Abstract
The acidic soluble fraction of whole saliva of type 1 diabetic children was analyzed by reversed phase (RP)1–HPLC-ESI-MS and compared with that of sex- and age-matched control subjects. Salivary acidic proline-rich phosphoproteins (aPRP), histatins, α-defensins, salivary cystatins, statherin, proline-rich peptide P-B (P-B), beta-thymosins, S100A8 and S100A9*(S100A9* corresponds to S100A9 vairant lacking the first four amino acids), as well some naturally occurring peptides derived from salivary acidic proline-rich phosphoproteins, histatins, statherin, and P-B peptide, were detected and quantified on the basis of the extracted ion current peak area. The level of phosphorylation of salivary acidic proline-rich phosphoproteins, histatin-1 (Hst-1), statherin and S100A9* and the percentage of truncated forms of salivary acidic proline-rich phosphoproteins was also determined in the two groups. The study revealed that statherin, proline-rich peptide P-B, P-C peptide, and histatins, were significantly less concentrated in saliva of diabetic subjects than in controls, while concentration of α-defensins 1, 2 and 4 and S100A9* was higher. The low concentration of P-C peptide was paralleled by high levels of some of its fragments. On the whole, the study highlighted the severe impairment of the repertoire of peptides involved in the safeguard of the oral cavity in children who have diabetes, as well as an higher concentration of the proinflammatory mediator S100A9* with respect to healthy children.
Type 1 diabetes mellitus is a disease with universal distribution, affecting all populations (1) with an incidence increasing in Europe (2). Because type 1 diabetes involves many organs and tissues, signs and symptoms of diabetes can occur in the oral cavity. Indeed, several studies have shown that the prevalence, severity, and progression of periodontal diseases are significantly increased in diabetics, and the pathology is considered an important risk factor for periodontitis (3). The study of Lalla et al. (4) showed an association between diabetes and the increased risk for periodontal destruction even very early in life. Flow rate and composition of saliva are crucial for the maintenance of oral cavity health, and both have been found altered in diabetic subjects, although with contradictory findings. For instance, several studies reported a reduced resting salivary flow rate in adults and children who have type 1 diabetes with respect to healthy subjects (5–10), but it was also observed that stimulated saliva of diabetics and controls did not significantly differ (11, 12). Total protein concentration of saliva was found either increased or not affected by the diabetic status. Lopez et al. (9) reported higher urea and total protein salivary content in diabetic children than in controls. Conversely, a study on 35 adult insulin-dependent diabetics demonstrated that they did not show a decreased saliva concentration of several innate antimicrobial factors (lysozyme, lactoferrin, salivary peroxidase, myeloperoxidase) and that protein synthesis in salivary glands, as judged by amylase assays, was within the normal range (11). In the same study, higher amounts of immunoglobulin A (IgA) and IgG in diabetics with respect to controls were detected. Similar results were obtained in type 1 diabetic children; diabetics had neither salivary total protein nor albumin concentrations that were significantly different from controls, however, IgA and IgG levels were increased (12). In a large study performed in non–insulin-dependent diabetic patients, a significant increase of the concentration of lactoferrin, myeloperoxidase, and salivary peroxidase in stimulated parotid saliva, and of total protein, albumin, lactoferrin, and secretory IgA in stimulated submandibular/sublingual saliva was shown with respect to controls (13). In the same study it was also established that submandibular/sublingual salivary cystatin concentration did not significantly differ between diabetics and controls. The finding of increased concentrations of salivary proteins in diabetics is puzzling given their tendency to be more predisposed to oral infection with respect to patients who do not have diabetes. In this study we investigated the composition of the acidic soluble fraction of whole saliva by RP-HPLC-ESI-MS in a group of children and adolescent diabetics in comparison with a sex- and age-matched healthy control group. The results highlighted that type 1 diabetes is associated with a deep quali-quantitative modification of the salivary peptidome.
EXPERIMENTAL PROCEDURES
Materials
All general chemicals and reagents were of analytical grade and were purchased from Farmitalia-Carlo Erba (Milan, Italy), Merck (Darmstadt, Germany), and Sigma-Aldrich (St. Louis, MO).
Subjects
The study protocol and written consent forms were approved by the Medical Ethics Committee of the Faculty of Medicine of the Catholic University of Rome (according to the instructions of the Declaration of Helsinki). The study protocol was explained to both parents and children, and informed written consent to participate in the study was obtained from a parent. Diabetic patients were enrolled at the Pediatric Department of Brotzu Hospital (Cagliari) and controls at the Department of Surgery and Odontostomatology Sciences of the Cagliari University. The subjects enrolled were 31 children (22 females and 9 males) aged 11.7 ± 3.6 yr (mean age ± S.D.) with diabetes onset at 5.1 ± 3.3 yr. Percentage of hemoglobin A1c, determined at the Centro Diabetologico, Brotzu Hospital, Cagliari, by using the HA8140 Instrument (Menarini diagnostic, Florence, Italy), was 7.8 ± 0.9% (mean value ± S.D.). The control group comprised the same number of subjects (22 females and 9 males) with the same mean age 11.7 ± 3.2 yr.
Sample Collection
Whole human saliva was collected according to a standard protocol. Donors did not eat or drink for 30 minutes before collection, which was performed between 10:00 am and 12:00 pm. Whole saliva was collected as it flowed into the anterior floor of the mouth with a soft plastic aspirator for less than one minute and transferred to a plastic tube. An acidic solution (0.2% TFA) was immediately added to salivary samples in ice bath in 1:1 v/v ratio and the solution centrifuged at 8,000 g at 4 °C for five minutes. The acidic supernatant was separated from the precipitate and either immediately analyzed by the HPLC-ESI-MS apparatus (100 μL, corresponding to 50 μL of saliva) or stored at - 80 °C until the analysis.
HPLC-ESI-IT-MS Analysis
The HPLC-ESI-MS apparatus was a Surveyor HPLC system (ThermoFisher, San Jose, CA) connected by a T splitter to a photodiode array detector and the electrospray ionization/ion trap mass spectrometer LCQ Deca XP Plus (ThermoFisher). The chromatographic column was either a Vydac (Hesperia, CA) C8 with a 5-μm particle diameter (column dimensions 150 × 2.1 mm), or a Vydac C18 with 5-μm particle diameter (column dimensions 150 × 2.1 mm), the latter used for the analysis of the digestion products of S100A9* isoforms. The following solutions were used for RP-HPLC-ESI-MS analysis: (eluent A) 0.056% (v/v) aqueous TFA and (eluent B) 0.05% (v/v) TFA in acetonitrile-water 80/20, and the flow rate was 0.30 mL/min. Salivary proteins were eluted using a linear gradient from 0 to 55% of B in 40 minutes, whereas for tryptic digests the gradient was from 0 to 65% of B in 40 minutes, and from 65% to 100% of B in five minutes. The T splitter permitted 0.20 mL/min to flow toward the diode array detector and 0.10 mL/min to flow toward the ESI source. During the first five minutes of the RP-HPLC separation, eluate was not addressed toward the MS apparatus to avoid instrument damage deriving from the high salt content. The photodiode array detector was set at 214 and 276 nm. Mass spectra were collected every three milliseconds in the positive ion mode. The MS spray voltage was 4.50 kV, the capillary temperature was 220 °C.
High-Resolution HPLC-ESI-MS Experiments
High resolution HPLC-ESI-MS and MS/MS experiments were performed using an Ultimate 3000 Nano/Micro-HPLC apparatus (Dionex, Sunnyvale, CA) equipped with an FLM-3000-Flow manager module, and coupled to an LTQ Orbitrap XL hybrid mass spectrometer (ThermoFisher), having a nano ESI source (Proxeon Biosystems, Odense, Denmark). Separations were performed by a Dionex 18 column (3-μm particle diameter; column dimension 300 μm i.d. × 15 cm), using the following eluents: (A) 0.05% (v/v) aqueous TFA and (B) 0.05% (v/v) TFA in acetonitrile. The applied gradient was 0–4 min 5% B, 4–34 minutes from 5 to 50% B (linear), 34–64 minutes from 50 to 90% B (linear), at a flow rate of 4.5 μL/min. High-resolution positive MS spectra were collected in full scan mode and in data-dependent acquisition mode, using the lock mass for internal mass calibration (polydimethyl cyclosiloxane, 445.1200 m/z) with the resolution of 60,000 and 30,000, respectively, and m/z range 350–2000. The three most intense multiply charged ions were selected and fragmented by using collision induced dissociation (35% normalized collision energy) and spectra were recorded in the Orbitrap. Tuning parameters were: capillary temperature 220 °C, source voltage 2.4 kV, capillary voltage 26 V, and tube lens voltage 245 V.
Characterization of Salivary Peptides and Proteins
Proteins (salivary acidic proline-rich phosphoproteins, histatins, α-defensins, salivary cystatins, statherin, proline-rich peptide P-B, and beta-thymosins) and naturally occurring peptides derived from salivary acidic proline-rich phosphoproteins, histatins, statherin, and P-B peptide have been already identified in previous studies (14–19). Characterization of salivary proteins and peptides followed a top down approach. The accurate mass measurement of intact pure proteins/peptides (or semi-purified fractions) made it possible also to identify multiple forms of post-translational modification products. Structural characterization was based on tandem-MS analysis and automated amino acid sequencing of entire proteins, as well as of proteolytic fragments obtained after different enzymatic treatments of pure proteins. Deconvolution of averaged ESI-MS spectra was automatically performed using either the Bioworks Browser software provided with the LCQ Deca XP instrument or MagTran 1.0 software (20). Experimental mass values were compared with the theoretical ones found in Swiss-Prot Data Bank (http://us.expasy.org/tools) given the accession numbers listed in Tables I and II.
Table I. Elution times, experimental and theoretical average mass values (Da) of salivary acidic Proline-rich Phosphoproteins, Histatins, α-Defensins, Salivary Cystatins and derivatives.
| Protein (Swiss-Prot code or ref.) | Elution time (min) | Experimental av mass (theoretical av mass) |
|---|---|---|
| Salivary acidic proline-rich phosphoproteinsa | ||
| PRP-1 type diphos (P02810) | 22.9–23.3 | 15515 ± 2 (15514–15515) |
| PRP-1 type monophos (P02810) | 23.9–24.3 | 15435 ± 2 (15434–15435) |
| PRP-1 type triphos (P02810) | 22.6–22.9 | 15595 ± 2 (15594–15595) |
| PRP-3 type diphos (P02810) | 23.3–23.8 | 11161 ± 1 (11161–11162) |
| PRP-3 type monophos (P02810) | 23.8–24.2 | 11081 ± 1 (11081–11082) |
| PRP-3 type diphos Des-Arg106 (P02810) | 23.5–23.8 | 11004 ± 1 (11005–11006) |
| P-C peptide (P02810) | 13.6–14.5 | 4370.9 ± 0.4 (4370.8) |
| P-C peptide Des-Gln44 (25) | 13.8–14.4 | 4242.7 ± 0.4 (4243.0) |
| Histatins | ||
| Hst-1 (P15515) | 23.3–23.8 | 4928.2 ± 0.5 (4928.2) |
| Hst-1 nonphos (P15515) | 23.4–23.8 | 4848.2 ± 0.5 (4848.2) |
| Hst-3 (P15516) | 17.6–17.9 | 4062.2 ± 0.4 (4062.4) |
| Hst-3 1/24 (P15516) | 14.2–14.7 | 3036.5 ± 0.3 (3036.3) |
| Hst-3 1/25 (P15516) | 14.0–14.4 | 3192.4 ± 0.3 (3192.5) |
| α-Defensins | ||
| α-defensin 1 (P59665) | 23.2–24.2 | 3442.1 ± 0.4 (3442.1) |
| α-defensin 2 (P59665 and P59666) | 23.2–24.2 | 3371.0 ± 0.4 (3371.0) |
| α-defensin 3 (P59666) | 23.2–24.2 | 3486.1 ± 0.4 (3486.1) |
| α-defensin 4 (P12838) | 27.0–27.7 | 3709.4 ± 0.5 (3709.5) |
| Salivary cystatins (17) | ||
| S nonphos (P01036) | 36.5–37.1 | 14186 ± 2 (14185) |
| S monophos at Ser3 (P01036) | 36.6–37.1 | 14266 ± 2 (14265) |
| S diphos at Ser1 and Ser3 (P01036) | 36.8–37.2 | 14346 ± 2 (14345) |
| SN (P01037) | 34.8–35.2 | 14312 ± 2 (14313) |
| SA (P09228) | 38.4–38.9 | 14347 ± 2 (14346) |
a The term PRP-1 type includes the three entire isoforms PRP-1, PRP-2, and Pif-s, with a mass difference of 1 Da. The term PRP-3 type includes the truncated isoforms PRP-3, PRP-4 and Pif-f (Ref. 14).
Table II. Elution times, experimental and theoretical average mass values (Da) of statherin, proline-rich peptide P-B, beta-thymosins, S100A8, S100A9* proteins and derivatives.
| Protein (Swiss-Prot code or reference number) | Elution time (min) | Experimental av mass (theoretical av mass) |
|---|---|---|
| Statherin and derivatives | ||
| statherin diphos (P02808) | 28.9–29.5 | 5380.0 ± 0.5 (5379.7) |
| statherin monophos (18) | 28.7–29.1 | 5299.9 ± 0.5 (5299.7) |
| statherin nonphos (18) | 28.4–28.8 | 5220.5 ± 0.5 (5219.7) |
| SV1 (statherin Des-Phe43) (18) | 27.6–28.0 | 5232.4 ± 0.5 (5232.5) |
| statherin Des-Thr42Phe43 (18) | 27.7–28.1 | 5131.2 ± 0.5 (5131.4) |
| statherin Des-Asp1 (18, 51) | 28.5–28.9 | 5264.7 ± 0.5 (5264.6) |
| statherin Des1–9 (18) | 27.5–28.8 | 4127.9 ± 0.4 (4127.6) |
| statherin Des1–10 (18) | 27.8–28.2 | 3971.3 ± 0.4 (3971.4) |
| statherin Des1–13 (18) | 27.8–28.3 | 3645.2 ± 0.4 (3645.0) |
| Proline-rich peptide P-B and derivatives | ||
| P-B (P02814) | 29.4–30.5 | 5792.9 ± 0.5 (5792.7) |
| P-B Des1–5 (18) | 29.9–30.7 | 5215.0 ± 0.5 (5215.1) |
| P-B Des1–7 (23) | 29.6–30.5 | 5061.7 ± 0.5 (5060.9) |
| P-B Des1–4 (23) | 29.6–30.2 | 5370.7 ± 0.5 (5371.3) |
| P-B Des1–12 (23) | 27.2–27.7 | 4549.4 ± 0.5 (4549.3) |
| Thymosins (19) | ||
| Thymosin β4 (P62328) | 20.7–21.0 | 4963.7 ± 0.4 (4963.5) |
| Thymosin β10 (P63313) | 21.7–22.2 | 4936.8 ± 0.4 (4936.5) |
| Thymosin β4 sulfoxide | 18.0–18.5 | 4979.7 ± 0.4 (4979.5) |
| S100A proteins | ||
| S100A8 (P05109) | 38.8–39.4 | 10832.2 ± 1 (10834.5) |
| S100A9* monophos (P06702) | 41.4–42.1 | 12770.2 ± 1 (12769.2) |
| S100A9* monophos ox | 41.4–42.1 | 12786.5 ± 1 (12785.2) |
| S100A9* nonphos (P06702) | 41.4–42.1 | 12690.1 ± 1 (12689.2) |
| S100A9* nonphos ox | 41.4–42.1 | 12706.9 ± 1 (12705.2) |
To confirm the structure of the salivary proteins and fragments not previously characterized by us, some samples were also submitted to high-resolution HPLC-ESI-MS/MS experiments (LTQ Orbitrap XL), according to the conditions above reported. Tandem-MS spectra were analyzed by the Proteome Discoverer 1.0 program, based on SEQUEST cluster as search engine (University of Washington, Seattle, licensed to Thermo Electron Corp., San Jose, CA) against the Swiss-Prot human proteome (March 10, 2010, release; uniprot-taxonomy-9606-AND-reviewed-yes.fasta; 34756 non redundant protein sequences). The following limits were used for peptide matching: Xcorr scores greater than 1.5 for singly charged peptide ions and 2.0 and 2.5 for doubly and triply charged ions, respectively, one missed cleavage site. Sequences were also confirmed by manual comparison of experimental MS/MS spectra, obtained by using the program Extract_msn (ThermoFisher) and default parameters, with the theoretical spectra generated using the MS-Product program available at the Protein Prospector site (http://us.expasy.org/tools). The match was considered positive when all the experimental m/z values with a relative abundance higher than 10% were present in the theoretical fragmentation spectrum and when the differences between the experimental and theoretical values were less than ± 0.03 m/z in high-resolution MS/MS spectra. Annotated MS/MS spectra are reported as supplemental material. Structures of S100A9* and derivatives were also confirmed by trypsin digestion and low resolution HPLC-ESI-MS/MS analysis of digestion mixture.
Trypsin Digestion of S100A9* and Derivatives
S100A9* isoforms were isolated from a salivary sample by preparative RP-HPLC using a preparative Vydac C8 Column (250 × 10 mm 5 μm), a flow rate of 2.8 mL/min, and the following eluents: (eluent A) 0.056% (v/v) aqueous TFA and (eluent B) 0.05% (v/v) TFA in acetonitrile-water 80/20. The gradient was from 0% to 60% of B in 37 minutes and from 60% to 100% B in 12 minutes. The chromatographic peak eluting at 68% of B, collected and analyzed by HPLC-ESI-MS, corresponded to the mixture of S100A9* isoforms. Lyophilized sample was submitted to digestion by using the kit “Trypsin Singles Proteomic Grade” (Sigma-Aldrich) according to the manufacturer instructions. The reaction was stopped with TFA (0.1% final concentration) after six hours of incubation and analyzed by RP-HPLC-ESI-MS, as above reported. RP-HPLC-ESI-MS analysis showed the presence of four different fragments 89–109 (T12). Three of them were submitted to tandem-MS analysis, performed on the triply charged ions at 726.40 m/z, 731.53 m/z, and 752.87 m/z (see supplemental material). The intensity of the fourth T12 peptide did not allow sequencing. Isolation width of 3.0 m/z, normalized collision energy of 50%, activation Q of 0.25 and activation time of 30 milliseconds were applied. Experimental MS/MS spectra were compared with the theoretical spectra generated using the MS-Product program available at the Protein Prospector site (http://us.expasy.org/tools). The match was considered positive when all the experimental m/z values with a relative abundance higher than 10% were present in the theoretical fragmentation spectrum and when the differences between the experimental and theoretical values were less than ± 0.5 m/z.
Quantification
Quantification was based on the area of the RP-HPLC-ESI-MS extracted ion current (XIC) peaks, considered when the signal-to-noise ratio was at least 5. Under constant analytical conditions, the area of the XIC peaks is proportional to the peptide/protein concentration (21, 22). The XIC analysis selectively reveals a protein in the chromatographic profile by extracting the ion current associated with the multiply charged ions characteristic of the protein. The ions used to quantify the proteins/peptides were carefully selected to exclude values in common with other co-eluting proteins, and, with the exception of S100A8 and S100A9* isoforms and the new fragments characterized in this work, they were reported previously (16, 19, 23). The following ions were used to quantify S100A8 and S100A9* isoforms: S100A8, 985.9 (+11), 1084.4 (+10), 1204.8 (+9), and 1355.3 m/z (+8); S100A9* nonphosphorylated, 977.2 (+13), 1058.5 (+12), 1154.6 (+11), 1270.0 (+10), 1411.0 (+9); S100A9* phosphorylated, 983.3(+13), 1065.2 (+12), 1161.9 (+11), 1278.0 (+10), 1419.9 (+9); S100A9* nonphosphorylated oxidized, 978.4 (+13), 1059.8 (+12), 1156.1 (+11), 1271.6 (+10), 1412.8 (+9); S100A9* phosphorylated oxidized, 984.5 (+13), 1066.5 (+12), 1163.4 (+11), 1279.6 (+10), 1421.7 (+9). Percentages of monophosphorylated statherin, nonphosphorylated Hst-1, monophosphorylated aPRP-1 type proteins and nonphosphorylated S100A9* (oxidized plus not oxidized) were calculated on the basis of the following XIC peak area ratios: percentage of monophosphorylated statherin = 100 × monophosphorylated statherin/(monophosphorylated statherin + 2 × diphosphorylated statherin), percentage of nonphosphorylated Hst-1 = 100 × nonphosphorylated Hst-1/(monophos Hst-1 + nonphosphorylated Hst-1), percentage of monophosphorylated aPRP-1 type proteins = 100 × monophosphorylated proline-rich phosphoprotein (PRP)–1/(monophosphorylated PRP-1 + 2 × diphosphorylated PRP-1 + 3 × triphosphorylated PRP-1), and percentage of nonphosphorylated S100A9* = 100 × nonphosphorylated S100A9*/(monophosphorylated S100A9* + nonphosphorylated S100A9*) (nonphosphorylated and monophosphorylated forms comprised both oxidized and not oxidized derivatives). The percentage of truncated aPRPs (PRP-3 types; see Table I for definition) was calculated on the basis of the following XIC peak area ratio: 100 × (total PRP-3 types)/(total PRP-3 types + total PRP-1 types).
Data Analysis
The software GraphPad Prism (version 4.0) was used for statistical analysis. Ranges, medians, means, standard deviations, and standard errors were calculated for all the peptides/proteins XIC peak area. Means and standard errors are reported in Tables III and IV. Standard errors take into account both the value of standard deviation and sample size and are shown to give the precision on population means. The non-parametric Mann-Whitney test was used to compare the two groups. Statistical analysis was considered significant when the p value was less than 0.05 (two-tailed).
Table III. Families of salivary peptides showing different extracted ion current peak areas between diabetics and controls. Mean value ± S.E. (×106).
| Peptide or derivative | Diabetics | Controls | p value |
|---|---|---|---|
| Statherin diphos | 190 ± 26 | 590 ± 67 | <0.0001↓ |
| Statherin monophos | 4.1 ± 0.7 | 12.0 ± 1.4 | <0.0001↓ |
| Statherin nonphos | 0.5 ± 0.2 | 2.8 ± 0.9 | 0.004↓ |
| Statherin Des-Phe43 (or SV1) | 20.7 ± 3.1 | 22.9 ± 3.5 | Ns |
| Statherin Des-Thr42-Phe43 | 12.4 ± 2.8 | 8.4 ± 1.3 | Ns |
| Statherin Des-Asp1 | 18.9 ± 2.7 | 20.9 ± 3.3 | Ns |
| Statherin Des1–9 | 14.2 ± 2.2 | 30.6 ± 6.1 | 0.003↓ |
| Statherin Des1–10 | 8.7 ± 1.3 | 10.8 ± 1.7 | Ns |
| Statherin Des1–13 | 5.2 ± 0.8 | 7.7 ± 1.4 | Ns |
| P-B | 177 ± 29 | 411 ± 57 | <0.0001↓ |
| P-B Des1–4 | 23.3 ± 3.2 | 19.1 ± 4.2 | Ns |
| P-B Des1–7 | 42.1 ± 5.5 | 79.5 ± 10.0 | 0.0005↓ |
| P-B Des1–12 | 26.9 ± 4.8 | 23.8 ± 4.9 | Ns |
| P-C peptide | 189 ± 30 | 417 ± 87 | 0.0008↓ |
| P-C peptide Des-Gln44 | 4.3 ± 1.8 | 8.5 ± 2.1 | 0.004↓ |
| Hst-1 | 91.6 ± 14.9 | 288.3 ± 44.9 | <0.0001↓ |
| Hst-1 nonphos | 14.8 ± 3.1 | 49.7 ± 8.2 | <0.0001↓ |
| Hst-3 | 25.4 ± 5.9 | 51.3 ± 13.0 | 0.03↓ |
| Hst-3 1/24 | 68.6 ± 12.6 | 135.7 ± 32.3 | 0.04↓ |
| Hst-3 1/25 | 27.7 ± 4.6 | 48.7 ± 10.4 | Ns |
| a-defensin 1 | 48.0 ± 7.2 | 25.6 ± 5.5 | 0.007↑ |
| a-defensin 2 | 34.2 ± 5.1 | 22.1 ± 4.4 | 0.02↑ |
| a-defensin 4 | 8.2 ± 1.3 | 4.3 ± 1.1 | 0.01↑ |
| S100A9* totala | 91.2 ± 13.6 | 58.6 ± 15.2 | 0.01↑ |
| S100A9* phosb | 40.2 ± 6.3 | 21.4 ± 6.2 | 0.004↑ |
| S100A8 | 18.2 ± 3.8 | 12.7 ± 3.8 | Ns |
The arrow shows the variation of diabetics vs. controls.
a The four isoforms of S100A9*.
b The two isoforms oxidized and not oxidized. Ns: not significative.
Table IV. Extracted ion current peak area (mean value ± S.E. (×107)) of new fragments of salivary peptides and proteins showing a significant different concentration between diabetics and controls.
| Peptide fragment | Diabetics | Controls | p value |
|---|---|---|---|
| P-C peptide fr. 1–14 | 1.3 ± 0.3 | 0.7 ± 0.2 | 0.001↑ |
| P-C peptide fr. 1–25 | 2.2 ± 0.3 | 1.3 ± 0.3 | 0.003↑ |
| P-C peptide fr. 5–25 | 2.4 ± 0.4 | 1.1 ± 0.4 | 0.02↑ |
| P-C peptide fr. 15–44 | 1.3 ± 0.2 | 0.7 ± 0.2 | 0.002↑ |
| P-C peptide fr. 26–35 | 4.5 ± 0.8 | 2.4 ± 0.7 | 0.01↑ |
| P-C peptide fr. 26–44 | 1.5 ± 0.2 | 0.7 ± 0.2 | 0.004↑ |
| P-C peptide fr. 36–44 | 8.1 ± 1.1 | 4.1 ± 1.0 | 0.002↑ |
| PRP-1/PRP-3 fr. 94–105 | 1.2 ± 0.2 | 0.5 ± 0.1 | 0.0003↑ |
| PRP-1 fr. 94–110 | 0.4 ± 0.07 | 0.07 ± 0.02 | 0.0001↑ |
| N.I. peptide 559.3 Da | 0.2 ± 0.1 | 0.1 ± 0.02 | 0.03↑ |
| N.I. peptide 1237.7 Da | 1.3 ± 0.3 | 0.4 ± 0.1 | 0.02↑ |
| N.I. peptide 1238.4 Da | 1.5 ± 0.2 | 0.7 ± 0.2 | 0.0005↑ |
| N.I. peptide 2542.3 Da | 0.9 ± 0.2 | 0.4 ± 0.1 | 0.04↑ |
| N.I. peptide 2485.9 Da | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.016↑ |
The arrow shows the variation of diabetics vs. controls.
RESULTS
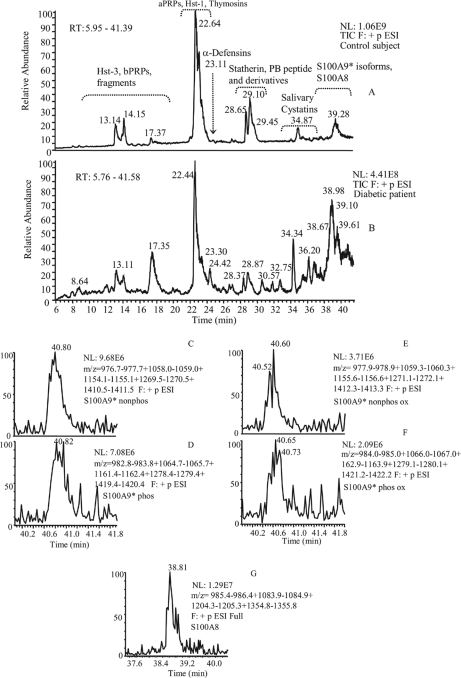
According to the guidelines for the management of type 1 diabetes mellitus (24) and on the basis of hemoglobin A1c levels less than 9.9% the group of diabetics enrolled for the study comprised well-to-moderately controlled patients. For ethical reasons it was not possible to collect selected saliva from gland ducts of the young donors, thus, the present study was performed on whole saliva. However, contribution of the submandibular/sublingual glands was slightly higher than parotid glands because saliva was collected from the floor of the mouth close to orifices of Wharton's ducts. HPLC-ESI-MS analysis of whole human saliva allows detecting more than 120 salivary components (25). In the present study, we investigated the following already characterized salivary proteins: salivary acidic proline-rich phosphoproteins, histatins, α-defensins, salivary cystatins (Table I), statherin, proline-rich peptide P-B, and beta-thymosins (Table II), as well as several derivatives generated by post-translational modifications occurring before and after secretion, such as phosphorylated isoforms and cleavage products. We also detected and quantified some proteins belonging to the S100 family, not previously evidenced in saliva by the HPLC-ESI-MS approach. They were S100A8 and four different forms of S100A9*. The chromatographic position of these calcium binding proteins is evidenced in Figure 1. The figure shows that the four isoforms of S100A9* co-eluted. Two of them corresponded to S100A9* nonphosphorylated and phosphorylated at Thr-108 (26). The remaining two isoforms were the corresponding oxidized derivatives (Δmass = 16.0 Da). Structure of S100A8 and S100A9* were confirmed by tandem-MS experiments (annotated spectra are reported as supplemental material). The structures of S100A9* and its phosphorylated, oxidized, and phosphorylated-oxidized derivatives were confirmed by peptide mapping analysis. The results of RP-HPLC-ESI-MS analysis of the tryptic digestion mixture of the four isoforms, as well as tandem-MS spectra of the tryptic fragment T12, carrying the modifications, were in agreement with the assigned structures and allowed to locate the phosphorylation at Thr-108 residue and oxidation at Met-89 residue (annotated spectra are reported as supplemental material).
Fig. 1.
Typical RP-HPLC-ESI profile of the acidic soluble fraction of human whole saliva of a control subject (A) and a Type 1 diabetic patient (B). Elution ranges of the principal classes of human salivary proteins/peptides are indicated. The other panels show the extracted ion current (XIC) peaks of nonphosphorylated S100A9* (C), phosphorylated S100A9* (D), nonphosphorylated oxidized S100A9* (E), phosphorylated oxidized S100A9* (F), and S100A8 (G).
Quantitation and Comparison of Salivary Peptide Levels Between Diabetics and Controls
The acidic treatment with aqueous TFA, performed immediately after collection to minimize protein degradation, caused precipitation of a pellet mainly constituted by high molecular weight proteins. As previously shown, precipitation did not affect significantly the quali/quantitative profile of acid-soluble proteins (23). Levels of salivary proteins and naturally occurring peptides were determined by HPLC-ESI-MS analysis, and the typical chromatographic profile obtained under our experimental conditions in the two groups under study is shown in Figure 1. Mann-Whitney test of the XIC peak areas revealed that statherin (diphosphorylated and monophosphorylated), proline-rich peptide P-B, P-C peptide, Hst-1, and nonphosphorylated Hst-1 were significantly less concentrated in saliva of diabetic patients than in controls, whereas the levels of α-defensins 1, 2, and 4 were significantly lower in saliva of control subjects (Table III). We also found that concentration of phosphorylated S100A9* (sum of oxidized and not oxidized forms) was significantly higher in diabetics than in controls (p = 0.004). The same result was obtained considering the totality of S100A9*forms (p = 0.01). Conversely, S100A8 levels were not significantly different in the two groups (p = 0.28). The reduced concentration of P-C peptide was intriguing because neither the level of PRP-1 type proteins, from which P-C peptide is originated by a pre-secretory cleavage, nor the level of the complementary fragments (PRP-3 type proteins), was significantly different in the two groups under investigation. To establish whether quantitative differences between diabetics and controls could be related to a different degree of proteolytic fragmentation occurring in the oral cavity, we searched for masses not yet characterized in the chromatographic profile of saliva of diabetics. Several masses were detected in the elution range of 5–30 minutes. Masses sporadically revealed were not further investigated, those detected in at least 9 of 31 samples were also searched in saliva chromatographic profile of controls, and quantified. The analysis by Mann-Whitney test of the XIC peak areas showed that 14 peptides were significantly more concentrated in saliva of diabetics than in controls (Table IV). The primary structure of these peptides (Table V) was determined by selected ion monitoring MS/MS experiments. Seven of them corresponded to the following fragments of P-C peptide: P-C peptide fr. 1–14 (exptl av mass: 1470.8 Da), P-C peptide fr. 1–25 (exptl av mass: 2521.4 Da), P-C peptide fr. 5–25 (exptl av mass: 2083.3 Da), P-C peptide fr. 26–44 (exptl av mass: 1867.2 Da), P-C peptide fr. 26–35 (exptl av mass: 990.1 Da), P-C peptide fr. 36–44 (exptl av mass: 894.9 Da), P-C peptide fr. 15–44 (exptl av mass: 2917.4 Da). Two peptides corresponded to fragment 94–105 of PRP-1/PRP-3 type proteins and 94–110 of PRP-1 type proteins. These two fragments accounted for approximately 0.8% of intact PRP-1/PRP-3 type proteins. The low extent of fragmentation is in agreement with the lack of significant differences in the XIC peak areas of acidic PRPs between diabetics and controls. Although the last five fragments have not been identified, MS/MS spectra allowed an origin from salivary secretory peptides/proteins to be excluded. Differently from P-C peptide, the lower concentration of statherin in saliva of diabetics with respect to controls (p < 0.0001, Table III) was not paralleled by an higher concentration of statherin fragments, and rather the level of statherin Des1–9, a fragment generated by a pre-secretory cleavage, was found to be lower in diabetics (p = 0.003). Serine phosphorylation level of statherin, Hst-1, and aPRPs, evaluated by the percentages of monophosphorylated statherin, nonphosphorylated Hst-1, and monophosphorylated aPRPs (entire isoforms), did not show significant differences in the two groups under study. Conversely, the percentage of total nonphosphorylated S100A9* (oxidized plus not oxidized) was significantly lower in the patient group (diabetics: 55.0 ± 3%, controls: 72.5 ± 4.5%, p = 0.0006). At this regard, it is relevant to outline that the site of phosphorylation of S100A9* is a threonine. The percentage of truncated salivary acidic proline-rich phosphoproteins did not show significant differences in the two groups under study.
Table V. Fragments of salivary peptides and proteins found in saliva of diabetics at higher concentration than in controls.
| Protein (Swiss-Prot code) | Elution time (min) | Experimental av mass (theoretical av mass) | m/z values of multiply charged ions used to reveal XIC peaks | Sequence |
|---|---|---|---|---|
| P-C peptide fr. 1–14 (P02810) | 8.5–8.9 | 1470.8 ± 0.4 (1471.6) | 1471.8 (+1), 736.4 (+2) | GRPQGPPQQGGHQQ |
| P-C peptide fr. 1–25 | 11.6–12.0 | 2521.4 ± 0.4 (2521.8) | 1261.9 (+2), 841.6 (+3) | GRPQGPPQQGGHQQGPPPPPPGKPQ |
| P-C peptide fr. 5–25 | 11.1–11.5 | 2083.3 ± 0.4 (2083.3) | 1042.6 (+2), 695.4 (+3) | GPPQQGGHQQGPPPPPPGKPQ |
| P-C peptide fr.15–44 | 12.3–12.8 | 2917.4 ± 0.4 (2917.2) | 1459.7 (+2), 973.5 (+3) | GPPPPPPGKPQGPPPQGGRPQGPPQGQSPQ |
| P-C peptide fr. 26–35 | 7.9–8.5 | 990.1 ± 0.3 (990.1) | 991.1 (+1), 496.1 (+2) | GPPPQGGRPQ |
| P-C peptide fr. 26–44 | 9.2–9.8 | 1867.2 ± 0.4 (1867.0) | 934.6 (+2), 623.4 (+3) | GPPPQGGRPQGPPQGQSPQ |
| P-C peptide fr. 36–44 | 6.5–7.3 | 894.9 ± 0.3 (894.9) | 895.9 (+1), 448.5 (+2) | GPPQGQSPQ |
| PRP-1/PRP-3 fr. 94–105 (P02810) | 10.8–11.3 | 1223.8 ± 0.4 (1224.4) | 1224.8 (+1), 612.9 (+2) | GPPQQGGHPRPP |
| PRP-1 fr. 94–110 | 10.7–11.5 | 1818.9 ± 0.4 (1819.0) | 910.5 (+2), 607.3 (+3) | GPPQQGGHPRPPRGRPQ |
| N.I. | 6.6–7.3 | 559.3 | 560.3 (+1) | |
| N.I. | 12.0–12.5 | 1237.7 | 1238.7 (+1), 619.9 (+2) | |
| N.I. | 12.7–13.4 | 1238.4 | 1239.4 (+1), 620.2 (+2) | |
| N.I. | 25.0–25.5 | 2542.3 | 1272.2 (+2), 848.4 (+3) | |
| N.I. | 19.0–19.6 | 2485.9 | 1244.0 (+2), 829.6 (+3) |
N.I.: not identified.
DISCUSSION
In a previous work devoted to the investigation of the influence of the age on salivary protein composition, we evidenced that the expression of basic PRPs is age-related and that these salivary proteins reach the concentration found in adults only after puberty (12–17 years of age) (23). Since the age of the subjects enrolled for this study was comprised between 7–18 years, basic PRPs (bPRPs) were not considered in the present investigation.
This study revealed that protein composition of saliva of diabetic patients is different from that of sex- and age-matched controls, both from a qualitative and from a quantitative point of view. Statherin, proline-rich peptide P-B, P-C peptide, and Hst-1, were found significantly less concentrated in saliva of diabetics, whereas α-defensins 1, 2, and 4, S100A9* and several small peptides, most likely originated by post-secretory proteolytic cleavages occurring in the mouth, showed a higher concentration.
Tandem-MS experiments allowed establishing that these peptides were mainly represented by P-C peptide fragments. Fragments 1–14, 1–25, 5–25, 26–35, 26–44, and 36–44 of P-C peptide have been already detected in saliva from healthy subjects (27). An association of fragments 1–14 and 26–44 to high numbers of dental caries and the presence of fragment 1–14 in saliva of subjects affected by Sjogrens syndrome were also reported (28). The same authors also detected fragments 1–25 and 15–44 in whole saliva of normal subjects, whereas fragments 1–14 e 26–44 were found in parotid saliva in another study (29). The higher concentration of P-C fragments in diabetics is consistent with the observed decreased concentration of the intact peptide. This finding suggests that the low concentration of P-C peptide should not reflect a lower activity of the pro-protein convertases acting on aPRPs before secretion, and generating P-C peptide and the complementary PRP-3 type proteins. Indeed, we did not find any significant difference in salivary concentration of PRP-1 type proteins and PRP-3 type proteins between diabetics and controls. Fragmentation of P-C peptide, occurring mainly at the XPQ↓G site (where X is preferably K, but often S and R), seems to be related to a glutamine endoprotease activity probably of microbial origin, as reported by Helmerhorst et al. (27). Our data suggest that in the oral environment of diabetic subjects the activity of this proteolytic enzyme could be higher than in healthy subjectsl. A reduced concentration of P-C peptide in saliva of type 2 diabetics with respect to healthy subjects has been reported by Kimura et al. (30). Low concentration of P-C peptide in saliva of diabetics is particularly intriguing and stimulates further deep studies, in consideration of its potentiating effect on glucose and arginine induced insulin release shown in vitro in perfused rat pancreas (31).
Differently from P-C peptide, the lower concentration of statherin and statherin Des1–9, a fragment of pre-secretory origin, were not paralleled by higher concentrations of other post-secretory fragments in saliva of diabetics. Thus, it may be hypothesized that the low concentration might be connected either to a different degree of statherin expression in salivary glands of diabetics or to the recruitment of this peptide to accomplish specific roles in the oral cavity of diabetics. In this regard, it is known that statherin, a tyrosine-rich 43 residue phosphopeptide involved in oral calcium homeostasis and teeth mineralization (32), is an in vivo constituent of the human acquired enamel pellicle (33). This protein film is important for the integrity of tooth enamel because it acts as a boundary lubricant on the enamel surface (34), and it has been shown that interactions between pellicle proteins and bacterial surfaces are responsible for specificity of the bacterial colonization during the earliest stage of plaque formation (35). Similar to statherin, P-B peptide showed low levels in saliva of diabetics. P-B peptide is usually included into the basic proline-rich protein family. However, unlike other bPRPs it is not a generated by the proteolytic cleavage of a larger pro-protein, but it is a mature protein itself, expressed by PROL3 gene located on chromosome 4q13.3, very close to STATH gene (36). P-B peptide shares more structural similarities with statherin. Similar to statherin, it is secreted both from parotid and submandibular/sublingual glands and the correlation between the two peptides in whole saliva is highly significant (18).
Also, in the case of Hst-1, the low levels found in saliva of diabetic patients should not be related only to a different degree of fragmentation of the peptide, which was shown to be almost stable in the environment of the oral cavity (37). Indeed, fragments of Hst-1 were not significantly more represented in saliva of diabetics than in controls. Recently it has been shown that Hst-1 plays an important role in the oral cavity because of its wound healing properties (38). The lower concentration of Hst-1 indicates that this role could not be fully achieved in the mouth of diabetics. It has been also reported that Hst-3 1/24 (Hst-5) displays the highest antifungal activity among the histatins (39), and it has been shown that it is an inhibitor of both host and bacterial enzymes implicated in periodontal disease (40).
In our study we showed that salivary levels of the major α-defensins are higher in diabetics than in controls. This result is in agreement with literature data. In fact, Seraheimo et al. (41) observed that α-defensin (1, 2, and 3) levels were increased in a group of type 1 diabetic patients with nephropathy with respect to normoalbuminuric patients. Otherwise, plasma defensin levels of the latter group were approximately five times higher than non-diabetics, which had α-defensins serum level of approximately 120 μg/L (42). Thus, saliva reflects plasma concentration of α-defensins. This finding is of particular interest in consideration of the study of Joseph et al. (43) that not only confirmed the higher α-defensin levels in type 1 diabetic patients with than without microalbuminuria, but also suggested that plasma α-defensins may serve as clinical risk marker for cardiovascular morbility and mortality in these patients.
Also, concentration of S100A9* was higher in saliva of diabetics than in controls. S100A9 represents 45% of the cyplasmatic fraction of neutrophils together with S100A8 underlining its importance in inflammation (44). Extracellularly its function includes chemotactic activity, triggering local inflammation, regulation of adhesion, transendothelial migration of leukocytes, and striking antimicrobial activity (45). The level of S100A8 did not increase along with S100A9, even though the two proteins are frequently co-expressed and can form a noncovalent heterodimer protein complex (calprotectin) which antagonizes the monomer function (46). However, Bouma et al. also observed increased levels of S100A9 and S100A8/S100A9 heterodimer, but not of S100A8, in sera of type 1 diabetic patients and showed that this variation is specific of this subtype of diabetes (47). Thus, the present data suggest that saliva and serum concentrations of the two calcium binding proteins correlate.
The proteolytic cleavages occurring before secretion did not show significant differences in the two groups under study, suggesting that the activity of the involved pro-protein convertases is not affected by the pathology.
Different results were obtained on phosphorylation levels. In fact, phosphorylation of serine residues, such as those present in aPRPs, statherin, and Hst-1, showed a similar extent in diabetics and controls, suggesting that the diabetic status should not affect the activity of the Golgi-casein kinase involved in the pre-secretory phosphorylation of serine residues. Conversely, phosphorylation level of S100A9* was higher in diabetic subjects. In this regard, it is relevant to outline that the site of phosphorylation of S100A9* is represented by a threonine residue, and that the phophorylation is under the control of p38 MAPK pathway (48). It has been suggested that phosphorylation of S100A9 by p38 MAPK contributes to p38 MAPK regulation of cytoskeletal reorganization necessary for exocytosis (48). Moreover, it has been reported an increased phosphorylation of S100A9* during human neutrophil activation (49) and that the phosphorylation event is connected with the regulation of translocation of the peptide to the plasma membrane. The present data are in agreement with the increased monocytic activity and with the increased expression of pro-inflammatory mediators observed in patients who have type 1 diabetes (50).
CONCLUSION
Collectively, our findings highlight that quantitative and qualitative differences occur in the salivary peptidome of type 1 diabetic subjects, with particular regard to peptides involved in inflammation and oral cavity host defense. The increased levels of several proteolytic fragments of P-C peptide in diabetics indicate a possible variation of exogenous proteinases in the oral cavity of diabetics. This observation is a stimulus for further studies to establish a possible connection between peptide fragments and oral microflora in diabetics. The decreased concentration of statherin, P-B peptide, and histatins suggests to verify if the low concentration of these peptides may contribute to the major incidence of dental and periodontal diseases in the type 1 diabetic population, possibly allowing the formulation of specific aids for their oral health.
The present results provide the basis for further studies on large cohorts of diabetic subjects to discover potential salivary biomarkers, which should be extensively validated by antibody-based methods, such as ELISA or Western blot. Thus, new and noninvasive tests might be developed for monitoring type 1 diabetes complications.
Supplementary Material
Acknowledgments
We acknowledge the financial support of Università di Cagliari, Università Cattolica in Rome, Italian National Research Council (CNR), Regione Sardegna, Fondazione Banco di Sardegna, International Scientific Institute “Paolo VI” (ISI), MIUR. We thank their programs of scientific research promotion and diffusion.
Footnotes
1 The abbreviations used are:
- aPRPs
- salivary acidic proline-rich phosphoproteins
- bPRPs
- basic salivary proline-rich proteins
- P-B
- proline-rich peptide P-B
- RP
- reversed phase
- Ig
- immunoglobulin
- XIC
- extracted ion current
- PRP
- proline-rich proteins
- Hst-1
- histatin-1.
REFERENCES
- 1.Soltesz G., Patterson C. C., Dahlquist G.EURODIAB Study Group (2007) Worldwide childhood type 1 diabetes incidence–what can we learn from epidemiology? Pediatr. Diabetes 8 (Suppl 6), 6–14 [DOI] [PubMed] [Google Scholar]
- 2.Patterson C. C., Dahlquist G. G., Gyürüs E., Green A., Soltész G.EURODIAB Study Group (2009) Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 373, 2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Mealey B. L., Ocampo G. L. (2007) Diabetes mellitus and periodontal disease. Periodontol. 2000 44, 127–153 [DOI] [PubMed] [Google Scholar]
- 4.Lalla E., Cheng B., Lal S., Kaplan S., Softness B., Greenberg E., Goland R. S., Lamster I. B. (2007) Diabetes mellitus promotes periodontal destruction in children. J. Clin. Periodontol. 34, 294–298 [DOI] [PubMed] [Google Scholar]
- 5.Ben-Aryeh H., Cohen M., Kanter Y., Szargel R., Laufer D. (1988) Salivary composition in diabetic patients. J. Diabetes Complications 2, 96–99 [DOI] [PubMed] [Google Scholar]
- 6.Ben-Aryeh H., Serouya R., Kanter Y., Szargel R., Laufer D. (1993) Oral health and salivary composition in diabetic patients. J. Diabetes Complications 7, 57–62 [DOI] [PubMed] [Google Scholar]
- 7.Moore P. A., Guggenheimer J., Etzel K. R., Weyant R. J., Orchard T. (2001) Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 92, 281–291 [DOI] [PubMed] [Google Scholar]
- 8.Siudikiene J., Machiulskiene V., Nyvad B., Tenovuo J., Nedzelskiene I. (2006) Dental caries and salivary status in children with type 1 diabetes mellitus, related to the metabolic control of the disease. Eur. J. Oral Sci. 114, 8–14 [DOI] [PubMed] [Google Scholar]
- 9.López M. E., Colloca M. E., Páez R. G., Schallmach J. N., Koss M. A., Chervonagura A. (2003) Salivary characteristics of diabetic children. Braz. Dent. J. 14, 26–31 [DOI] [PubMed] [Google Scholar]
- 10.Thorstensson H., Falk H., Hugoson A., Olsson J. (1989) Some salivary factors in insulin-dependent diabetics. Acta Odontol. Scand. 47, 175–183 [DOI] [PubMed] [Google Scholar]
- 11.Tenovuo J., Lehtonen O. P., Viikari J., Larjava H., Vilja P., Tuohimaa P. (1986) Immunoglobulins and innate antimicrobial factors in whole saliva of patients with insulin-dependent diabetes mellitus. J. Dent. Res. 65, 62–66 [DOI] [PubMed] [Google Scholar]
- 12.Belazi M. A., Galli-Tsinopoulou A., Drakoulakos D., Fleva A., Papanayiotou P. H. (1998) Salivary alterations in insulin-dependent diabetes mellitus. Int. J. Paediatr. Dent. 8, 29–33 [DOI] [PubMed] [Google Scholar]
- 13.Dodds M. W., Yeh C. K., Johnson D. A. (2000) Salivary alterations in type 2 (non-insulin-dependent) diabetes mellitus and hypertension. Community Dent. Oral Epidemiol. 28, 373–381 [DOI] [PubMed] [Google Scholar]
- 14.Inzitari R., Cabras T., Onnis G., Olmi C., Mastinu A., Sanna M. T., Pellegrini M., Castagnola M., Messana I. (2005) Different isoforms and post-translational modifications of human salivary acidic proline-rich proteins. Proteomics 5, 805–815 [DOI] [PubMed] [Google Scholar]
- 15.Castagnola M., Inzitari R, Rossetti D. V., Olmi C., Cabras T., Piras V., Nicolussi P., Sanna M. T., Pellegrini M., Giardina B., Messana I. (2004) A cascade of 24 Histatins (Histatin 3 fragments) in human saliva: suggestions for a pre-secretory sequential cleavage pathway. J. Biol. Chem. 279, 41436–41443 [DOI] [PubMed] [Google Scholar]
- 16.Pisano E., Cabras T., Montaldo C., Piras V., Inzitari R., Olmi C., Castagnola M., Messana I. (2005) Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur. J. Oral. Sci. 113, 462–468 [DOI] [PubMed] [Google Scholar]
- 17.Lupi A., Messana I., Denotti G., Schinina‘ M. E., Gambarini G., Fadda M. B., Vitali A., Cabras T., Piras V., Patamia M., Cordaro M., Giardina B., Castagnola M. (2003) Identification of the human salivary Cystatin complex by the coupling of high-performance liquid chromatography and ion-trap mass spectrometry. Proteomics 3, 461–467 [DOI] [PubMed] [Google Scholar]
- 18.Inzitari R., Cabras T., Rossetti D. V., Fanali C., Vitali A., Pellegrini M., Paludetti G., Manni A., Giardina B., Messana I., Castagnola M. (2006) Detection in human saliva of different statherin and P-B fragments and derivatives. Proteomics 6, 6370–6379 [DOI] [PubMed] [Google Scholar]
- 19.Inzitari R., Cabras T., Pisano E., Fanali C., Manconi B., Scarano E., Fiorita A., Paludetti G., Manni A., Nemolato S., Faa G., Castagnola M., Messana I. (2009) HPLC-ESI-MS analysis of oral human fluids reveals that gingival crevicular fluid is the main source of oral thymosins beta(4) and beta(10). J. Sep. Sci. 32, 57–63 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z., Marshall A. G. (1998) A universal algorithm for fast and automated charge state deconvolution of electrospray mass-to-charge ratio spectra. J. Am. Soc. Mass Spectrom. 9, 225–233 [DOI] [PubMed] [Google Scholar]
- 21.Ong S. E., Mann M. (2005) Mass spectrometry-based proteomics turns quantitative. Nat. Chem. Biol. 1, 252–262 [DOI] [PubMed] [Google Scholar]
- 22.Messana I., Inzitari R., Fanali C., Cabras T., Castagnola M. (2008) Facts and artifacts in proteomics of body fluids. What proteomics of saliva is telling us? J. Sep. Sci. 31, 1948–1963 [DOI] [PubMed] [Google Scholar]
- 23.Cabras T., Pisano E., Boi R., Olianas A., Manconi B., Inzitari R., Fanali C., Giardina B., Castagnola M., Messana I. (2009) Age-Dependent Modifications of the Human Salivary Secretory Protein Complex. J. Proteome Res. 8, 4126–4134 [DOI] [PubMed] [Google Scholar]
- 24.International Society for Pediatric and Adolescent Diabetes: ISPAD Consensus guidelines for the management of Type 1 Diabetes Mellitus in Children and Adolescent Guidelines 2000, pp. 34–39 Swift PGF. Ed. Zeist, the Netherlands [Google Scholar]
- 25.Messana I., Cabras T., Pisano E., Sanna M. T., Olianas A., Manconi B., Pellegrini M., Paludetti G., Scarano E., Fiorita A., Agostino S., Cóntucci A. M., Calò L., Picciotti P. M., Manni A., Bennick A., Vitali A., Fanali C., Inzitari R., Castagnola M. (2008) Trafficking and postsecretory events responsible for the formation of secreted human salivary peptides: a proteomics approach. Mol. Cell. Proteomics 7, 911–926 [DOI] [PubMed] [Google Scholar]
- 26.Edgeworth J., Freemont P., Hogg N. (1989) Ionomycin-regulated phosphorylation of the myeloid calcium-binding protein p14. Nature 342, 189–192 [DOI] [PubMed] [Google Scholar]
- 27.Helmerhorst E. J., Sun X., Salih E., Oppenheim F. G. (2008) Identification of Lys-Pro-Gln as a novel cleavage site specificity of saliva-associated proteases. J. Biol. Chem. 283, 19957–19966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huq N. L., Cross K. J., Ung M., Myroforidis H., Veith P. D., Chen D., Stanton D., Huiling H., Ward B. R., Reynolds E. C. (2007) A review of the salivary proteome and peptidome and saliva-derived peptide therapeutics. Int. J. Pept. Res. Ther. 13, 547–564 [Google Scholar]
- 29.Hardt M., Thomas L. R., Dixon S. E., Newport G., Agabian N., Prakobphol A., Hall S. C., Witkowska H. E., Fisher S. J. (2005) Toward defining the human parotid gland salivary proteome and peptidome: identification and characterization using 2D SDS-PAGE, ultrafiltration, HPLC, and mass spectrometry. Biochemistry 44, 2885–2899 [DOI] [PubMed] [Google Scholar]
- 30.Kimura I., Sasamoto H., Sasamura T., Sugihara Y., Ohgaku S., Kobayashi M. (2001) Reduction of incretin-like salivatin in saliva from patients with type 2 diabetes and in parotid glands of streptozotocin-diabetic BALB/c mice. Diabetes Obes. Metab. 3, 254–258 [DOI] [PubMed] [Google Scholar]
- 31.Nakashima N., Kimura I., Kimura M. (1995) Salivary Peptide P-C potentiates insulin release and inhibits glucagon release from isolated perfused pancreas of the diabetic GK rat. Jpn. J. Pharmacol. 67, 15–20 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz S. S., Hay D. I., Schluckebier S. K. (1992) Inhibition of calcium phosphate precipitation by human salivary statherin: structure-activity relationships. Calcif. Tissue Int. 50, 511–517 [DOI] [PubMed] [Google Scholar]
- 33.Li J., Helmerhorst E. J., Yao Y., Nunn M. E., Troxler R. F., Oppenheim F. G. (2004) Statherin is an in vivo pellicle constituent: identification and immuno-quantification. Arch. Oral. Biol. 49, 379–385 [DOI] [PubMed] [Google Scholar]
- 34.Douglas W. H., Reeh E. S., Ramasubbu N., Raj P. A., Bhandary K. K., Levine M. J. (1991) Statherin: a major boundary lubricant of human saliva. Biochem. Biophys. Res. Commun. 180, 91–97 [DOI] [PubMed] [Google Scholar]
- 35.Hay D. I. (1995) Salivary factors in caries models. Adv. Dent. Res. 9, 239–243 [DOI] [PubMed] [Google Scholar]
- 36.Isemura S. (2000) Nucleotide sequence of gene PBII encoding salivary proline-rich protein P-B. J. Biochem. 127, 393–398 [DOI] [PubMed] [Google Scholar]
- 37.Campese M., Sun X., Bosch J. A., Oppenheim F. G., Helmerhorst E. J. (2009) Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Arch. Oral Biol. 54, 345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudhoff M. J., Kroeze K. L., Nazmi K., van den Keijbus P. A., van 't Hof W., Fernandez-Borja M., Hordijk P. L., Gibbs S., Bolscher J. G., Veerman E. C. (2009) Structure-activity analysis of histatin, a potent wound healing peptide from human saliva: cyclization of histatin potentiates molar activity 1,000-fold. FASEB J. 23, 3928–3935 [DOI] [PubMed] [Google Scholar]
- 39.Xu T., Levitz S. M., Diamond R. D., Oppenheim F. G. (1991) Anticandidal activity of major human salivary histatins. Infect. Immun. 59, 2549–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gusman H., Travis J., Helmerhorst E. J., Potempa J., Troxler R. F., Oppenheim F. G. (2001) Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect. Immun. 69, 1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saraheimo M., Forsblom C., Pettersson-Fernholm K., Flyvbjerg A., Groop P. H., Frystyk J.FinnDiane Study Group (2008) Increased levels of alpha-defensin (-1, -2 and -3) in type 1 diabetic patients with nephropathy. Nephrol. Dial. Transplant. 23, 914–918 [DOI] [PubMed] [Google Scholar]
- 42.Sthoeger Z. M., Bezalel S., Chapnik N., Asher I., Froy O. (2009) High alpha-defensin levels in patients with systemic lupus erythematosus. Immunology 127, 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph G., Tarnow L., Astrup A. S., Hansen T. K., Parving H. H., Flyvbjerg A., Frystyk J. (2008) Plasma alpha-defensin is associated with cardiovascular morbidity and mortality in type 1 diabetic patients. J. Clin. Endocrinol. Metab. 93, 1470–1475 [DOI] [PubMed] [Google Scholar]
- 44.Edgeworth J., Gorman M., Bennett R., Freemont P., Hogg N. (1991) Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 266, 7706–7713 [PubMed] [Google Scholar]
- 45.Kerkhoff C., Klempt M., Kaever V., Sorg C. (1999) The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J. Biol. Chem. 274, 32672–32679 [DOI] [PubMed] [Google Scholar]
- 46.Newton R. A., Hogg N. (1998) The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J. Immunol. 160, 1427–1435 [PubMed] [Google Scholar]
- 47.Bouma G., Coppens J. M., Lam-Tse W. K., Luini W., Sintnicolaas K., Levering W. H., Sozzani S., Drexhage H. A., Versnel M. A. (2005) An increased MRP8/14 expression and adhesion, but a decreased migration towards proinflammatory chemokines of type 1 diabetes monocytes. Clin. Exp. Immunol. 141, 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lominadze G., Rane M. J., Merchant M., Cai J., Ward R. A., McLeisch K. R. (2005) Myeloid-related protein-14 is a p38MAPK substrate in human neutrophils. J. Immunol. 174, 7257–7267 [DOI] [PubMed] [Google Scholar]
- 49.Guignard F., Mauel J., Markert M. (1996) Phosphorylation of myeloid-related proteins MRP-14 and MRP-8 during human neutrophil activation. Eur. J. Biochem. 241, 265–271 [DOI] [PubMed] [Google Scholar]
- 50.Devaraj S., Glaser N., Griffen S., Wang-Polagruto J., Miguelino E., Jialal I. (2006) Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55, 774–779 [DOI] [PubMed] [Google Scholar]
- 51.Vitorino R., Lobo M. J., Duarte J. R., Ferrer-Correia A. J., Domingues P. M., Amado F. M. (2004) Analysis of salivary peptides using HPLC-electrospray mass spectrometry. Biomed. Chromatogr. 18, 570–575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.