Abstract
Chronic lymphocytic leukemia (CLL) is an incurable progressive disease for which new therapies are required. Therapy with monoclonal antibodies (mAbs) has improved the outcome of patients with CLL, making further investigation of novel antibodies directed against alternative and specific targets on B cells an important area of translational research. We now describe functional properties of an antagonistic humanized mAb to CD74, milatuzumab, showing that milatuzumab combined with a crosslinking antibody induces cytotoxicity in vitro in CLL cells in a caspase- and stromal-independent manner associated with aggregation of CD74 on the cell surface. Furthermore, incorporation of milatuzumab into an immunoliposome induces even more of a cytotoxic response than in vitro crosslinking, representing a novel therapeutic formulation for this mAb. Based on these data, future development of the milatuzumab-immunoliposome formulation as a therapeutic agent for CLL is warranted.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia, and is a progressive and incurable disease. CLL treatments include alkylating drugs, purine analogs, and more recently, monoclonal antibodies (mAbs). mAbs such as rituximab that target the CD20 antigen selectively expressed on CLL cells augment the cytotoxicity of traditional chemotherapy agents, and are associated with improved response and progression-free survival.1–4 However, nearly all patients eventually relapse after such treatments, indicating a need for novel and specific therapeutic agents.
CD74 is a type II transmembrane protein expressed on B cells that has recently been pursued as a target for antibody-mediated therapy.5 It associates with the α and β chains of HLA-DR, and normally functions as a major histocompatibility complex class II chaperone. Signaling through CD74 is also implicated in B-cell proliferation, nuclear factor κB activation, and cell survival.6,7 CD74 expression is increased on the surface of leukemic B cells, making it an attractive target for CLL and other B-cell malignancies. CD74 signaling is initiated after engagement with macrophage migration-inhibitory factor (MIF) and subsequent activation of survival pathways to inhibit apoptosis and stimulate proliferation.8,9 In addition, a recent study demonstrates that CD74 signaling induces TAp63 and VLA-4 to enhance CLL cell survival and homing to the bone marrow.10 Therefore, disruption of CD74 signaling represents a potential therapeutic option in CLL and other CD74-expressing malignancies.5
Here we describe an antagonistic humanized mAb to CD74, milatuzumab. Milatuzumab has demonstrated antiproliferative activity in non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) cell lines and extends the survival of severe combined immune-deficient (SCID) mice injected with NHL and MM cells.5,7,11 However, little is known about the efficacy of milatuzumab in CLL. Our data demonstrate that milatuzumab mediates direct cytotoxicity in CLL cells by a mechanism involving aggregation of CD74 on the cell surface. Furthermore, incorporation of milatuzumab into a liposome potentiates the cytotoxic effect of this antibody, suggesting a novel therapeutic formulation.
Methods
Patients, cell separation, culture conditions, and reagents
For in vitro studies, written, informed consent was obtained in accordance with the Declaration of Helsinki to procure cells from patients with previously diagnosed CLL, as defined by the modified National Cancer Institute criteria, under an Institutional Review Board–approved protocol at The Ohio State University.12 Patient characteristics are available in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Isolated mononuclear cells were negatively B-cell selected and placed in culture, as previously described by our group.13 HS-5 stromal cells were obtained from ATCC. CD40L was purchased from PeproTech. Milatuzumab was provided by Immunomedics Inc. Goat anti–human IgG antibody (Fc gamma fragment-specific, anti-Fc) was purchased from Jackson ImmunoResearch Laboratories. Q-VD-OPH pan-caspase inhibitor was purchased from MP Biomedicals.
Flow cytometric assays
Viability was determined by flow cytometry using propidium iodide (PI). For surface staining, CLL cells were washed in phosphate-buffered saline and stained with antibodies to CD20 or CD74 (BD Biosciences).
Immunoblot analysis
Immunoblots were performed as described.14 Antibodies used included PARP (Calbiochem); caspase 3 and 9 (R&D Systems), caspase 2, 6 and 8 (Cell Signaling), and tubulin (Santa Cruz Biotechnology).
Preparation of ILs
Immunoliposomes (ILs) were prepared as previously described.15 A postinsertion method was used to incorporate milatuzumab into preformed liposomes, and targeted milatuzumab-IL was prepared with an antibody-to-lipid ratio of 1:1000. Further details are available in supplemental Methods.
Statistical analysis
All reported statistical evaluations were performed by the Center for Biostatistics at The Ohio State University. Because the observations from the same patient are correlated, linear mixed models were used for analysis to take account of this within patient correlation. Treatment differences were estimated and tested from these models. The Holm step-down procedure was used to adjust for multiple comparisons or multiple endpoints when necessary. P values less than .05 for single comparisons or after adjustment for multiple comparisons were considered significant.
Results and discussion
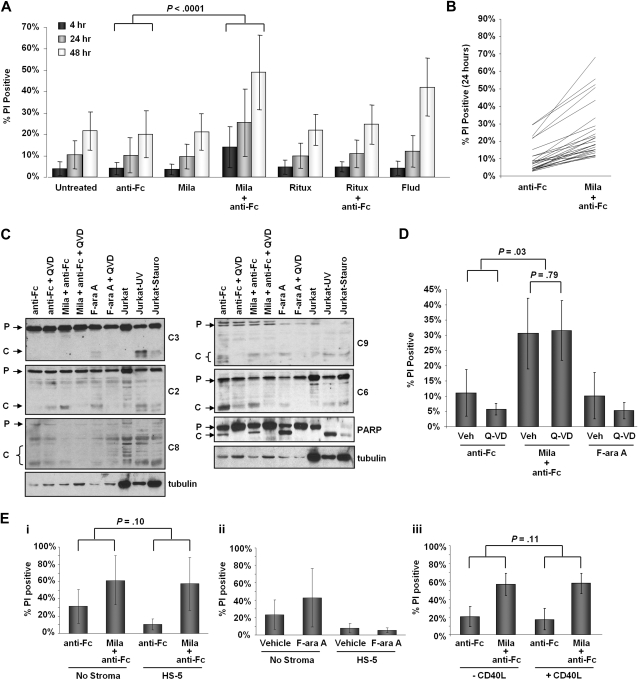
We first determined the in vitro survival of primary CLL cells after milatuzumab treatment. As shown in Figure 1A and B, milatuzumab (mila) + anti-Fc crosslinker rapidly induces significant cell death compared with anti-Fc alone (difference of 18% averaged across time points; N = 26; P < .0001). This result was verified by MTT assay (supplemental Figure 1). This effect is dependent on crosslinking, since milatuzumab alone had no cytotoxic effect on the cells, and the effect of mila + anti-Fc was greater than that of rituximab + anti-Fc. Milatuzumab-mediated killing appears to be caspase-independent, because treatment does not increase cleavage of caspases 3, 6, 8, or 9 or the caspase substrate PARP relative to the Fc crosslinker control, although processing of caspase 2 is observed (Figure 1C). While the pan-caspase inhibitor, Q-VD-OPH, is able to block cleavage of both of PARP and caspase 2, it has no significant effect on milatuzumab-induced cell death (Figure 1D; N = 5; P = .03).
Figure 1.
Milatuzumab mediates direct cytotoxicity in CLL cells. (A) Viability of chronic lymphocytic leukemia (CLL) patient cells at 4, 24, and 48 hours by propidium iodide (PI) staining. Cells were untreated, treated with goat anti–human Fc crosslinker alone, or treated with 5 μg/mL milatuzumab (Mila) or 10 μg/mL rituximab (Ritux) in the presence or absence of Fc crosslinker (in 5 × excess of binding antibody; N = 26; P < .0001 for anti-Fc vs mila + anti-Fc). Y-axis indicates the percent of PI positive cells and the active metabolite of fludarabine (2-F-ara A; Flud) was used as a positive control for cell death. (B) Viability of CLL patient cells at 24 hours by PI staining (N = 26 from panel A; each line represents individual patient sample). (C) Immunoblots for caspases-2, -3, -8, -6, -9, and PARP in whole cell lysates isolated from CLL cells treated with anti-Fc, 5 μg/mL mila + anti-Fc, or 5μM fludarabine in the presence or absence of 20μM caspase inhibitor, Q-VD-OPH, for 24 hours. Jurkat cells treated with ultraviolet (UV) or staurosporine were used as controls for caspase cleavage. Pro-caspase (P) and cleaved (C) forms are indicated. (D) Viability of CLL patient cells by PI staining treated with anti-Fc, 5 μg/mL mila + anti-Fc, or 5μM fludarabine in the presence or absence of 20μM caspase inhibitor, Q-VD-OPH, for 24 hours (N = 5; P = .03). (Ei) Viability of CLL patient cells treated with anti-Fc alone or 5 μg/mL mila + anti-Fc in the presence or absence of stroma coculture. After 6 hours in drug, cells were washed and cultured in fresh media alone or in the presence of HS-5 stromal cells. Viability was determined by PI staining after 48 hours of coculture (N = 11; P = .10). (ii) Viability of CLL patient cells treated with vehicle or 1μM 2-F-ara A in the presence or absence of stroma coculture. After 6 hours in drug cells were washed and cultured in fresh media alone or in the presence of HS-5 cells. Viability by PI staining was determined after 48 hours of coculture (N = 6). (iii) Viability of CLL patient cells treated with anti-Fc alone or 5 μg/mL mila + anti-Fc in the presence or absence of 500 ng/mL soluble CD40L. Viability by PI staining was determined at 48 hours (N = 17; P = .11).
The role of microenvironmental factors in the survival and drug resistance of CLL cells is becoming increasingly studied.16 Importantly, the effect of milatuzumab is not significantly diminished by coculture with a stromal cell line (Figure 1Ei; 37.3% more cell death with mila + anti-Fc vs anti-Fc alone in the absence of HS-5; 48.5% more cell death with mila + anti-Fc vs anti-Fc alone in the presence of HS-5; N = 11; P = .10). In contrast, fludarabine-induced cytotoxicity is noticeably diminished by coculture with stroma despite the variation in fludarabine responsiveness among individual samples (Figure 1Eii; 19.3% more cell death with fludarabine vs vehicle control in the absence of HS-5; 2.2% more cell death with fludarabine vs vehicle control in the presence of HS-5). Furthermore, treatment with CD40L, commonly found to protect CLL cells from cell death, is unable to prevent milatuzumab-induced cytotoxicity (Figure 1Eiii; 23% more cell death with mila + anti-Fc vs anti-Fc alone in the absence of CD40L; 29% more cell death with mila + anti-Fc vs anti-Fc alone in the presence of CD40L; N = 17; P = .09; N = 17; P = .11). Together, these data indicate that milatuzumab may be effective in vivo despite the presence of an intact microenvironment.
We next investigated whether milatuzumab was able to mediate antibody-dependent cellular cytotoxicity (ADCC). Similar to reports in lymphoma cell lines,17 no ADCC was detected with either mononuclear cells or granulocytes at any effector to CLL target cell ratio (data not shown). These findings indicate that direct cell death via CD74 ligation is likely the principal contributor to milatuzumab efficacy.
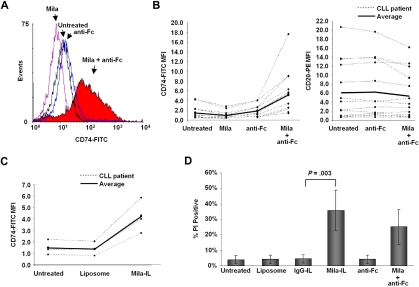
We next sought to determine how CD74 ligation by milatuzumab promoted cell death. We observed that upon milatuzumab treatment in the presence of crosslinking, CLL cells aggregated in culture (supplemental Figure 2) and the mean fluorescent intensity (MFI) of CD74 on the cell surface increased significantly (Figure 2A-B left; 1.50 vs 5.03 MFI [anti-Fc vs mila + anti-Fc]; N = 14; P = .0003). No significant change in surface expression of CD74 was observed after treatment with anti-Fc alone, while milatuzumab alone lead to a slight decrease in surface CD74, potentially due to increased receptor internalization in the absence of crosslinker. In addition, significant surface retention of CD20 was not observed after in vitro crosslinking with milatuzumab (Figure 2B right; 6.36 vs 5.56 MFI [anti-Fc vs mila + anti-Fc] N = 14; P = .14), indicating that the increased surface CD74 is antigen-specific and not due to nonspecific antibody trapping between clustered cells. These data suggest that milatuzumab promotes the maintenance and/or accumulation of CD74 on the cell surface, which likely initiates downstream signaling pathway(s) leading to cell death. The lack of this effect after treatment with milatuzumab alone indicates that crosslinking is necessary for milatuzumab-induced cell death. However, association with Fc receptors on other cells in the microenvironment may not be sufficient to mediate this effect, as indicated by the lack of ADCC with milatuzumab.17 We therefore investigated whether other methods to promote receptor accumulation with milatuzumab induce a similar in vitro cytotoxic effect as crosslinking antibody. Similar to previous studies with the anti-CD22 mAb, epratuzumab,18 we found that milatuzumab immobilized on a plastic cell culture plate increased cell death compared with the nonimmobilized antibody, but this was not as active in cell killing compared with using soluble anti-Fc (supplemental Figure 3; 18.2% vs 43.2% PI positive, immobilized mila vs mila + anti-Fc averaged across time points; N = 6; P < .0001). These results suggest that association of multiple receptors may be required to initiate a death signal, which is limited when antibodies are fixed on cell culture plates. Enhanced cell death through Fas signaling has been described for CD44,19 a binding partner for CD74, and is a suggested mechanism for milatuzumab.20 Therefore activation of this signaling pathway in CLL after milatuzumab treatment is currently being evaluated.
Figure 2.
Milatuzumab immunoliposome increases CD74 on the surface of CLL cells and induces cell death. (A) Mean fluorescent intensity (MFI) of surface CD74 in CLL patient cells either untreated, treated with 25 μg/mL anti-Fc, or 5 μg/mL milatuzumab with or without 25 μg/mL anti-Fc for 1 hour. Histogram shown is representative of 14 patients. (B left) Mean fluorescent intensity of CD74 in CLL patient cells either untreated, treated with 25 μg/mL anti-Fc, or 5 μg/mL milatuzumab with or without 25 μg/mL anti-Fc for 1 hour (N = 14; P = .0003). (Right) MFI of CD20 in CLL patient cells either untreated, or treated with 5 μg/mL milatuzumab with or without 25 μg/mL anti-Fc for 1 hour (N = 14; P = .14). (C) MFI of CD74 in CLL patient cells either untreated, or treated with empty liposome or milatuzumab-immunoliposome (mila-IL) for 1 hour (N = 4; P = .0003). (D) Viability by PI staining in CLL patient cells either untreated, or treated with empty liposome, IgG-immunoliposome (IgG-IL), mila-IL, 25 μg/mL anti-Fc, or 5 μg/mL milatuzumab with 25 μg/mL anti-Fc for 24 hours (N = 11; P = .0003).
Incorporation of internalizing antibodies into liposomes has been described as a method for targeted drug delivery in B-cell malignancies.15,21 We found that incorporation of milatuzumab into a liposome (mila-IL) was able to mediate the same receptor aggregation on the cell surface as milatuzumab with anti-Fc (Figure 2C), an effect that was not evident with liposome alone (mila-IL vs liposome; 4.23 vs 1.39 MFI; N = 4; P = .0003). Furthermore, mila-IL induced significantly more cell death in CLL cells compared with IgG incorporated liposomes (Figure 2D; mila-IL vs IgG-IL; 36% vs 4.8% PI positive; N = 11; P < .0001). Importantly, cell death induced by mila-IL was significantly higher than that caused by milatuzumab plus crosslinking in vitro (Figure 2D; mila-IL vs mila + anti-Fc; 36% vs 25.5% PI positive; N = 11; P = .0003). This cytotoxicity was evident even without packaging the immunoliposome with a chemotherapeutic agent, such as doxorubicin, which has been described previously with this antibody.21
Here, we provide evidence that the incorporation of milatuzumab into a liposome may induce cell death without a dependence on other cell types in the microenvironment. Together, these data support the clinical application of mila-IL-based therapy in CLL and possibly other CD74-positive malignancies.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants (T-32-5CA009338, E.H.; P01 CA95426, J.C.B.; P01 CA103985, D.M.G.; P50-CA140158, J.C.B., R.J.L.), CLL Research Consortium (P01 CA81534-02, J.C.B.), the National Cancer Institute, the Leukemia & Lymphoma Society (J.C.B.), and The D. Warren Brown Foundation (J.C.B.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.H. designed the research, performed experiments, analyzed data, wrote the manuscript, and approved the final version of the manuscript; G.T. designed the research, performed experiments, analyzed data, reviewed drafts, and approved the final version of the manuscript; E.J.S. and J.D.H. performed experiments and approved the final version of the manuscript; X.Z. and D.J. performed statistical analysis and approved the final version of the manuscript; D.M.L. and N.M. designed components of the research, reviewed drafts, and approved the final version of the manuscript; D.M.G. provided reagents, reviewed drafts, and approved the final version of the manuscript; R.J.L. reviewed drafts and approved the final version of the manuscript; and J.C.B. designed and supervised the research, obtained funding for the research work, reviewed drafts, and approved the final version of the manuscript.
Conflict-of-interest disclosure: D.M.G. is an officer and member of the Board of Directors of Immunomedics Inc, which owns milatuzumab. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, MD, Division of Hematology, Department of Internal Medicine, the Comprehensive Cancer Center at The Ohio State University, B302 Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.
References
- 1.Robak T, Moiseev SI, Dmoszynska A, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fingerle-Rowson G, Fink A-M, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R; FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL). ASH Annual Meeting Abstracts. 2008;112(11):325. [Google Scholar]
- 3.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105(1):49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 5.Stein R, Mattes MJ, Cardillo TM, et al. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13(18 Pt 2):5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 6.Binsky I, Haran M, Starlets D, et al. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci U S A. 2007;104(33):13408–13413. doi: 10.1073/pnas.0701553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starlets D, Gore Y, Binsky I, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107(12):4807–4816. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 8.Gore Y, Starlets D, Maharshak N, et al. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283(5):2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 9.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197(11):1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binsky I, Lantner F, Grabovsky V, et al. TAp63 Regulates VLA-4 Expression and Chronic Lymphocytic Leukemia Cell Migration to the Bone Marrow in a CD74-Dependent Manner. J Immunol. 2010;184(9):4761–4769. doi: 10.4049/jimmunol.0904149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths GL, Mattes MJ, Stein R, et al. Cure of SCID mice bearing human B-lymphoma xenografts by an anti-CD74 antibody-anthracycline drug conjugate. Clin Cancer Res. 2003;9(17):6567–6571. [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997. [PubMed] [Google Scholar]
- 13.Lucas DM, Edwards RB, Lozanski G, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113(19):4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AJ, Lucas DM, Muthusamy N, et al. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood. 2006;108(4):1334–1338. doi: 10.1182/blood-2005-12-011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62(24):7190–7194. [PubMed] [Google Scholar]
- 16.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein R, Qu Z, Cardillo TM, et al. Antiproliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood. 2004;104(12):3705–3711. doi: 10.1182/blood-2004-03-0890. [DOI] [PubMed] [Google Scholar]
- 18.Carnahan J, Stein R, Qu Z, et al. Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol Immunol. 2007;44(6):1331–1341. doi: 10.1016/j.molimm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Mielgo A, Brondani V, Landmann L, et al. The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis. 2007;12(11):2051–2061. doi: 10.1007/s10495-007-0115-3. [DOI] [PubMed] [Google Scholar]
- 20.Berkova Z, Tao RH, Samaniego F. Milatuzumab - a promising new immunotherapeutic agent. Expert Opin Investig Drugs. 2010;19(1):141–149. doi: 10.1517/13543780903463854. [DOI] [PubMed] [Google Scholar]
- 21.Sapra P, Stein R, Pickett J, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11(14):5257–5264. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.