Abstract
Caveolae are organelles abundant in the plasma membrane of many specialized cells including endothelial cells (ECs), epithelial cells, and adipocytes, and in these cells, caveolin-1 (Cav-1) is the major coat protein essential for the formation of caveolae. To identify proteins that require Cav-1 for stable incorporation into membrane raft domains, a quantitative proteomics analysis using isobaric tagging for relative and absolute quantification was performed on rafts isolated from wild-type and Cav-1-deficient mice. In three independent experiments, 117 proteins were consistently identified in membrane rafts with the largest differences in the levels of Cav-2 and in the caveola regulatory proteins Cavin-1 and Cavin-2. Because the lung is highly enriched in ECs, we validated and characterized the role of the newly described protein Cavin-1 in several cardiovascular tissues and in ECs. Cavin-1 was highly expressed in ECs lining blood vessels and in cultured ECs. Knockdown of Cavin-1 reduced the levels of Cav-1 and -2 and weakly influenced the formation of high molecular weight oligomers containing Cav-1 and -2. Cavin-1 silencing enhanced basal nitric oxide release from ECs but blocked proangiogenic phenotypes such as EC proliferation, migration, and morphogenesis in vitro. Thus, these data support an important role of Cavin-1 as a regulator of caveola function in ECs.
Caveolae have been extensively studied and shown to play an important role in the regulation of many cellular functions including cell signaling, vesicular transport, and lipid metabolism. Caveolae are especially abundant in adipocytes, endothelial cells (ECs),1 fibroblasts, and smooth muscle cells (1–3), and there is evidence that the loss of caveola function may contribute to dyslipidemia, muscular dystrophy, cancer, diabetes, and cardiovascular diseases (1, 4–7). Caveolae are a subset of lipid rafts characterized by a particularly high content of cholesterol and sphingolipids that contain the protein caveolin. There are three isoforms of caveolin, caveolin-1 (Cav-1), Cav-2, and Cav-3, that have been extensively studied (1, 4). Cav-1 and Cav-3 are required for caveola formation in different tissues (8–10). Although Cav-2 is not required for caveola formation per se, (11) it regulates aspects of lung morphogenesis. Although the roles of caveolins in caveola formation are well known, the requirement of other proteins regulating caveolin function and thus caveola formation has been recently addressed (12–14).
To identify proteins that stably interact and are regulated by Cav-1, we used an unbiased proteomics approach using isobaric tagging for relative and absolute quantification (iTRAQ). iTRAQ is a powerful quantitative proteomics tool for the relative quantification of proteins in complex mixtures by mass spectrometry (15), and this technology has been successfully used for global comparison of protein expression in many cells systems and in different tissues (16, 17). As caveolae and Cav-1 are present in the endothelium lining all blood vessels of the body (18, 19) and play an important role in the regulation of several EC functions (8, 20–22), we examined the protein expression profile of proteins localized in detergent-resistant membranes (DRMs) prepared from the lungs of wild-type (WT) and Cav-1 KO mice via iTRAQ labeling and multidimensional LC and MS/MS analysis. Here, we show that Cav-1 was required for the appearance of Cav-2, polymerase I and transcript release factor (PTRF)/Cavin-1, and serum deprivation response protein (SDPR; also known as Cavin-2) in isolated DRMs from lungs, confirming the initial discovery of these proteins as regulators of Cav-1-dependent caveola function. Isolated as a functional complex in cells, Cavin-1 co-fractionated into DRMs with Cav-1 and -2 but Cavin-1 formed high molecular weight homo-oligomers separated from Cav-1 and Cav-2 using sedimentation velocity gradients. Knockdown of Cavin-1 in ECs affected several functions including nitric oxide production, migration, and morphogenesis. Thus, Cavin-1 has a critical role in regulating several aspects of caveola function in ECs.
EXPERIMENTAL PROCEDURES
Isolation of Detergent-insoluble Rafts from Lung Tissue
Membrane rafts were isolated as described previously (23) with minor modifications. Briefly, lungs from three mice (female, 8–10 weeks old) were isolated, and tissue was minced with scissors on ice and transferred into a tube. Lungs were adjusted up to a 3-ml volume with cold Mes-buffered saline (MBS; 25 mm Mes, pH 6.5, 150 mm NaCl) containing protease inhibitors and homogenized using a Dounce homogenizer. The homogenate was adjusted to 3.25 ml with MBS, and 3.25 ml of MBS containing 2% Triton X-100 was added and homogenized. The sample was rocked for 30 min at 4 °C. Next, the suspension was mixed with an equal volume of 85% sucrose in MBS. 2 ml of the 42.5% sucrose suspension was overlaid with 8 ml of 35% sucrose and 2 ml of 5% sucrose in an ultracentrifuge tube and centrifuged for 18 h at 35,000 rpm at 4 °C in an SW40 rotor. 12 fractions of 1 ml were collected from the top and mixed with SDS-PAGE loading buffer, and equal volumes were loaded, separated by SDS-12% PAGE, and Western blotted. For iTRAQ analysis, fractions 1 and 2 were pooled together, sucrose was diluted to ½ with cold water, and the pooled sample was split in SW28 rotor ultracentrifuge tubes and centrifuged for 90 min at 28,000 rpm at 4 °C. The pellets were pooled, precipitated with chloroform:methanol, and submitted to iTRAQ analysis.
Protein Digest, iTRAQ Labeling, and LC-MS Analysis
200 μg of protein pellets was dissolved in the solution buffer and reduced, and cysteines were blocked as described in the protocol of the iTRAQTM kit (Applied Biosystems). In total, three biological replicates were performed, and each 4-plex iTRAQ had an experimental (technical) replicate for both the wild-type and knock-out samples. Briefly, after overnight trypsin digestion, 100 μg of the samples was labeled with iTRAQ tags as follow: two WT samples with iTRAQ 114 and iTRAQ 115 reagents, respectively, and two Cav-1 KO samples with iTRAQ 116 and iTRAQ 117 reagents, respectively. Labeled samples were pooled and purified using a strong cation exchange column (Applied Biosystems) and separated into 20 fractions.
For QSTAR XL LC-MS/MS analysis, each cation exchange fraction was dried and resuspended in 10 μl of 0.1% formic acid in preparation for reverse phase LC with the LC Packings UltiMate work station, allowing us to preconcentrate the 10-μl samples on a Waters 5-mm C18 Symmetry 300 trap column. The individual peptides were then separated at a flow rate of 450 nl/min on an in-line 100-μm × 15-cm Waters Atlantis C18 column equilibrated with 0.5% acetic acid, 5% acetonitrile and eluted with a 60-min acetonitrile gradient. Data collection was performed by electrospray ionization of the eluent with data-dependent acquisition on an Applied Biosystems API QSTAR XL mass spectrometer.
Data Analysis and Statistics
Each of the QSTAR XL mass spectrometer spectra files (*.wiff) was processed with Mascot Distiller version 2.1, the resulting peak lists were combined, and the database was searched using Mascot Server 2.1. The search parameters included trypsin with one miss cleavage, static modifications carbamidomethyl (Cys) and iTRAQ reagents (N terminus and Lys), and variable modification oxidation (Met). Data analysis on the resulting LC-MS and MS/MS data sets was accomplished using a dual processor Dell 650 Workstation. The search results for each fraction were analyzed using the IPI mouse 3.27 database (released March 27, 2007 and contained 53,831 sequences). After Mascot analysis, PeptideProphet and ProteinProphet (Institute for Systems Biology) analyses (24, 25) were performed using the Trans-Proteomic Pipeline version 2.7 MIST Revision 2, Build 200601091318. PeptideProphet and ProteinProphet compute the probabilities for both individually searched peptides and the resulting proteins, respectively. The protein validation was performed using both PeptideProphet and ProteinProphet values such that a protein was validated if it had at least two top ranked peptides with each peptide probability score above 95% and above 94%, respectively (supplemental Fig. S1). The ProteinProphet probability cutoff corresponded to a 0.004, 0.003, and 0.003 false positive error rate for all three biological replicates (supplemental Table S1). Finally, all Trans-Proteomic Pipeline identifications were submitted to Yale Proteomics Expression Database web site for further user analysis.
iTRAQ quantitation and secondary protein identification were performed using the ParagonTM search algorithm (26) in the ProteinPilot 2.0 software. The IPI mouse 3.27 database (released March 27, 2007) was used using “thorough search.” The “iTRAQ 4-plex peptide-labeled” sample type and a “biological modification ID focus” were selected in the analysis method. Trypsin was selected as the digestion enzyme with cysteine alkylation by methyl methanethiosulfonate as a modification. Raw data that included, but were not limited to, reporter ion peak areas, reporter ion peak area error, peptide assignment, and confidence were exported from ProteinPilot (tab-delimited) without the ProteinPilot autobias correction applied (non-normalized data) so we could perform quantile normalization as described below. The tab-delimited ProteinPilot results were then uploaded into our Yale Proteomics Expression Database. For secondary protein identification, each protein had to have also been identified by Paragon and had to have two or more identified peptides and a ProteinPilot confidence score >2 (99% confidence level). To ensure that we only compared data that ProteinPilot includes in its quantitation analysis, peptides had to be classified as “used,” which requires the presence of an iTRAQ label and at least one valid iTRAQ reporter ion ratio. Additionally, ProteinPilot only uses high quality reporter ions for the peak area measurements to calculate iTRAQ peptide ratios. To remove low intensity reporter ion ratios from the exported raw data sets, we used the non-modifiable criterion of ProteinPilot that requires that valid iTRAQ reporter ion ratios must contain two iTRAQ reporter ions with a summed signal to noise ratio >9 to be included in the analysis. All raw data (mzXML), peak list (mgf), Mascot search results (dat), ProteinPilot search results, PeptideProphet (pepXML), and ProteinProphet (protXML) files are publicly available through http://yped.med.yale.edu/repository (access code, aSKezE).
The Yale Proteomics Expression Database has additional features, which enabled us to perform sample comparisons and Panther classification. Quantile normalization of the iTRAQ data was performed to correct the variation of the protein abundance using the statistical package R. Principal component analysis was used to examine the consistency of the technical replicates and biological replicates. Among 425 unique proteins observed in the experiments, there were 117 proteins identified in common through the experiments. A cutoff was set at 0.8 for down-regulated proteins and at 1.2 for up-regulated proteins as reported for other works (27, 28). Protein expression values from technical and biological replicates were analyzed under the analysis of variance model. Nitric oxide production was compared using t test and pairwise comparisons by the Mann-Whitney test. Effects on protein or gene expression were analyzed by analysis of variance, and comparisons between each experimental condition and the control were made by the confidence interval method. All values are presented as the mean ± S.E., and a p value of less than 0.05 was considered statistically significant. Calculations were performed using GraphPad Prism 4 software (San Diego, CA) or SigmaStat Statistical Analysis, System Version 1.00 (Jandel Corp., San Rafael, CA).
Antibodies
Mouse SDPR antibody was a kind gift from Prof. R. G. W. Anderson (University of Texas Southwestern Medical Center). The following antibodies were obtained from commercial sources: rabbit anti-caveolin (610060, BD Biosciences), mouse anti-caveolin 2 (610685, BD Biosciences), mouse anti-HSP90 (610419, BD Biosciences), mouse anti-endothelial nitric-oxide synthase (eNOS) (610297, BD Biosciences), mouse anti-PTRF (611259, BD Biosciences), rabbit anti-PTRF (A301-271A and A301-270A, Bethyl Laboratories), rabbit anti-phospho-eNOS (36-9100; Zymed Laboratories Inc.), rabbit anti-NOS (sc-653, Santa Cruz Biotechnology), rabbit anti-caveolin-1 (sc-894, Santa Cruz Biotechnology), mouse anti-caveolin-1 (NB 100-615, Novus Biologicals), and mouse anti-SDPR (H08436-B01, Novus Biologicals).
Cell Culture
COS-7, HEK293, and EAhy.926 were maintained in high glucose DMEM supplemented with 10% FBS; l-glutamine; antibiotics; and hypoxanthine, aminopterin, and thymidine supplement (EAhy.926) at 37 °C in a humidified atmosphere of 5% CO2. Human umbilical ECs (HUVECs) were maintained in M199 medium, and only passages 2–3 were used for experiments. Mouse lung endothelial cells isolated from WT, Cav-1 KO, Cav-1 RC, and Cav-1 transgenic mice were maintained in EBM-2 medium supplemented with EGM-2 MV SingleQuots.
Immunufluorescence Microscopy
After dissection, aortas (from 8-week-old mice) were fixed with 4% paraformaldehyde for 10 min at 4 °C and then dehydrated in 15% sucrose overnight at 4 °C. The vessels were then embedded in OCT (Sakura) and frozen. Serial 10-μm sections were blocked with 3% goat serum. Slides were incubated with either rabbit anti-Cavin-1 (Bethyl Laboratories) or rabbit anti-Cav-1 antibody (BD Biosciences) at a 1:100 dilution each at 4 °C overnight. Alexa Fluor 488 or 594 anti-rabbit IgG (Invitrogen) was used as the secondary antibody (1:250 dilution at room temperature for 1 h). For cells, COS-7 and EA.hy.926 cells grown on coverslips were fixed with 4% paraformaldehyde for 5 min, rinsed with PBS, permeabilized with 0.1% Triton X-100 for 10 min, washed with PBS, and blocked with 5% goat serum for 45 min at room temperature. Cells were incubated with the primary antibodies (diluted 1:200) overnight at 4 °C and washed twice with blocking solution followed by a 45-min incubation with appropriate secondary antibodies conjugated to immunofluorescent dye Alexa Fluor 488 or Alexa Fluor 594 (diluted 1:250) at room temperature. After washing three times, coverslips were mounted on slides with gelvatol/DAPI (Sigma-Aldrich) and analyzed with an epifluorescence microscope (Axiovert, Carl Zeiss MicroImaging, Inc.). Images were acquired using a charge-coupled device camera (Axio, Carl Zeiss MicroImaging, Inc.). Analysis of different images was performed using OpenLab software (Improvision) after subtracting background.
Real Time RT-PCR Analysis
Total RNA was extracted with TRIzol reagent using RNeasy columns (Qiagen). Reverse transcription was performed using 2 μg of total RNA using TaqMan reverse transcription reagents (Applied Biosystem), and quantitative PCR was performed using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer's protocol on an iCycler quantitative PCR analyzer (Bio-Rad).
siRNA, Plasmids, and Cell Transfection
The Cavin-1 target sequence against 5′-CAACTTTAAAGTCATGATCTA-3′ was obtained from Qiagen (high performance-guaranteed siRNA), and a scrambled siRNA was used as a negative nonsilencing control (NS) (5′-AATTCTCCGAACGTGTCACGT-3′). ECs at 50% confluence were transfected with RNAi construct (75 nm) using Oligofectamine (Invitrogen) according to the manufacturer's instructions for 8 h in Opti-MEM (Invitrogen) and then incubated in full medium for 48 h. A second 8 h of transfection was performed, and then either HUVEC or EAhy.926 growth medium was added for an additional 48 h. After reaching 40% confluence in a 6-well tissue culture plate, HEK293 or COS-7 cells were transfected with 1 μg of cDNA plasmid for 8 h with FuGENE 6 reagent in Opti-MEM. Then, medium was replaced with DMEM, 10% FBS for 36 h. cDNAs for human HA-tagged Cav-1 or Myc-tagged Cav-2 were constructed and subcloned in pcDNA3 vector (Invitrogen).
Non-detergent-based Fractionation and Sucrose Gradient
Cells (150-mm dish) were washed twice with PBS; scraped into 2 ml of ice-cold 500 mm sodium carbonate, pH 11 supplemented with 1 mg/ml protease inhibitor mixture (Roche Applied Science); homogenized using a Dounce homogenizer; and sonicated (three 20-s bursts at 30% maximal power). The homogenate was then adjusted to 42.5% sucrose by the addition of 2 ml of 85% sucrose prepared in 25 mm Mes, pH 6.5, 0.5 m NaCl and placed at the bottom of an ultracentrifuge tube. A 5–30% discontinuous sucrose gradient was formed (3 ml of 5% sucrose and 5 ml of 30% sucrose, both in Mes containing 250 mm sodium carbonate) and centrifuged at 35,000 rpm for 18 h in an SW40 rotor (Beckman Coulter). 12 gradient fractions (1 ml) were collected from the top and mixed with SDS-PAGE loading buffer, and equal volumes were loaded, separated by SDS-12% PAGE, and Western blotted. The percentage of total proteins in different fractions was determined by densitometry and plotted as percentage of total protein (NIH Image program).
Immunoprecipitation and Western Blot Analysis
Lysed cells were solubilized with either 60 mm octyl glucoside or 1% Triton X-100 for 1 h at 4 °C, and immunoprecipitation was performed on 500 μg of total cell protein using antibodies or nonspecific normal rabbit IgG (4 μg) for 2 h at 4 °C. Protein G-Sepharose (Sigma) was then added and incubated for 1 h. Beads were precipitated by centrifugation, and the supernatant was collected. The immune complexes were washed three times with immunoprecipitation buffer, and both the supernatants and immunoprecipitates were boiled in SDS sample buffer before resolving by SDS-PAGE and Western blotting using the antibodies described above.
Velocity Gradient Centrifugation and Cross-linking
Velocity Gradient Centrifugation
The influence of Cavin in caveolin oligomerization was analyzed as described for caveolin-1 (29) and caveolin-2 (30) with minor modifications. Briefly, sample were dissociated with 700 μl of MBS plus 60 mm n-octyl-β-d-glucopyranoside). Solubilized material was then loaded atop a 5–50% linear sucrose gradient (12 ml) and centrifuged at 42,000 rpm for 16 h in an SW40 rotor (Beckman Coulter). After centrifugation, 12 1-ml gradient fractions were collected from the top and mixed with SDS-PAGE loading buffer, and equal volumes were loaded, separated by SDS-12% PAGE, and Western blotted. Molecular mass standards for velocity gradient centrifugation were as follows: carbonic anhydrase (29 kDa), BSA (66 kDa), β-amylase (200 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa) (Sigma). The linear sucrose gradient was performed with 5 and 50% sucrose solutions prepared with MBS, 60 mm octyl glucoside using a Gradient Station ip (BioComp Instruments).
Chemical Cross-linking
Chemical cross-linking was performed in vivo on intact cells as described previously (31). Confluent cells were washed with KCl/HEPES buffer (90 mm KCl, 50 mm HEPES, pH 7.5) and incubated with DSP at a final concentration of 2 mm. After incubation for 30 min at room temperature, the reaction was terminated by adding 20 mm Tris-HCl, pH 8, 50 mm glycine. Cells were lysed followed by separation on a 4–20% continuous gradient precast gel (Invitrogen) and Western blot analysis.
NO Release Analysis
After 96 h of Cavin-1 silencing, the medium was removed, and the cells were supplemented with serum-free DMEM for 8 h. Nitrite (NO2−) was measured by an NO-specific chemiluminescence analyzer (Sievers) as described previously (32). NO release was normalized to protein levels, and values are expressed as μmol of nitrite/mg of cell protein.
Proliferation Assay
HUVECs were seeded in 12-well plates and treated with siRNA sequences as described above. At the indicated times, cells were trypsinized and counted using a hemocytometer. Viability was determined by trypan blue dye exclusion.
Cell Migration Assay
Cell migration was evaluated with a modified Boyden chamber (Corning). Transwell inserts were coated with a 0.1% gelatin solution at 4 °C overnight. After Cavin silencing, subconfluent ECs were serum-starved overnight and suspended in DMEM containing 0.1% FBS, and 100 μl of cell suspension containing 50 × 103 ECs was added to the upper chamber. The bottom chamber contained either 50 ng/ml VEGF, 200 nmol/liter sphingosine 1-phosphate, or 10% FBS prepared in DMEM. After 5 h of incubation at 37 °C, cells on both sides of the membrane were fixed and stained with the Diff-Quik staining kit (Baxter Health). Non-migrated cells were removed with a cotton swab, and the average number of migrated cells per field from five high power (×400) fields was determined by counting.
Tube Formation Assay
After Cavin-1 silencing, 60 × 103 ECs were seeded on top in a 24-well plate coated with growth factor-reduced Matrigel (BD Biosciences) in DMEM containing 0.1% FBS with or without 50 ng/ml VEGF or 200 nmol/liter sphingosine 1-phosphate. After 5 h of incubation at 37 °C, sprout length of capillary-like structures was quantified by measuring the mean cumulative tube length using OpenLab software (Improvision) in a computer-assisted microscope (Axiovert, Carl Zeiss MicroImaging, Inc.). Images were taken in five fields for each well, and each condition was performed in triplicate per experiment.
RESULTS
Quantitative Proteomics Examining Proteins in Lipid Rafts Isolated from WT and Cav-1 KO Lungs
To examine the proteins that interact with and stabilize caveolin-1, DRMs (or raft domains) were isolated using sucrose gradient fractionation from lung tissues prepared from WT and Cav-1 KO mice. Sucrose gradient centrifugation of raft domains (fractions 1 and 2 from the top of gradient reflecting glycolipid- and cholesterol-rich fractions) verified the distribution of Cav-1 and flotillin-1 in WT extracts and flotillin-1 in KO extracts (Fig. 1A, left panel). The raft fractions were pooled, and protein was precipitated with methanol:chloroform, reduced, alkylated, trypsinized, and labeled with iTRAQ reagents for analysis by LC-MS/MS. As variations in protein isolation, enzymatic digestion, and isotopic labeling can contribute to the variation in iTRAQ data, consistency of the protein abundance within an experiment or across experiments can be a concern. Three independent experiments using four different isotopic labeling reagents for each experiment were performed (supplemental Fig. S2A). Although the ratio of WT to KO obtained across experiments was highly correlated (r2 = 0.909), some variation within an experiment and across experiments was observed (supplemental Fig. S2B), and quantile normalization was performed to minimize variation. As depicted in supplemental Fig. S2C, the alignment of the protein abundance for KOs labeled with 116 reagent and 117 reagent within an experiment was improved after normalization. Principal component analysis was performed to examine the clustering patterns of the technical replicates and biological replicates (supplemental Fig. S2D). 70.99% of the data were explained by the first component, and 15.51% of the data were explained by the second component. Actin γ cytoplasmic 1 with a score of −9.29 and probable phospholipid-transporting ATPase IA with a score of 5.04 are the top two proteins driving the separation along the first principal component. Although the variation due to protein isolation and enzymatic digestion still exists, the variation in isotopic labeling was corrected.
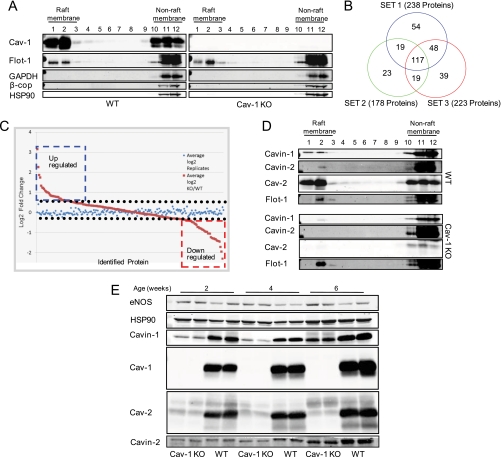
Fig. 1.
iTRAQ-based approach to quantitative analysis of lung proteins within caveolar membrane rafts. A, mouse lung membrane raft fractions from caveolin-1 KO and WT animals were isolated by solubilization by Triton X-100 at 4 °C, and after centrifugation, 12 sucrose gradient fractions were collected and analyzed by immunoblotting. Raft fractions 1 and 2 were labeled with iTRAQ reagents and analyzed by LC-MS/MS. B, number of proteins identified in lung DRMs of WT and Cav-1 KO mice. Three sets of experiments were performed each in duplicate, and common proteins were analyzed for quantitative differences. C, log2-transformed values of the normalized data from the ratio of WT/KO and the internal replicate illustrated for the means of the relative protein expression levels. D, Western blot confirmation of proteins levels reduced in raft fractions of Cav-1 KO mouse lung (fractions 1 and 2). Flotillin-1 (Flot-1) was used as raft protein marker. E, lung tissue lysate from Cav-1 KO animals of different ages were separated by SDS-PAGE and analyzed by immunoblotting for caveolins and Cavin levels.
After protein identification and relative quantification using the criteria (described under “Experimental Procedures”), around 200 proteins were identified from each of the three sets of independent experiments performed (238, 178, and 223 proteins from experiments 1–3, respectively). Protein lists generated from the three experiments were combined and analyzed, and 117 proteins were in common in all experiments (Fig. 1B). For the 117 proteins common to the three DRM iTRAQ replicates, log2-transformed values of the normalized data from the ratio of WT/KO and the internal replicate were determined and are illustrated for the means of the relative protein expression levels in Fig. 1C. Statistical analysis of the relative expression of these proteins was performed, and the -fold change was calculated. The ratio of average expression in KO and in WT was defined as -fold changes. The average expression in KO and in WT was calculated as the average of mean expression of the technical replicates across the biological replicates. These resulted in a list of 61 proteins that showed marked changes in lipid rafts isolated from WT versus Cav-1 KO mice (p < 0.05) as listed in Tables I and II. Additionally, 56 proteins showed no significant changes (supplemental Table S2) in WT versus Cav-1 KO, suggesting that enrichment of these proteins is not dependent on the absence or presence of Cav-1. However, we have to consider that some of these proteins may be contaminants that partially copurify with DRM/raft preparations (33, 34) such as electron transport proteins, hemoglobins, and histones proteins. To confirm the iTRAQ results, we performed semiquantitative Western blotting on certain proteins observed to be reduced in caveola/lipid raft fractions from Cav-1 KO mice and rationalized based on the literature. As seen in Fig. 1D, the levels of Cavin-1, SDPR (also known as Cavin-2), and Cav-2 (a known interacting protein stabilized by Cav-1) were reduced in raft fractions from Cav-1 KO mice in accordance with our proteomics data set (Table I). The loss of Cavin-1 and -2 are consistent with data suggesting that these proteins regulate aspects of caveola formation (12–14, 35, 36). As seen Fig. 1E, Cavin-1, Cavin-2, and Cav-2 levels were reduced in total cell lysates prepared from lung tissue, supporting the idea that the reduction of Cavins and Cav-2 in raft fractions is due to destabilization of the protein complex.
Table I. Quantitative analysis of proteins reduced in lung membrane rafts of caveolin-1 KO versus WT mice.
-Fold change reflects data from three independent iTRAQ experiments. Proteins in bold font were confirmed by post hoc Western blotting. Each protein was identified in three separate biological replicates and by the Paragon and Mascot database search engines using the IPI mouse database. False discovery rates were determined by running PeptideProphet and ProteinProphet analyses on Mascot results. Raw data and data results are available at http://yped.med.yale.edu/repository (access code, aSKezE).
| Protein ID | Protein name | No. of distinct peptides in ratios | ProteinProphet score | ProteinPilot protein score | -Fold change | Grp |
|---|---|---|---|---|---|---|
| IPI00125832 | Caveolin-2 | 6 | 1 | 2 | 0.17340 | 0.005515 |
| IPI00117689 | Polymerase I and transcript release factor (Cavin-1) | 14 | 1 | 22.38 | 0.242523 | 1.23e−09 |
| IPI00400016 | Laminin γ-1 chain precursor | 27 | 1 | 56.25 | 0.332421 | 1.53e−07 |
| IPI00135660 | Serum deprivation response protein (SDPR, Cavin-2) | 14 | 1 | 23.34 | 0.356752 | 1.54e−08 |
| IPI00338452 | Collagen a-2(IV) chain precursor | 27 | 1 | 53.35 | 0.404912 | 8.81e−09 |
| IPI00119065 | Laminin β-2 chain precursor | 22 | 1 | 47.94 | 0.42949 | 7.08e−09 |
| IPI00117115 | Laminin γ-2 chain precursor | 9 | 1 | 17.78 | 0.439639 | 1.97e−05 |
| IPI00649506 | POEM-nephronectin | 11 | 1 | 20.88 | 0.460032 | 7.16e−07 |
| IPI00229542 | Histone protein Hist1h2aa | 5 | 1 | 11.41 | 0.487956 | 9.90e−07 |
| IPI00339885 | Collagen a-1(VI) chain precursor | 7 | 1 | 16.15 | 0.520209 | 2.22e−05 |
| IPI00222188 | Procollagen, type I, a2 | 50 | 1 | 80.49 | 0.541152 | 3.40e−06 |
| IPI00223713 | Histone H1.2 | 4 | 1 | 7.98 | 0.57921 | 6.42e−06 |
| IPI00329872 | Isoform 1 of collagen a-1(I) chain precursor | 58 | 1 | 102.35 | 0.58872 | 4.39e−07 |
| IPI00230133 | Histone H1.5 | 3 | 1 | 9.13 | 0.605466 | 2.29e−05 |
| IPI00753139 | AHNAK | 2 | 1 | 2.13 | 0.626105 | 0.003095 |
| IPI00223714 | Histone H1.4 | 4 | 1 | 24.08 | 0.63929 | 4.29e−05 |
| IPI00553798 | AHNAK nucleoprotein isoform 1 | 263 | 1 | 419.45 | 0.64112 | 0.002718 |
| IPI00230702 | Icam1 | 2 | 0.8 | 4.34 | 0.66573 | 0.047698 |
| IPI00338785 | Laminin B1 subunit 1 | 7 | 0.94 | 8.94 | 0.66879 | 0.047449 |
| IPI00421223 | Tropomyosin a-4 chain | 7 | 1 | 11.81 | 0.66896 | 0.007480 |
| IPI00474554 | MfAP4 | 3 | 1 | 2.66 | 0.69693 | 2.41e−05 |
| IPI00227299 | Vimentin | 29 | 1 | 69.7 | 0.722867 | 1.90e−05 |
| IPI00229703 | Vesicle-associated membrane protein 2 | 2 | 1 | 7.77 | 0.733982 | 0.0001236 |
| IPI00135971 | Tight junction protein ZO-1 | 23 | 1 | 38.27 | 0.740322 | 0.0319772 |
| IPI00130102 | Desmin | 9 | 1 | 20.79 | 0.786906 | 0.0003938 |
Table II. Quantitative analysis of proteins increased in lung membrane rafts of caveolin-1 KO versus WT mice.
-Fold change reflects data from three independent experiments. Each protein was identified independently both by Paragon and Mascot database search engines using the IPI mouse database. False discovery rates were determined by running PeptideProphet and ProteinProphet analyses on Mascot results. Raw data and data results are available at http://yped.med.yale.edu/repository (access code, aSKezE).
| Protein ID | Protein name | No. of distinct peptides in ratios | ProteinProphet score | ProteinPilot protein score | -Fold change | Grp |
|---|---|---|---|---|---|---|
| IPI00323230 | Spectrin a chain | 6 | 1 | 218.04 | 1.214358 | 1.14e−08 |
| IPI00307966 | ADP-ribosyl cyclase 1 | 11 | 1 | 17.07 | 1.215031 | 1.63e−07 |
| IPI00117735 | Myelin P0 protein precursor | 6 | 1 | 6.48 | 1.221400 | 0.000288 |
| IPI00679092 | Similar to spectrin a 1 | 5 | 1 | 8.36 | 1.225561 | 1.75e−08 |
| IPI00131176 | Cytochrome c oxidase subunit 2 | 4 | 1 | 8.56 | 1.243182 | 0.003629 |
| IPI00109727 | Thy-1 membrane glycoprotein precursor | 3 | 1 | 6 | 1.261946 | 0.000248 |
| IPI00123746 | Cadherin-13 precursor | 10 | 1 | 19.23 | 1.317579 | 0.000150 |
| IPI00223047 | Cytoskeleton-associated protein 4 | 13 | 1 | 20.8 | 1.335605 | 2.63e−06 |
| IPI00120719 | Cytochrome c oxidase, subunit Va | 20 | 1 | 32.69 | 1.38002 | 1.03e−05 |
| IPI00131695 | Serum albumin precursor | 27 | 1 | 51.18 | 1.385615 | 9.60e−09 |
| IPI00121550 | Na+/K+-transporting ATPase subunit β-1 | 5 | 1 | 10.26 | 1.39774 | 0.001573 |
| IPI00116154 | Cytochrome c oxidase, subunit Vb | 7 | 1 | 14 | 1.42061 | 1.64e−07 |
| IPI00128642 | Lung carbonyl reductase | 5 | 1 | 12.19 | 1.446933 | 9.37e−06 |
| IPI00133956 | Gpihbp1 | 2 | 1 | 3.1 | 1.453672 | 2.84e−05 |
| IPI00225390 | Cytochrome c oxidase subunit VIb isoform 1 | 8 | 1 | 16.44 | 1.460724 | 6.27e−07 |
| IPI00170093 | Ndufs8 | 5 | 1 | 5.73 | 1.489794 | 2.96e−05 |
| IPI00121209 | Apolipoprotein A-I precursor | 25 | 1 | 26.07 | 1.407229 | 2.56e−05 |
| IPI00121319 | Cysteine-rich protein 2 | 4 | 0.94 | 5.55 | 1.513123 | 0.000772 |
| IPI00133562 | Chchd3 | 10 | 1 | 16.1 | 1.533374 | 3.45e−07 |
| IPI00230540 | VDAC1 | 7 | 1 | 15.4 | 1.544335 | 2.38e−08 |
| IPI00133006 | Acyl carrier protein | 6 | 1 | 10.28 | 1.562844 | 2.72e−05 |
| IPI00121288 | Ndufb10 | 2 | 0.97 | 4.47 | 1.641092 | 2.13e−05 |
| IPI00126208 | Hemoglobin, β adult major chain | 3 | 1 | 14.03 | 1.66103 | 8.25e−09 |
| IPI00315480 | Surfactant-associated protein A | 13 | 1 | 20.97 | 1.661763 | 4.56e−05 |
| IPI00132474 | Itgb1 | 8 | 1 | 14.96 | 1.663839 | 5.88e−07 |
| IPI00311682 | Atp1a1 | 13 | 1 | 22.67 | 1.727181 | 1.71e−05 |
| IPI00229008 | Ndufs4 | 6 | 1 | 11.07 | 1.81813 | 8.10e−09 |
| IPI00115522 | Fibrinogen, a polypeptide | 3 | 0.94 | 5.53 | 1.915084 | 4.08e−06 |
| IPI00119618 | Calnexin precursor | 10 | 1 | 20.67 | 2.138918 | 0.000461 |
| IPI00316491 | Hemoglobin β-2 subunit | 6 | 1 | 29.92 | 2.096561 | 3.92e−11 |
| IPI00110658 | Hemoglobin, β adult major chain | 3 | 1 | 18.65 | 2.17418 | 5.65e−09 |
| IPI00230113 | Cytochrome b5 | 6 | 1 | 10.33 | 2.204051 | 1.93e−11 |
| IPI00469114 | Hemoglobin a subunit | 3 | 1 | 2.36 | 2.230882 | 8.95e−09 |
| IPI00387318 | Tmem30a | 3 | 1 | 5.15 | 3.114185 | 5.10e−08 |
| IPI00623553 | ATP synthase D chain, mitochondrial | 10 | 1 | 16.4 | 3.389328 | 7.95e−10 |
| IPI00555000 | Uqcrb | 8 | 1 | 10.79 | 4.012617 | 1.58e−09 |
Cavin-1 Is Enriched in Blood Vessels and Endothelial Cells and Is Regulated by Cav-1
Cav-1 in blood vessels is critical for mechanosensing (22) and vascular function (20, 37); thus, we examined the localization of Cavin-1 in intact mouse aorta and cultured ECs. As seen in Fig. 2A, using immunofluorescence microscopy, Cavin-1 was highly expressed in all layers of the blood vessel (top panel), and the loss of Cav-1 reduced immunoreactive Cavin-1 throughout the vessel (bottom panel). To examine the relationship between Cav-1 and Cavin-1 levels in more detail, we examined the protein levels in heart, lung, and aortic extracts from WT, Cav-1 KO, and Cav-1 RC mice. Cav-1 RC is a strain of mice in which global Cav-1 KO mice were reconstituted with an endothelium-specific transgene of Cav-1 (20, 22, 38). As seen in Fig. 2B, the loss of Cav-1 markedly diminished Cav-2 levels and partially reduced Cavin-1 levels in all tissues examined. Re-expression of Cav-1 in ECs (Cav-1 RC) dramatically rescued Cav-2 in the lung and aorta and to a lesser extent in the heart while modestly increasing Cavin-1 levels in lung and aorta but not in the heart. Because lungs have the highest abundance of ECs in the body, the re-expression of Cav-1 in ECs stabilizes Cav-2 and Cavin-1 best in this tissue followed by the aorta. The lack of significant rescue in the heart is likely due to the expression of Cav-1/Cavin-1 in cardiac fibroblasts and the high abundance of Cav-3/Cavin-1 in cardiac myocytes, the primary cell type in the heart, where the re-expression of Cav-1 in ECs would not be expected to rescue the loss of Cavin-1 in cardiac fibroblasts or myocytes (39).
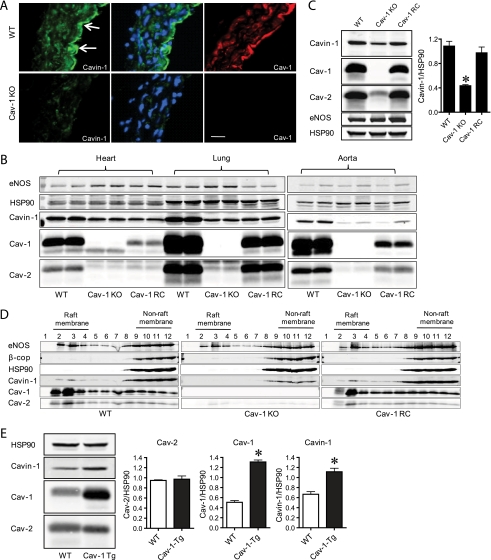
Fig. 2.
Cavin-1 levels are regulated by Cav-1 in endothelium. A, Cavin-1 is found in intact blood vessels. Representative immunofluorescence analysis of serial cross-sections (10 μm) from the mouse aorta stained for Cavin-1 (green), nuclei (DAPI; blue), and Cav-1 (red) in vessels from WT and Cav-1 KO mice is shown. The scale bar represents 100 μm. B, levels of eNOS, Hsp90 (loading control), caveolin-1 and -2, and Cavin-1 proteins in heart, lung, and aortic extracts from WT, Cav-1 KO, and Cav-1 RC mice. C, protein levels of Cavin-1 and caveolins in ECs isolated from WT, Cav-1 KO, and Cav-1 RC mice as quantified in the graph on right inset. D, Cavin-1 and caveolin localization to raft membrane fractions analyzed by sucrose gradient fractionation of ECs isolated from WT, Cav-1 KO, and Cav-1 RC mice. β-cop and HSP90 were used as markers for non-raft fractions. E, Cavin-1 and caveolin levels in ECs isolated from control (WT) and transgenic mice overexpressing Cav-1 (Cav-1 Tg) in the endothelium. Data are the mean ± S.E. of three independent experiments. *, p < 0.05 versus control (WT).
To examine the effects of Cav-1 on Cav-2 and Cavin-1 levels in a cell that expresses all three proteins, lung ECs from the three strains of mice were cultured. Similar to data in lung lysates, the loss of Cav-1 markedly reduced Cav-2 levels and partially reduced Cavin-1 levels (by ≈60%; see inset graph in Fig. 2C). Again, reconstitution of Cav-1 strongly enhanced the levels of Cav-2 and partially enhanced Cavin-1 levels (Fig. 2C). These data suggest that the stability of Cavin-1 in cells may be regulated differently than that of the Cav-1·Cav-2 complex perhaps due to independent trafficking of caveolins versus Cavin in cells, resulting in less than stoichiometric interactions of Cavin-1 with the caveolins.
Next, we determined the distribution of Cavin-1 in isolated ECs using sodium carbonate lysis and a discontinuous sucrose gradient to separate buoyant membranes (enriched in Cav-1; fractions 2–4) from bulk cellular proteins (fractions 8–12). As depicted in Fig. 2D (top panel), Cavin-1 was present in raft (fractions 2 and 3) and non-raft fractions (8–12) and co-distributed with Cav-1, Cav-2, and eNOS. The presence of caveolins and Cavin-1 in non-raft membranes (Figs. 1D and 2D) likely reflects the distribution of these proteins in domains distinct from rafts membranes. These proteins have also been localized to Cav-1-positive endocytic vesicles (40) and newly assembled caveolins en route to the plasma membrane (41), and Cavin-1 may also be found in other subcellular fractions (42). The loss of Cav-1 (Cav-1 KO; middle panel) reduced Cavin-1 and Cav-2 levels, whereas eNOS remained unchanged. As can be seen in Figs. 1D and 2D, Cav-2 was drastically reduced in both raft and non-raft fractions, whereas Cavin-1 protein levels were mainly reduced in raft fractions relative to non-raft fractions. Reconstitution of Cav-1 (Cav-1 RC; bottom panel) increased the recovery of Cavin-1 and Cav-2 in light membranes, indicating that Cavin-1 is a membrane raft constituent that colocalizes with Cav-1 and Cav-2 in ECs.
Finally, to examine whether Cav-1 overexpression (on a WT background) in ECs increases Cavin-1 levels, ECs from mice that transgenically overexpress Cav-1 in ECs (43) were isolated and assayed for the levels of Cavin-1 and caveolins. Transgenic overexpression of Cav-1 increased basal levels of Cavin-1 but not caveolin-2 levels (Fig. 2E).
Role of Cavin-1 on Cav-1 and Cav-2 in ECs
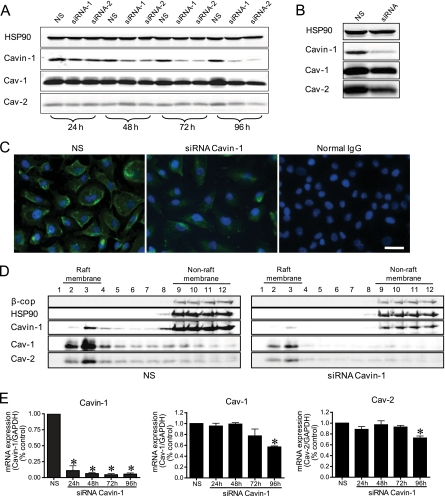
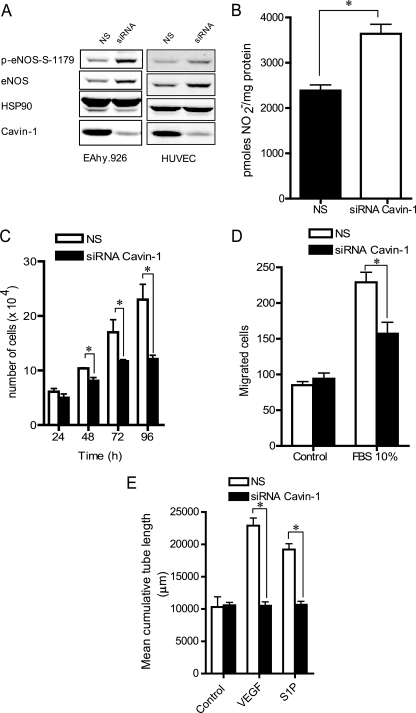
Cavin-1 is a critical component of caveolae, and reducing its levels decreases global caveolin levels and caveola formation in all tissues examined to date (12–14); however, the role of Cavin-1 in ECs has not been examined. To test the functions of Cavin-1 in ECs, Cavin-1 levels were suppressed using RNAi. Two different RNAi sequences were initially tested in EAhy.926 cells (a human EC line that has ample Cav-1 and caveolae by EM). A time course of Cavin-1 silencing showed a reduction in Cavin-1 expression at 24 h followed by a reduction in Cav-1 and Cav-2 protein levels at a later time point (96 h; Fig. 3, A and B). siRNA sequence 2 (siRNA-2) reduced Cavin-1 by ≈75% in primary cultures of HUVECs (Fig. 3B) and by ≈60% in primary bovine aortic endothelial cells (supplemental Fig. S3A, bottom panel). These data suggest that a kinetic lag exists between the loss of Cavin-1 and quantitative changes in Cav-1/Cav-2 levels and that the changes in caveolins due to the loss of Cavin-1 vary in different EC lines. The reduction in Cavin-1 levels were clearly observable by immunofluorescence microscopy in ECs (Fig. 3C). As expected, using sucrose fractionation, the loss of Cavin-1 also reduced its levels in buoyant membranes and the levels of Cav-1 and -2 (Fig. 3D) but to a lesser extent than observed for other cells types such as NIH 3T3 fibroblasts (12, 14). After knockdown of Cavin-1, the reduction of Cav-1 and Cav-2 levels was mainly observed in the plasma membrane as imaged via immunofluorescence microscopy (supplemental Fig. S3B). The reduction in Cavin-1 mRNA levels by RNAi also reduced Cav-1 mRNA and to a lesser extent Cav-2 mRNA levels at later time points (Fig. 3E). It should be noted that the levels of Cavin-1 mRNA levels decreased dramatically in the first 24 h after siRNA treatment, whereas the reduction in protein levels were much slower, suggesting that Cavin-1 has a long biosynthetic half-life. As previously shown for adipocytes (14), Cav-1 colocalized with Cavin-1 in the plasma membrane of ECs (supplemental Fig. S3C).
Fig. 3.
Characterization of Cavin-1 silencing in human ECs. Cavin-1 silencing in Eahy926 cells (A) and HUVECs (B). Cells were transfected with NS or with two independent siRNAs for Cavin-1 (siRNA-1 or siRNA-2), and at the indicated time points, protein levels were evaluated by immunoblotting. C, immunofluorescence microscopy of Cavin-1 (green) in Eahy926 cells in the absence or presence of Cavin-1 siRNA treatment. Blue reflects DAPI staining of nuclei, and the scale bar represent 25 μm. D, raft distribution of Cavin-1 and caveolins in control and Cavin-1-silenced Eahy926 cells. β-cop and HSP90 were used as markers for non-raft fractions. E, time course of mRNA expression of Cavin-1 and caveolins during Cavin-1 silencing. Gene expression data are expressed taking the control as reference (equal to 1). Values are the mean ± S.E. of three independent experiments. *, p < 0.05 (comparisons versus NS).
Next, we examined whether Cavin-1 existed in a protein complex with caveolins via co-immunoprecipitation (IP) experiments. As shown in supplemental Fig. S3D, IP of either Cav-1 or Cav-2 from EC lysates solubilized in β-octyl glucoside buffer resulted in weak co-immunoprecipitation of Cavin-1 compared with IgG control antibody. Longer exposure of the membrane showed the presence of the band of Cav-1 in the Cavin-1 IP. Using a 1% Triton X-100 buffer, the complex of Cavin-1, Cav-1, and Cav-2 appeared to be better stabilized. To decipher whether this interaction is direct or indirect, we used a recombinant GST-Cav-1 as bait for in vitro binding (44). Supplemental Fig. S3E shows the expression of GST and several distinct modules of GST-Cav-1 (amino acids 1–61, 61–101, 135–178, and full length 1–178). Incubation of EC lysates with these fusion proteins demonstrated that GST-Cav-1(135–178) was sufficient to interact with endogenous Cav-1, and GST-Cav-1(1–178) could interact with Cav-1 and Cav-2, but no interactions were observed with Cavin-1 under these conditions (supplemental Fig. S3F). Overall, these data confirm previous data showing that the interaction of Cavin-1 with caveolins is likely indirect or dependent on caveola structure (14).
Cavin-1 Exists as a Unique Oligomer but Does Not Sediment with Cav-1 and Cav-2
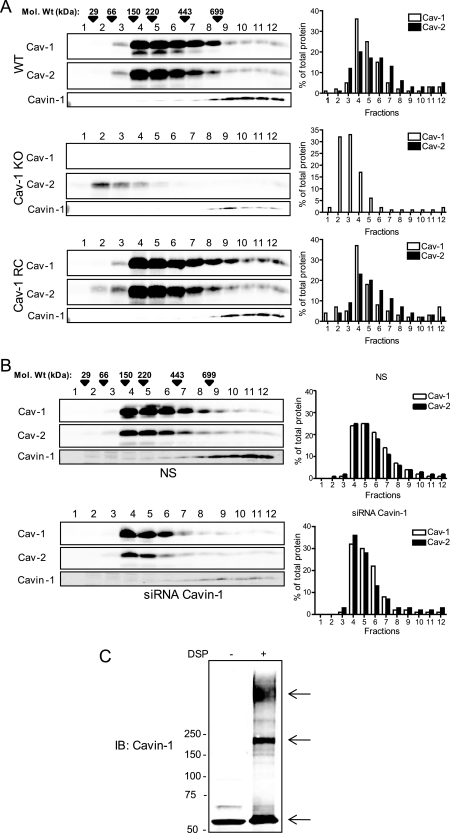
It is well recognized that Cav-1 exists as high molecular mass homo-oligomers of ≈200–350 kDa (29), and Cav-1 is necessary for the hetero-oligomerization of Cav-2 within the isolated complex (44, 45). To examine whether Cavin-1 is part of this oligomeric complex, we performed velocity sedimentation gradients using a 5–50% linear sucrose gradient and assessed the flotation of Cav-1, Cav-2, and Cavin-1 using lysates from WT, Cav-1 KO, and Cav-1 RC ECs. As seen in Fig. 4A, oligomeric Cav-1 and Cav-2 complexes were enriched in high molecular mass fractions between 150 and 220 kDa. Surprisingly, Cavin-1 was virtually undetectable in these fractions but resided in a high molecular mass complex of greater than 669 kDa. Because the predicted molecular mass of Cavin-1 is 60 kDa, >10 monomers of Cavin-1 are suggested to exist in the complex, but the complex did not co-sediment with Cav-1 and Cav-2. In the absence of Cav-1, the levels of Cav-2 and Cavin-1 were reduced, but the residual Cav-2 was left shifted (Fig. 4A, see graph on the right), whereas the oligomerization of Cavin-1 was intact. However, reconstitution of Cav-1 in the Cav-1 KO background rescued this phenotype.
Fig. 4.
Oligomerization of caveolins and Cavin-1. The oligomerization state of caveolins and Cavin-1 was examined by velocity centrifugation gradients (calibrated with known molecular mass markers depicted by arrowheads) in extracts from WT (top panel), Cav-1 KO (middle panel), and Cav-1 RC (bottom panel) ECs. The graph on the right denotes the distribution of Cav-1 (white bars) and Cav-2 (black bars) in the gradient. B, Cavin-1 silencing weakly reduces high molecular mass caveolin oligomers in human ECs. C, in situ oligomerization of Cavin-1 by chemical cross-linking with DSP in intact cells. Arrows indicate Cavin-1-containing oligomers or protein complexes. IB, immunoblot.
Next, we examined whether Cavin-1 modulates the oligomerization of Cav-1 and Cav-2 in ECs. siRNA knockdown of Cavin-1 (Fig. 4B, bottom panels) weakly reduced high molecular mass Cav-1 and Cav-2 oligomers (from fraction 6 onward) as compared with NS (Fig. 4B, top panels); however, the functional relevance of this is not known. To independently test the possible role of Cavin-1 in caveolin oligomerization, reconstitution experiments were performed in HEK293 cells, which exhibit very low levels of Cav-1, Cav-2, and Cavin (supplemental Fig. S4A). Transfection of Cav-1 alone resulted in homo-oligomer formation (corresponding to molecular masses of at least 220 kDa), whereas transfection of Cav-2 alone resulted in the formation of lower molecular weight complexes (supplemental Fig. S4B, left panel, middle). As previously shown, co-expression of Cav-1 and -2 resulted in the incorporation of Cav-2 into high molecular weight oligomers (supplemental Fig. S4B, left panel, bottom). Transfection of Cavin-1 alone resulted in the formation of high molecular weight homo-oligomers, similar to the finding in cells with endogenously expressed Cavin (supplemental Fig. S4B, right panel, top). However, co-expression of Cav-1, Cav-2, and Cavin-1 together weakly increased the amount of high molecular weight oligomeric Cav-1 and Cav-2 (supplemental Fig. S4B, right panel, bottom) compared with that of Cav-1 and Cav-2 alone. It has been shown that Cavin-1 is associated to caveolae after caveolins arrive to the plasma membrane as a preassembled complex, and it is possible that Cavin may stabilize caveolin oligomers in the context of the caveola structure (46). These results are consistent with the slight reduction in the high molecular mass oligomeric Cav-1·Cav-2 complex after Cavin-1 silencing in ECs (Fig. 4B). Moreover, although Cav-2 alone formed lower molecular weight complexes, co-expression with Cavin-1 (supplemental Fig. S4B, right panel, middle) did not result in the incorporation of Cav-2 into high molecular weight oligomers. To confirm the latter findings, HEK923 cells transfected with either Cav-1, Cav-2, or Cavin were analyzed by confocal imaging. As previously shown, transfection with Cav-1 or Cav-2 showed that Cav-1 was localized both in the plasma membrane and perinuclear region, whereas Cav-2 was localized exclusively in the perinuclear region (supplemental Fig. S4C, left panels). Co-transfection of Cav-1 and Cav-2 caused a redistribution of Cav-2 from the perinuclear region to the plasma membrane (supplemental Fig. S4C, third panel), whereas co-transfection of Cavin-1 with Cav-2 did not influence Cav-2 targeting (supplemental Fig. 4C, right panel).
Interestingly, in all of the above experiments using velocity gradients, Cavin-1 was detected in higher order homo-oligomers in the absence or presence of caveolins. To test whether oligomeric Cavin-1 exists in intact cells, a cell-permeable chemical cross-linking approach was used as described previously (31). DSP is a membrane-permeable cross-linker with an arm length of 12 Å that stabilizes high molecular weight oligomers; it can be analyzed by SDS-PAGE and detected by Western blotting. As shown in Fig. 4C, in the absence of DSP, Cavin ran as a 60-kDa protein on SDS-PAGE, whereas in the presence of DSP, different higher order species of Cavin-1 were found. The band at ∼180 kDa is consistent with at least three monomers of Cavin-1, whereas the molecular masses of the uppers bands are not discernible.
Cavin-1 Regulates Several EC Phenotypes
eNOS is negatively regulated via an interaction with Cav-1 (47, 48). To evaluate the functional role of Cavin-1 silencing in ECs, eNOS levels and NO release were evaluated. Knockdown of Cavin-1 increased eNOS protein levels and its phosphorylation on Ser-1177 (Fig. 5A) in EAhy.926 cells and HUVECs. The increase in eNOS levels was accompanied by a trend of increasing eNOS mRNA levels as detected by quantitative PCR (supplemental Fig. S5A) and a ≈40% increase in NO levels (measured as NO2−) in the medium (Fig. 5B).
Fig. 5.
Cavin-1 regulates several endothelial cell phenotypes. A, Cavin-1 silencing (96 h) increases the levels and phosphorylation of eNOS at serine 1177 (p-eNOS-S-1177) in Eahy926 cells and HUVECs. Blots are representative of different experiments with similar results. HSP90 is used as a loading control. B, loss of Cavin-1 enhances basal nitric oxide release from Eahy926 cells. The total nitrite accumulation was quantified over 8 h. Data are the mean ± S.E. of three independent experiments performed in triplicate. *, p < 0.05 versus control (NS). Cavin-1 silencing reduces cell proliferation (C), migration (D), and tube formation (E) in ECs. Data are the mean ± S.E. of three independent experiments performed in quadruplicate (comparisons versus control (NS) at each time point). *, p < 0.05 versus control (NS). S1P, sphingosine 1-phosphate.
In addition to an effect on eNOS, Cav-1 is important for other aspects of angiogenesis including cell proliferation, migration, and morphogenesis, functions important for angiogenesis in vivo. Knockdown of Cavin-1 reduced cell growth, migration, and growth factor-induced tube formation in response to VEGF and sphingosine 1-phosphate (Fig. 5, C–E). Collectively, these data indicate that Cavin-1 is necessary for regulation of eNOS function and several angiogenic phenotypes in vitro.
DISCUSSION
The central purpose of this study was to identify proteins that are co-isolated with or require an interaction with endogenous Cav-1 for their fractionation into DRMs or lipid rafts. To this end, we performed a quantitative comparison of proteins isolated in lipid raft domains in the presence or absence of endogenous Cav-1 using iTRAQ analysis. Up to 290 proteins were identified, some of which have been identified previously in rafts or DRMs of cultured ECs or other tissues (12, 16, 49–50). After establishing stringent criterion for filtering the peptide data, statistical analysis was performed with 117 proteins reproducibly recovered in DRMs from three independent experiments. 25 proteins were significantly reduced, and 36 proteins were found to be increased in DRMs isolated from Cav-1 KO mice compared with WT mice, suggesting that the loss of Cav-1 reduces the partitioning and/or expression of some proteins while increasing the enrichment/expression of others in isolated raft domains. However, as biochemical isolation of membrane microdomains typically never yields an absolutely homogenous preparation (33), we could not discard copurifying contaminants during the DRM isolation such as those from the mitochondrial complex (34, 51). From a biological interest perspective, we validated certain proteins that were reduced following the loss of Cav-1, and our results confirmed the identification of Cavin-1, Cavin-2, and Cav-2 as Cav-1-interacting proteins in vivo.
It is well appreciated that Cav-1 and Cav-3 are necessary for caveola formation in mammalian cells. However, recent data suggest that certain accessory molecules are necessary for stabilizing Cav-1 or Cav-3 functions and to form a caveola in specific tissues. Four proteins recently termed Cavins, Cavin-1 (also known as PTRF and Cav pp60), Cavin-2 (SDPR), Cavin-3 (protein kinase C δ-binding protein or SRBC), and Cavin-4 (PTRF/SDPR family protein or MURC), have been shown to interact with each other and require caveolins for membrane association (35, 52). Very recently, in the context of Cavin-1, Cavin-2, and Cav-1, the loss of either Cavin-1 or Cavin-2 was shown to reduce the levels of Cav-1, and overexpression of Cavin-2 is sufficient to stabilize the ternary complex and promote caveola function (35). Cavin-1 can directly interact with Cavin-2 in vitro and can form immunocomplexes with Cav-1. Our data support this idea because Cavin-1 co-precipitated with both Cav-1 and Cav-2, and the interaction with Cav-1 is likely indirect perhaps via Cavin-2 (35). In our analysis of isolated DRMs from WT and Cav-1 KO mice, only Cavin-1 and Cavin-2 were found to be significantly reduced in Cav-1 KO mice via iTRAQ analysis and semiquantitative Western blotting, thus providing a salient example of the interaction of endogenous Cav-1 with these accessory proteins in vivo. To better understand the interactions of caveolins with Cavin-1, velocity sedimentation analysis and cross-linking studies were performed. As expected for Cav-1 and Cav-2, they existed as higher order oligomers (>150 kDa), but unexpectedly, Cavin-1 sedimented as a high molecular mass oligomer in the absence or presence of endogenous Cav-1. The appearance of higher ordered oligomers of Cavin-1 were independently confirmed in unbroken cells using a cell-permeable cross-linking approach. Thus, although Cavin-1 can complex and colocalize with Cavin-2 and caveolins and the loss of either Cavin-1 or caveolins destabilizes the other protein, biochemically the Cav-1·Cav-2 oligomeric complex is distinct from Cavin-1.
Cavin-1, originally called PTRF, is a protein that governs the termination of transcription by RNA polymerase I (53) and has been localized to the cytoplasmic face of caveolae (42, 54, 55). Cavin-1 is required for the formation of caveolae in cultured cells (12, 14) and zebrafish embryos (12), and in all mammalian tissues that have caveolae (13) and in rodents, the distribution of Cavin-1 coincides with that of caveolins in various tissues (13, 56). Interestingly, Cavin-1 KOs exhibit hyperinsulinemia, reduced adiposity, elevated triglycerides, and glucose intolerance, phenotypes reminiscent of Cav-1 and/or Cav-3 KO mice. From a mechanistic perspective, it is not known how Cavin-1 functions to regulate the assembly and/or kinetics of caveolae, but recent data suggest that Cavin-2 recruits Cavin-1 to Cav-1/caveolae, permitting plasma membrane tubulation events to occur (35).
In the present study, we showed that Cavin-1 is highly expressed in ECs lining intact blood vessels and in cultured ECs, it colocalizes with Cav-1 both by sucrose gradient centrifugation and immunofluorescence microscopy, and its expression is dependent on the presence of Cav-1 in vitro and in vivo. Reconstitution of Cav-1 using EC-specific transgenesis in a Cav-1 KO background partially restored the Cavin-1 level in tissues where ECs are abundant (lung/aorta) as well as in cultured ECs from these animals. Whether the increase in both Cav-1 and Cavin-1 results in increased caveola structures in ECs is not known because we did not quantify the organelle in this study. However, we have previously shown by EM that increasing the levels of Cav-1 over and above endogenous levels does not increase the number of caveolae in the endothelium (43). A possible explanation of the effects of Cav-1 may be through the stabilization of Cav-2. We have previously shown that Cav-2 and specifically its phosphorylation at serine residues 23 and 36 are positive regulators of caveola biogenesis (30).
Apart from adipocytes and skeletal muscle, caveolae are highly abundant in ECs (3), and there are major cardiovascular phenotypes in Cav-1 KO mice such as defects in vascular function, angiogenesis, cardiac hypertrophy, and pulmonary hypertension. Mechanistically, the impaired vascular and pulmonary functions are due to dysregulation of eNOS, resulting in enhanced NO levels in Cav-1 KO mice (8, 20–22). Here, we show that a reduction in Cavin-1 increased NO production in ECs, and this was due presumably to the loss of the inhibitory influence of Cav-1 on eNOS. Also, a reduction in Cavin-1 reduced several in vitro paradigms of angiogenesis including EC proliferation, migration, and cord formation. Although the loss of Cav-1 can promote cell growth (21), it can stimulate or inhibit angiogenesis (57, 58); thus, it is possible that the decrease in angiogenic phenotypes after knockdown of Cavin-1 may reflect the complex actions of Cav-1 or be independent of Cav-1 and relate to its action in transcriptional termination processes (53).
In summary, using a comparative quantitative proteomics analysis of proteins within Cav-1-enriched membranes of intact tissue, we identified and characterized the role of Cavin-1 in ECs. Additional analyses are required to elucidate the relationships among Cav-1, Cavins, and intracellular targets such as eNOS and to characterize the relevance of proteins enriched in DRMs after the loss of Cav-1.
Supplementary Material
Acknowledgments
We thank Dr. Kenneth Williams for insights and support for this project.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL64793, R01 HL61371, R01 HL081190, and P01 HL70295 and Contract N01-HV-28186 (NHLBI-Yale Proteomics Contract) (to W. C. S.). This work was also supported by a scientist development grant from the American Heart Association (to C. F.-H.).
 This article contains supplemental Figs. S1–S5 and Tables S1 and S2.
This article contains supplemental Figs. S1–S5 and Tables S1 and S2.
1 The abbreviations used are:
- EC
- endothelial cell
- Cav
- caveolin
- PTRF
- polymerase I and transcript release factor
- DRM
- detergent-resistant membrane
- SDPR
- serum deprivation response protein
- NS
- negative nonsilencing control
- HUVEC
- human umbilical vein EC
- iTRAQ
- isobaric tagging for relative and absolute quantification
- MBS
- Mes-buffered saline
- IPI
- International Protein Index
- eNOS
- endothelial nitric-oxide synthase
- RC
- reconstitution
- DSP
- dithiobis(succinimidylpropionate)
- IP
- immunoprecipitation.
REFERENCES
- 1.Parton R. G., Simons K. (2007) The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 2.Pilch P. F., Souto R. P., Liu L., Jedrychowski M. P., Berg E. A., Costello C. E., Gygi S. P. (2007) Cellular spelunking: exploring adipocyte caveolae. J. Lipid Res. 48, 2103–2111 [DOI] [PubMed] [Google Scholar]
- 3.Gratton J. P., Bernatchez P., Sessa W. C. (2004) Caveolae and caveolins in the cardiovascular system. Circ. Res. 94, 1408–1417 [DOI] [PubMed] [Google Scholar]
- 4.Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. (2004) Role of caveolae and caveolins in health and disease. Physiol. Rev. 84, 1341–1379 [DOI] [PubMed] [Google Scholar]
- 5.Frank P. G., Pavlides S., Cheung M. W., Daumer K., Lisanti M. P. (2008) Role of caveolin-1 in the regulation of lipoprotein metabolism. Am. J. Physiol. Cell Physiol. 295, C242–C248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank P. G., Pavlides S., Lisanti M. P. (2009) Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 335, 41–47 [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Hernando C., Ackah E., Yu J., Suárez Y., Murata T., Iwakiri Y., Prendergast J., Miao R. Q., Birnbaum M. J., Sessa W. C. (2007) Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 6, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F. C., Schedl A., Haller H., Kurzchalia T. V. (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452 [DOI] [PubMed] [Google Scholar]
- 9.Fra A. M., Williamson E., Simons K., Parton R. G. (1995) De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. U.S.A. 92, 8655–8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galbiati F., Engelman J. A., Volonte D., Zhang X. L., Minetti C., Li M., Hou H., Jr., Kneitz B., Edelmann W., Lisanti M. P. (2001) Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J. Biol. Chem. 276, 21425–21433 [DOI] [PubMed] [Google Scholar]
- 11.Razani B., Wang X. B., Engelman J. A., Battista M., Lagaud G., Zhang X. L., Kneitz B., Hou H., Jr., Christ G. J., Edelmann W., Lisanti M. P. (2002) Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol. Cell. Biol. 22, 2329–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill M. M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S. J., Walser P., Abankwa D., Oorschot V. M., Martin S., Hancock J. F., Parton R. G. (2008) PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L., Brown D., McKee M., Lebrasseur N. K., Yang D., Albrecht K. H., Ravid K., Pilch P. F. (2008) Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 8, 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L., Pilch P. F. (2008) A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 283, 4314–4322 [DOI] [PubMed] [Google Scholar]
- 15.Ross P. L., Huang Y. N., Marchese J. N., Williamson B., Parker K., Hattan S., Khainovski N., Pillai S., Dey S., Daniels S., Purkayastha S., Juhasz P., Martin S., Bartlet-Jones M., He F., Jacobson A., Pappin D. J. (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 [DOI] [PubMed] [Google Scholar]
- 16.Guo Y., Singleton P. A., Rowshan A., Gucek M., Cole R. N., Graham D. R., Van Eyk J. E., Garcia J. G. (2007) Quantitative proteomics analysis of human endothelial cell membrane rafts: evidence of MARCKS and MRP regulation in the sphingosine 1-phosphate-induced barrier enhancement. Mol. Cell. Proteomics 6, 689–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralhan R., Desouza L. V., Matta A., Chandra Tripathi S., Ghanny S., Datta Gupta S., Bahadur S., Siu K. W. (2008) Discovery and verification of head-and-neck cancer biomarkers by differential protein expression analysis using iTRAQ labeling, multidimensional liquid chromatography, and tandem mass spectrometry. Mol. Cell. Proteomics 7, 1162–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank P. G., Woodman S. E., Park D. S., Lisanti M. P. (2003) Caveolin, caveolae, and endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 23, 1161–1168 [DOI] [PubMed] [Google Scholar]
- 19.Stan R. V., Roberts W. G., Predescu D., Ihida K., Saucan L., Ghitescu L., Palade G. E. (1997) Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae). Mol. Biol. Cell 8, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata T., Lin M. I., Huang Y., Yu J., Bauer P. M., Giordano F. J., Sessa W. C. (2007) Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J. Exp. Med. 204, 2373–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razani B., Engelman J. A., Wang X. B., Schubert W., Zhang X. L., Marks C. B., Macaluso F., Russell R. G., Li M., Pestell R. G., Di Vizio D., Hou H., Jr., Kneitz B., Lagaud G., Christ G. J., Edelmann W., Lisanti M. P. (2001) Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 276, 38121–38138 [DOI] [PubMed] [Google Scholar]
- 22.Yu J., Bergaya S., Murata T., Alp I. F., Bauer M. P., Lin M. I., Drab M., Kurzchalia T. V., Stan R. V., Sessa W. C. (2006) Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J. Clin. Investig. 116, 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrami L., Fivaz M., Kobayashi T., Kinoshita T., Parton R. G., van der Goot F. G. (2001) Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276, 30729–30736 [DOI] [PubMed] [Google Scholar]
- 24.Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 25.Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 26.Shilov I. V., Seymour S. L., Patel A. A., Loboda A., Tang W. H., Keating S. P., Hunter C. L., Nuwaysir L. M., Schaeffer D. A. (2007) The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteomics 6, 1638–1655 [DOI] [PubMed] [Google Scholar]
- 27.Salim K., Kehoe L., Minkoff M. S., Bilsland J. G., Munoz-Sanjuan I., Guest P. C. (2006) Identification of differentiating neural progenitor cell markers using shotgun isobaric tagging mass spectrometry. Stem Cells Dev. 15, 461–470 [DOI] [PubMed] [Google Scholar]
- 28.Seshi B. (2006) An integrated approach to mapping the proteome of the human bone marrow stromal cell. Proteomics 6, 5169–5182 [DOI] [PubMed] [Google Scholar]
- 29.Sargiacomo M., Scherer P. E., Tang Z., Kübler E., Song K. S., Sanders M. C., Lisanti M. P. (1995) Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc. Natl. Acad. Sci. U.S.A. 92, 9407–9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sowa G., Pypaert M., Fulton D., Sessa W. C. (2003) The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc. Natl. Acad. Sci. U.S.A. 100, 6511–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litman T., Jensen U., Hansen A., Covitz K. M., Zhan Z., Fetsch P., Abati A., Hansen P. R., Horn T., Skovsgaard T., Bates S. E. (2002) Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim. Biophys. Acta 1565, 6–16 [DOI] [PubMed] [Google Scholar]
- 32.Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster L. J., De Hoog C. L., Mann M. (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. U.S.A. 100, 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y. Z., Berg K. B., Foster L. J. (2009) Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 50, 988–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen C. G., Bright N. A., Howard G., Nichols B. J. (2009) SDPR induces membrane curvature and functions in the formation of caveolae. Nat. Cell Biol. 11, 807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mineo C., Ying Y. S., Chapline C., Jaken S., Anderson R. G. (1998) Targeting of protein kinase Calpha to caveolae. J. Cell Biol. 141, 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman A., Sward K. (2009) The role of caveolin-1 in cardiovascular regulation. Acta Physiol. 195, 231–245 [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Hernando C., Yu J., Suárez Y., Rahner C., Dávalos A., Lasunción M. A., Sessa W. C. (2009) Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 10, 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song K. S., Scherer P. E., Tang Z., Okamoto T., Li S., Chafel M., Chu C., Kohtz D. S., Lisanti M. P. (1996) Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 271, 15160–15165 [DOI] [PubMed] [Google Scholar]
- 40.Pelkmans L., Bürli T., Zerial M., Helenius A. (2004) Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118, 767–780 [DOI] [PubMed] [Google Scholar]
- 41.Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., Helenius A. (2005) Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 170, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aboulaich N., Vainonen J. P., Strålfors P., Vener A. V. (2004) Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem. J. 383, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer P. M., Yu J., Chen Y., Hickey R., Bernatchez P. N., Looft-Wilson R., Huang Y., Giordano F., Stan R. V., Sessa W. C. (2005) Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das K., Lewis R. Y., Scherer P. E., Lisanti M. P. (1999) The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J. Biol. Chem. 274, 18721–18728 [DOI] [PubMed] [Google Scholar]
- 45.Li S., Galbiati F., Volonte D., Sargiacomo M., Engelman J. A., Das K., Scherer P. E., Lisanti M. P. (1998) Mutational analysis of caveolin-induced vesicle formation. Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett. 434, 127–134 [DOI] [PubMed] [Google Scholar]
- 46.Hayer A., Stoeber M., Bissig C., Helenius A. (2010) Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11, 361–382 [DOI] [PubMed] [Google Scholar]
- 47.García-Cardeña G., Martasek P., Masters B. S., Skidd P. M., Couet J., Li S., Lisanti M. P., Sessa W. C. (1997) Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 272, 25437–25440 [DOI] [PubMed] [Google Scholar]
- 48.Michel J. B., Feron O., Sacks D., Michel T. (1997) Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 272, 15583–15586 [DOI] [PubMed] [Google Scholar]
- 49.Baruthio F., Quadroni M., Rüegg C., Mariotti A. (2008) Proteomic analysis of membrane rafts of melanoma cells identifies protein patterns characteristic of the tumor progression stage. Proteomics 8, 4733–4747 [DOI] [PubMed] [Google Scholar]
- 50.von Haller P. D., Donohoe S., Goodlett D. R., Aebersold R., Watts J. D. (2001) Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics 1, 1010–1021 [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y. Z., Foster L. J. (2009) Contributions of quantitative proteomics to understanding membrane microdomains. J. Lipid Res. 50(10), 1976–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bastiani M., Liu L., Hill M. M., Jedrychowski M. P., Nixon S. J., Lo H. P., Abankwa D., Luetterforst R., Fernandez-Rojo M., Breen M. R., Gygi S. P., Vinten J., Walser P. J., North K. N., Hancock J. F., Pilch P. F., Parton R. G. (2009) MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell Biol. 185, 1259–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansa P., Mason S. W., Hoffmann-Rohrer U., Grummt I. (1998) Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 17, 2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinten J., Johnsen A. H., Roepstorff P., Harpøth J., Tranum-Jensen J. (2005) Identification of a major protein on the cytosolic face of caveolae. Biochim. Biophys. Acta 1717, 34–40 [DOI] [PubMed] [Google Scholar]
- 55.Vinten J., Voldstedlund M., Clausen H., Christiansen K., Carlsen J., Tranum-Jensen J. (2001) A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res. 305, 99–106 [DOI] [PubMed] [Google Scholar]
- 56.Voldstedlund M., Vinten J., Tranum-Jensen J. (2001) cav-p60 expression in rat muscle tissues. Distribution of caveolar proteins. Cell Tissue Res. 306, 265–276 [DOI] [PubMed] [Google Scholar]
- 57.Williams T. M., Medina F., Badano I., Hazan R. B., Hutchinson J., Muller W. J., Chopra N. G., Scherer P. E., Pestell R. G., Lisanti M. P. (2004) Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J. Biol. Chem. 279, 51630–51646 [DOI] [PubMed] [Google Scholar]
- 58.Lin M. I., Yu J., Murata T., Sessa W. C. (2007) Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 67, 2849–2856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.