 days] without reinfection, p = 0.06). Of the patients with reinfection, 22% underwent at least one revision procedure between initial CSF shunt infection and CSF shunt reinfection. The median time to reinfection from infection or last surgery (whichever was last) was 19 days (IQR 8–60 days).
Some surgical approaches to the treatment of infection did demonstrate associations with reinfection, including nonsurgical management (p = 0.03) and complete shunt removal (p < 0.001) (Table 2). For ease of interpretation, Table 3 shows reinfection rates following each surgical approach to treatment of initial CSF shunt infection. Rates of reinfection were similar for shunt removal/new shunt placement (15.4% [95% CI 11.4%–20.1%]) and externalization (total 20.3% [95% CI 11.0%–32.8%]). Re-infection was higher but nonsignificantly so for the subset of children in whom externalization failed (29.4% [95% CI 10.3%–56.0%]) and who were treated nonsurgically (23.4% [95% CI 13.8%–35.7%]), respectively.
Significant differences were seen between surgical approaches in terms of LOS (Table 3). Shunt removal/new shunt placement and externalization demonstrated similar LOS (16 days [IQR 12–23 days] and 18 days [IQR 12–23 days], respectively). The LOS was longer but not significantly so for children whose externalization failed (23 days [IQR 18–28 days]). Children who were treated nonsurgically had significantly shorter LOS (3.5 days [IQR 3–7 days]). Those who underwent removal with no shunt replacement had similar LOS to other surgical approaches (13 days [IQR 10–18 days]).
Discussion
This large retrospective cohort study examined the treatment of 675 children who had uncomplicated initial CSF shunt placement and subsequently developed an initial CSF shunt infection. Surgical approaches to treatment of infection included shunt removal/new shunt placement (59.2%), externalization (12.2%), nonsurgical management (13.3%), and removal with no shunt replacement (15.3%). The median duration of intravenous antibiotic use was 9 days (IQR 0–16 days). Of these 675 children, 14.8% (100 of 675) developed a second CSF shunt infection within 6 months. None of the patient, hospital, and surgeon factors tested demonstrated a statistically significant association with reinfection. Shunt removal/new shunt placement and externalization did not demonstrate an association with reinfection, a difference in reinfection rates, or a difference in LOS. Failed externalization demonstrated a statistically nonsignificant higher reinfection rate and a statistically nonsignificant longer LOS. Nonsurgical management demonstrated an association with reinfection, a statistically nonsignificant higher reinfection rate, and a statistically significant shorter LOS. Complete shunt removal prevented reinfection and had similar LOS to other surgical approaches.
Comparison with Prior Recent Studies
By using administrative data, this study examined a substantially larger number of patients (675) undergoing treatment for their first CSF shunt infection than either of the 2 prior recent rigorous observational studies of CSF shunt infection (70 and 51 patients).18,19
This study population had significantly more variability in surgical approaches to treatment of CSF shunt infection than prior studies, whose cohorts were treated with primarily shunt removal/new shunt placement (71%18 and 55%19) and externalization (24%18 and 37%19). The variability to surgical approaches to CSF shunt infection treatment may reflect differences in study population. While this study was conducted across 41 US children’s hospitals, earlier work focused on 10 centers with voluntary participation or a single center.18,19 This study also distinguishes between first and recurrent shunt infections, whereas earlier work does not.18,19 This study may, therefore, reflect a more representative population of children undergoing treatment for first CSF shunt infection.
The median duration of intravenous antibiotic use in this study was 9 days (IQR 0–16 days), compared with 16.2–17.4 days18 and approximately 11.2 days in other studies.19 The shorter duration of intravenous antibiotic use in this study probably reflects the inclusion of substantial numbers of patients undergoing nonsurgical treatment of CSF shunt infection (13.3%). Nonsurgical treatment was associated with significantly shorter LOS (and therefore duration of intravenous antibiotics) than other surgical approaches.
This large cohort demonstrated a reinfection rate of 14.8% at 6 months, somewhat lower than previous work.18,19 The cause for this difference may be multifactorial. This study focuses on first CSF shunt infections; reinfection rates following recurrent CSF shunt infections may be higher than those following first CSF shunt infection. In addition, this cohort was derived from patients who underwent uncomplicated initial CSF shunt placement who may be at lower risk for recurrent CSF shunt infections.32 Here again, however, this study probably reflects a more representative population of children undergoing treatment for first CSF shunt infection.
New Contributions to Our Understanding of CSF Shunt Infection Treatment
Due to the larger number of patients in this cohort, this study was able to test the association of numerous patient, hospital, and surgeon factors with CSF shunt infection. Like earlier work,18,19 none of the patient, hospital, and surgeon factors tested demonstrated associations with reinfection. Also like earlier work, shunt removal/new shunt placement and externalization demonstrated no association with reinfection,19 and nonsurgical management demonstrated a trend toward an association with reinfection.37 Failed externalization demonstrated a higher but statistically nonsignificant reinfection rate. A new finding was the extent to which shunts were not replaced after removal (15.3%); as would be expected, this approach was effective at preventing CSF shunt reinfection. While nonsurgical management had a statistically significant shorter LOS, its benefit is arguably offset by the higher risk of reinfection.
Study Limitations
Data available from administrative data sets such as PHIS are limited. Cerebrospinal fluid shunt infection was determined by ICD-9-CM code. While some patient, hospital, and surgeon factors are available as covariates, many factors of clinical interest (for example, individual surgeon, surgeon experience, timing of antibiotics during previous surgeries, use of antibiotic impregnated shunts and/or previous externalized drains, and outpatient antibiotic use) are not available. The surgical approach was assigned by consensus, and significant numbers of patients (190 children) were not analyzed because the surgical approach could not be determined. In addition, the infecting organism at the time of first and second infections are of particular interest18,19 and are not available in the PHIS. Therefore, no analyses to distinguish between relapse and recurrence of infection were performed.27 In addition, use of intrathecal antibiotics for treatment of CSF shunt infection occurs,2–4,6,9,13,14,16,24,28–30,34,38 but we were not able to obtain this covariate. Some of the available covariates (indication for initial shunt and chronic medical condition count) are problematic given their dependence on ICD-9-CM diagnosis codes, which are less reliable than procedure codes.22 The study design assumes a patient is seen for follow-up at the same hospital as their CSF shunt placement, which may not be true; we assumed equal rates of drop-out across hospitals.
Finally, the restriction of our study population to only uncomplicated initial CSF shunt placements (due to ICD-9-CM coding) resulted in a relatively low-risk population, but, on the other hand, it allowed us to ensure a relatively generalizable study population.
Conclusions
This large, recent, and likely representative, retrospective cohort study examined the treatment of 675 children who underwent uncomplicated initial CSF shunt placement and subsequently developed an initial CSF shunt infection. Surgical approaches to treatment of infection included shunt removal/new shunt placement (59.2%), externalization (12.2%), nonsurgical management (13.3%), and removal with no shunt replacement (15.3%). Of the 675 children, 14.8% developed a second CSF shunt infection within 6 months. Despite a significantly larger study population than earlier work, none of the patient, hospital, and surgeon factors tested demonstrated a statistically significant association with reinfection. Complete shunt removal was used to treat significant numbers of patients (15.3%). Shunt removal/new shunt placement and externalization demonstrated no association with reinfection, and nonsurgical management demonstrated an association with reinfection.
Acknowledgments
The authors thank the Division of Inpatient Medicine for its support and feedback and Stephan J. Nemeth for his valuable feedback.
The Hydrocephalus Clinical Research Network consists of John Kestle, Jay Riva-Cambrin, Tamara Simon, Marion Walker, Tracey Bach, Marcie Langley, Jeff Yearley, Richard Holubkov: Primary Children’s Medical Center/University of Utah, Salt Lake City, Utah; Abhaya Kulkarni, James M. Drake, Lindsay O’Connor: Hospital for Sick Children/University of Toronto, Canada; Jerry Oakes, John Wellons, Courtney Shannon: Children’s Hospital of Alabama/University of Alabama at Birmingham, Alabama; William Whitehead, Thomas Luerssen, Sheila Nguyen Ryan: Texas Children’s Hospital/Baylor College of Medicine, Houston, Texas; and Sam Browd, Amy Anderson: Seattle Children’s Hospital/University of Washington, Seattle, Washington.
Abbreviations used in this paper
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- LOS
length of stay
- PHIS
Pediatric Health Information System
- VP
ventriculoperitoneal
Footnotes
Disclosure
Dr. Simon is a Hydrocephalus Clinical Research Network investigator, and her work is supported by Award K23NS062900 from the National Institute of Neurological Disorders And Stroke and in part by a Primary Children’s Medical Center Innovative Research Grant and by the Children’s Health Research Center, University of Utah.
Author contributions to the study and manuscript preparation include the following. Conception and design: Simon, Hall, Kestle, Riva-Cambrin. Acquisition of data: Hall. Analysis and interpretation of data: all authors. Drafting the article: Simon. Critically revising the article: Hall, Dean, Kestle, Riva-Cambrin. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Hall. Administrative/technical/material support: all authors. Study supervision: Simon.
Portions of this work were presented as an oral presentation at AANS/CNS Pediatric Section meeting in Boston in December 2009.
References
- 1.Albert JE, Simon TD, Hall M, Kestle J, Jeffries HE. Pediatric Academic Societies Annual Meeting. Honolulu, Hawaii: 2008. May 2–6, Improved identification of pediatric neurosurgical procedure infections. (Abstract) [Google Scholar]
- 2.Bayston R, Barnicoat M, Cudmore RE, Guiney EJ, Gurusinghe N, Norman PM. The use of intraventricular vancomycin in the treatment of CSF shunt-associated ventriculitis. Z Kinderchir. 1984;39 Suppl 2:111–113. doi: 10.1055/s-2008-1044299. [DOI] [PubMed] [Google Scholar]
- 3.Brown EM, Edwards RJ, Pople IK. Conservative management of patients with cerebrospinal fluid shunt infections. Neurosurgery. 2006;58:657–665. doi: 10.1227/01.NEU.0000204126.54417.46. [DOI] [PubMed] [Google Scholar]
- 4.Cruciani M, Navarra A, Di Perri G, Andreoni M, Danzi MC, Concia E, et al. Evaluation of intraventricular teicoplanin for the treatment of neurosurgical shunt infections. Clin Infect Dis. 1992;15:285–289. doi: 10.1093/clinids/15.2.285. [DOI] [PubMed] [Google Scholar]
- 5.Fan-Havard P, Nahata MC. Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm. 1987;6:866–880. [PubMed] [Google Scholar]
- 6.Fernández Guerrero ML, de Górgolas M, Fernández Roblas R, Campos JM. Treatment of cerebrospinal fluid shunt infections with teicoplanin. Eur J Clin Microbiol Infect Dis. 1994;13:1056–1058. doi: 10.1007/BF02111827. [DOI] [PubMed] [Google Scholar]
- 7.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 198–1997. Pediatrics. 2000;106:205–209. [PubMed] [Google Scholar]
- 8.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 9.Frame PT, McLaurin RL. Treatment of CSF shunt infections with intrashunt plus oral antibiotic therapy. J Neurosurg. 1984;60:354–360. doi: 10.3171/jns.1984.60.2.0354. [DOI] [PubMed] [Google Scholar]
- 10.Gardner P, Leipzig T, Phillips P. Infections of central nervous system shunts. Med Clin North Am. 1985;69:297–314. [PubMed] [Google Scholar]
- 11.Gardner P, Leipzig TJ, Sadigh M. Infections of mechanical cerebrospinal fluid shunts. Curr Clin Top Infect Dis. 1988;9:185–214. [PubMed] [Google Scholar]
- 12.George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979;51:804–811. doi: 10.3171/jns.1979.51.6.0804. [DOI] [PubMed] [Google Scholar]
- 13.Greene KA, Clark RJ, Zabramski JM. Ventricular CSF shunt infections associated with Corynebacterium jeikeium: report of three cases and review. Clin Infect Dis. 1993;16:139–141. doi: 10.1093/clinids/16.1.139. [DOI] [PubMed] [Google Scholar]
- 14.Jamjoom A, al-Abedeen Jamjoom Z, al-Hedaithy S, Jamali A, Naim-Ur-Rahman, Malabarey T. Ventriculitis and hydrocephalus caused by Candida albicans successfully treated by antimycotic therapy and cerebrospinal fluid shunting. Br J Neurosurg. 1992;6:501–504. doi: 10.3109/02688699208995043. [DOI] [PubMed] [Google Scholar]
- 15.Kanev PM, Sheehan JM. Reflections on shunt infection. Pediatr Neurosurg. 2003;39:285–290. doi: 10.1159/000075255. [DOI] [PubMed] [Google Scholar]
- 16.Katz MD, Rapp RP, Walsh JW. Infection in a functioning ventriculoperitoneal shunt treated with intraventricular gentamicin. Am J Hosp Pharm. 1980;37:268–271. [PubMed] [Google Scholar]
- 17.Kestle JR. Pediatric hydrocephalus: current management. Neurol Clin. 2003;21:883–895. vii. doi: 10.1016/s0733-8619(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 18.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, et al. Management of shunt infections: a multicenter pilot study. J Neurosurg. 2006;105 3 Suppl:177–181. doi: 10.3171/ped.2006.105.3.177. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni AV, Rabin D, Lamberti-Pasculli M, Drake JM. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg. 2001;35:66–71. doi: 10.1159/000050393. [DOI] [PubMed] [Google Scholar]
- 21.Li V, Dias MS. The results of a practice survey on the management of patients with shunted hydrocephalus. Pediatr Neurosurg. 1999;30:288–295. doi: 10.1159/000028813. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy EP, Iezzoni LI, Davis RB, Palmer RH, Cahalane M, Hamel MB, et al. Does clinical evidence support ICD-9-CM diagnosis coding of complications? Med Care. 2000;38:868–876. doi: 10.1097/00005650-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 23.McGirt MJ, Leveque JC, Wellons JC, III, Villavicencio AT, Hopkins JS, Fuchs HE, et al. Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg. 2002;36:248–255. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]
- 24.McLaurin RL. Treatment of infected ventricular shunts. Childs Brain. 1975;1:306–310. doi: 10.1159/000119580. [DOI] [PubMed] [Google Scholar]
- 25.Morissette I, Gourdeau M, Francoeur J. CSF shunt infections: a fifteen-year experience with emphasis on management and outcome. Can J Neurol Sci. 1993;20:118–122. doi: 10.1017/s0317167100047661. [DOI] [PubMed] [Google Scholar]
- 26.Nelson JD. Cerebrospinal fluid shunt infections. Pediatr Infect Dis. 1984;3 3 Suppl:S30–S32. doi: 10.1097/00006454-198405001-00011. [DOI] [PubMed] [Google Scholar]
- 27.Odio C, McCracken GH, Jr, Nelson JD. CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138:1103–1108. doi: 10.1001/archpedi.1984.02140500009004. [DOI] [PubMed] [Google Scholar]
- 28.Pickering LK, Ericsson CD, Ruiz-Palacios G, Blevins J, Miner ME. Intraventricular and parenteral gentamicin therapy for ventriculitis in children. Am J Dis Child. 1978;132:480–483. doi: 10.1001/archpedi.1978.02120300040007. [DOI] [PubMed] [Google Scholar]
- 29.Quinn AL, Parada JP, Belmares J, O’Keefe JP. Intrathecal colistin and sterilization of resistant Pseudomonas aeruginosa shunt infection. Ann Pharmacother. 2005;39:949–952. doi: 10.1345/aph.1E485. [DOI] [PubMed] [Google Scholar]
- 30.Segal-Maurer S, Mariano N, Qavi A, Urban C, Rahal JJ., Jr Successful treatment of ceftazidime-resistant Klebsiella pneumoniae ventriculitis with intravenous meropenem and intraventricular polymyxin B: case report and review. Clin Infect Dis. 1999;28:1134–1138. doi: 10.1086/514754. [DOI] [PubMed] [Google Scholar]
- 31.Sells CJ, Shurtleff DB, Loeser JD. Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics. 1977;59:614–618. [PubMed] [Google Scholar]
- 32.Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States Clinical article. J Neurosurg Pediatr. 2009;4:156–165. doi: 10.3171/2009.3.PEDS08215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr. 2008;1:131–137. doi: 10.3171/PED/2008/1/2/131. [DOI] [PubMed] [Google Scholar]
- 34.Swayne R, Rampling A, Newsom SW. Intraventricular vancomycin for treatment of shunt-associated ventriculitis. J Antimicrob Chemother. 1987;19:249–253. doi: 10.1093/jac/19.2.249. [DOI] [PubMed] [Google Scholar]
- 35.Venes JL. Infections of CSF shunt and intracranial pressure monitoring devices. Infect Dis Clin North Am. 1989;3:289–299. [PubMed] [Google Scholar]
- 36.Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22:692–697. doi: 10.1007/s00381-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 37.Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60:1014–1021. doi: 10.3171/jns.1984.60.5.1014. [DOI] [PubMed] [Google Scholar]
- 38.Wen DY, Bottini AG, Hall WA, Haines SJ. Infections in neurologic surgery. The intraventricular use of antibiotics. Neurosurg Clin N Am. 1992;3:343–354. [PubMed] [Google Scholar]
- 39.Whitehead WE, Kestle JR. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg. 2001;35:205–210. doi: 10.1159/000050422. [DOI] [PubMed] [Google Scholar]
- 40.Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, et al. Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. J Neurosurg. 2007 5 Suppl:345–357. doi: 10.3171/PED-07/11/345. [DOI] [PubMed] [Google Scholar]
- 41.Younger JJ, Christensen GD, Bartley DL, Simmons JC, Barrett FF. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987;156:548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]