Abstract
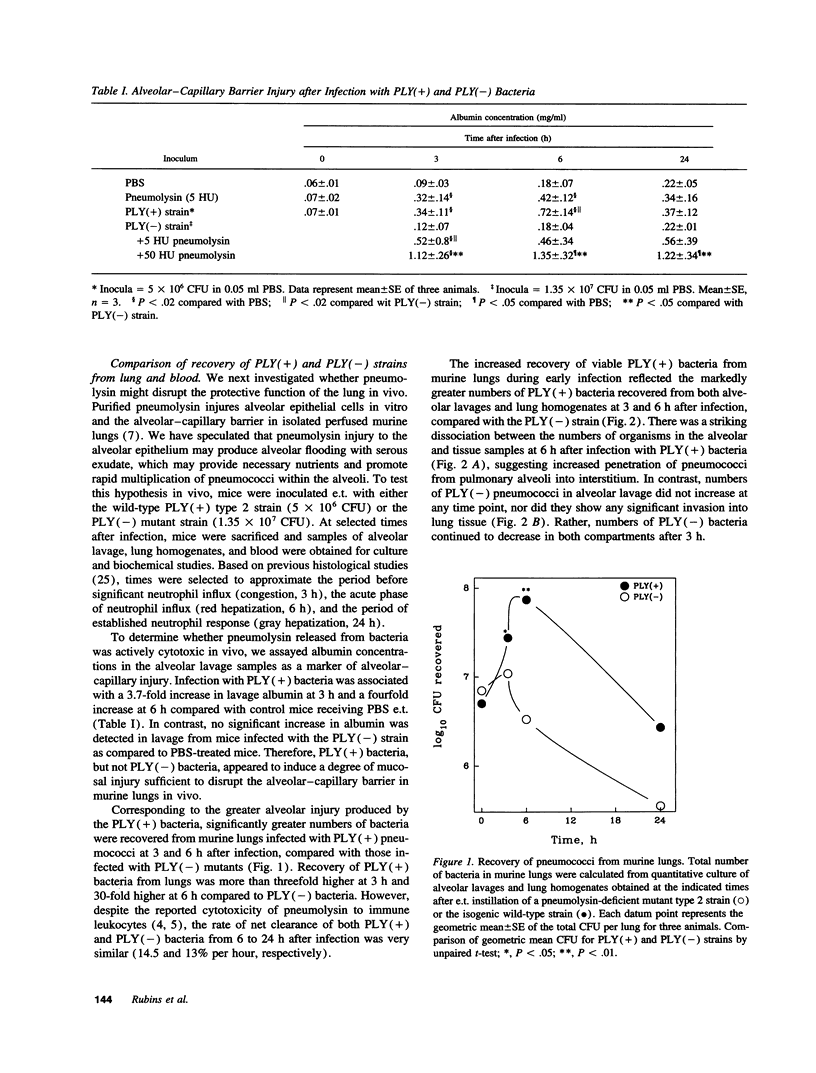
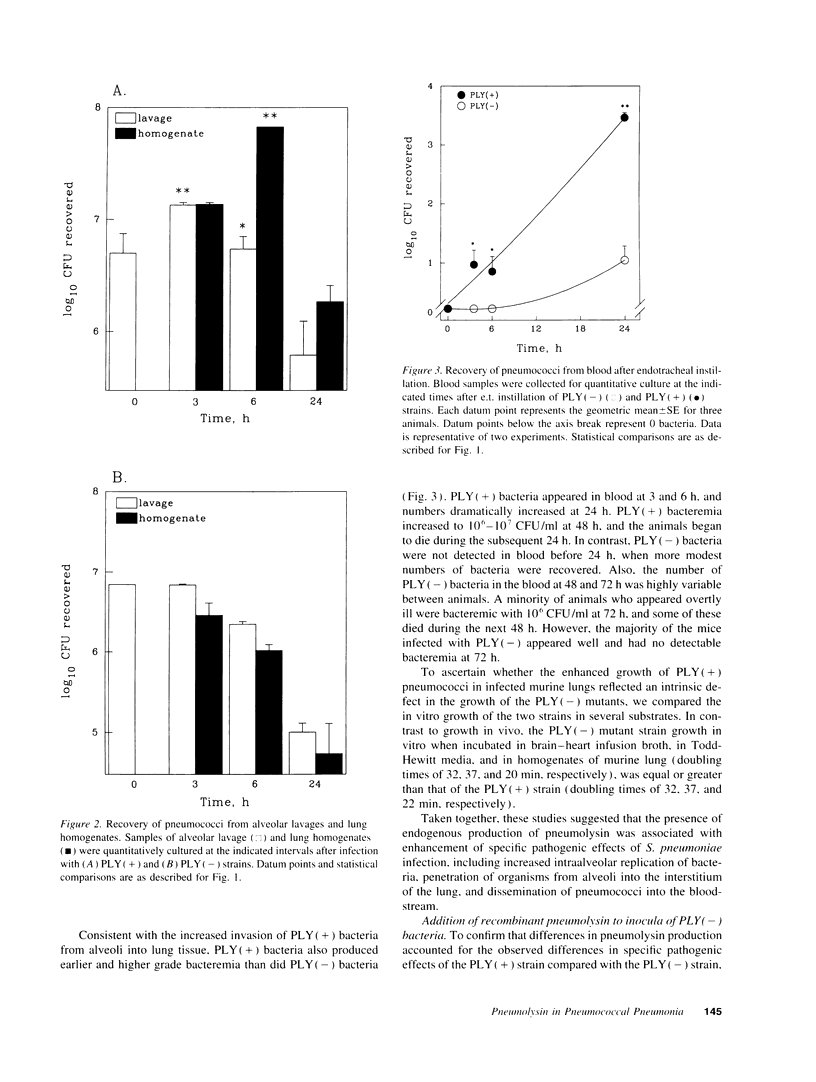
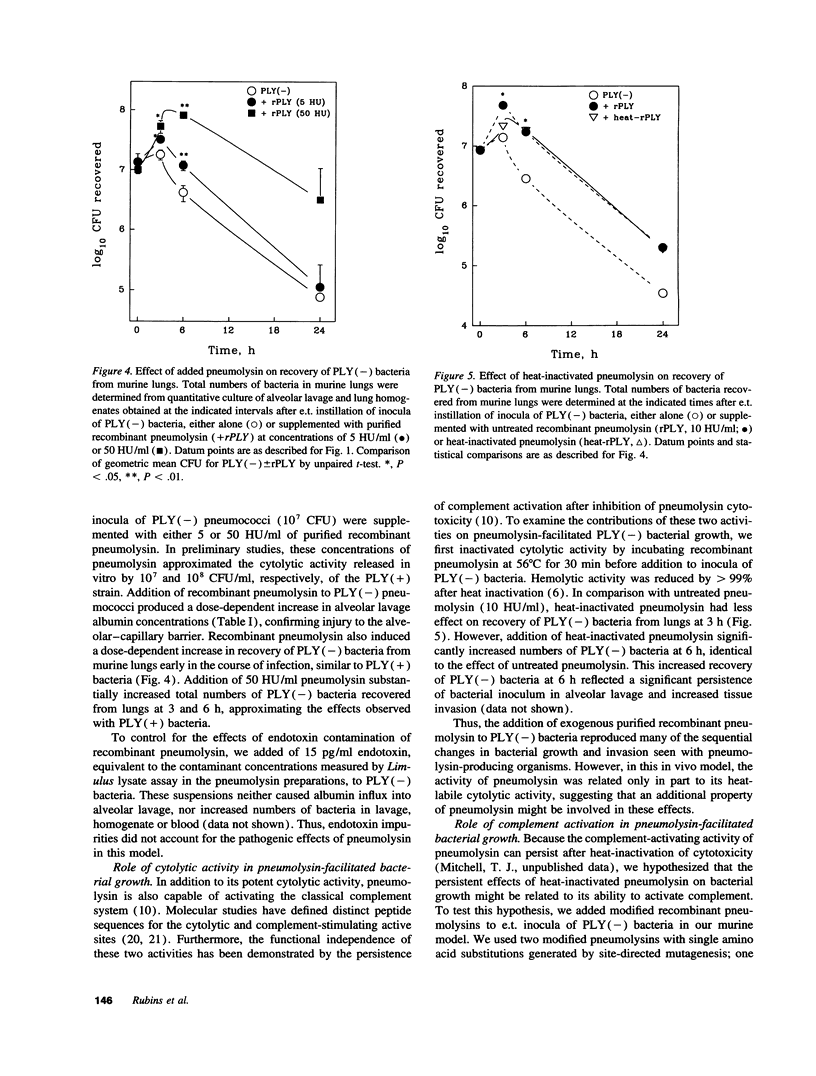
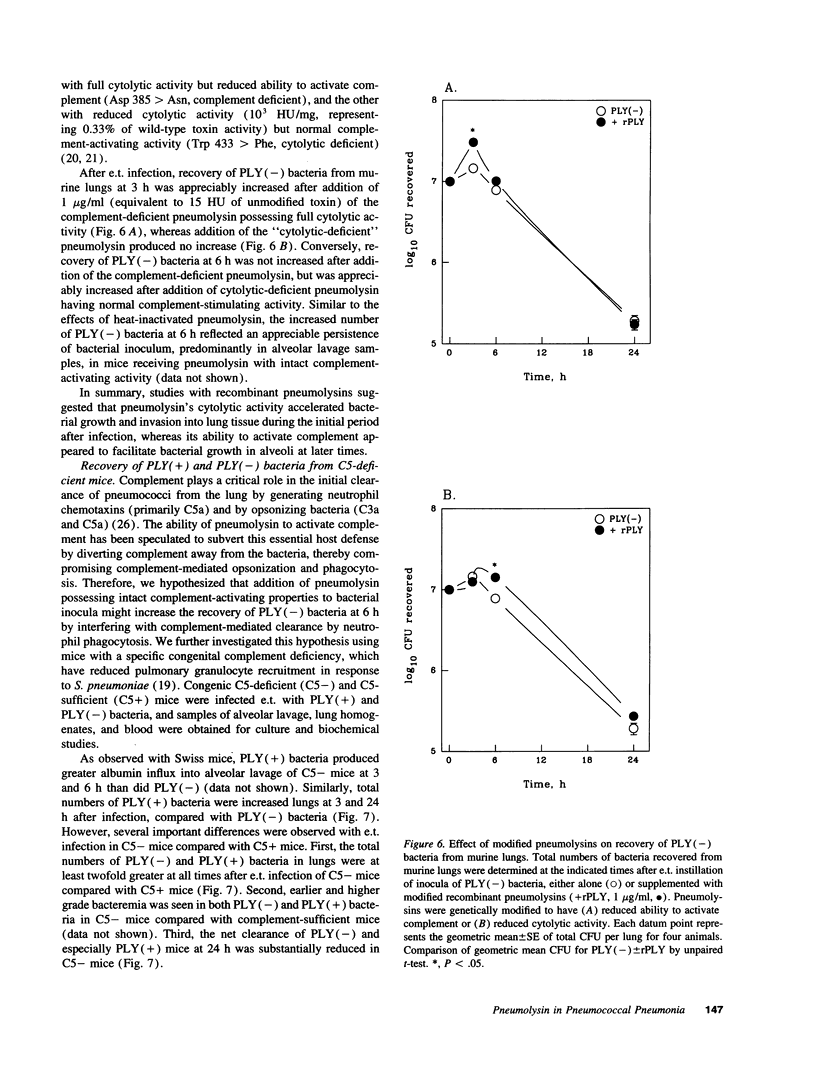
Streptococcus pneumoniae is one of the most common etiologic agents of community-acquired pneumonia, particularly bacteremic pneumonia. Pneumolysin, a multifunctional cytotoxin, is a putative virulence factor for S. pneumoniae; however, a direct role for pneumolysin in the early pathogenesis of pneumococcal pneumonia has not been confirmed in vivo. We compared the growth of a pneumolysin-deficient (PLY[-]) type 2 S. pneumoniae strain with its isogenic wild-type strain (PLY[+]) after direct endotracheal instillation of bacteria into murine lungs. Compared with PLY(-) bacteria, infection with PLY(+) bacteria produced greater injury to the alveolar-capillary barrier, as assayed by albumin concentrations in alveolar lavage, and substantially greater numbers of PLY(+) bacteria were recovered in alveolar lavages and lung homogenates at 3 and 6 h after infection. The presence of pneumolysin also contributed to the development of bacteremia, which was detected at 3 h after intratracheal instillation of PLY(+) bacteria. The direct effects of pneumolysin on lung injury and on the ability of pneumococci to evade local lung defenses was confirmed by addition of purified recombinant pneumolysin to inocula of PLY(-) pneumococci, which promoted growth of PLY(-) bacteria in the lung to levels comparable to those seen with the PLY(+) strain. We further demonstrated the contributions of both the cytolytic and the complement-activating properties of pneumolysin on enhanced bacterial growth in murine lungs using genetically modified pneumolysin congeners and genetically complement-deficient mice. Thus, pneumolysin facilitates intraalveolar replication of pneumococci, penetration of bacteria from alveoli into the interstitium of the lung, and dissemination of pneumococci into the bloodstream during experimental pneumonia. Moreover, both the cytotoxic and the complement-activating activities of pneumolysin may contribute independently to the acute pulmonary injury and the high rates of bacteremia which characterize pneumococcal pneumonia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry A. M., Paton J. C., Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992 Feb;12(2):87–93. doi: 10.1016/0882-4010(92)90111-z. [DOI] [PubMed] [Google Scholar]
- Berry A. M., Yother J., Briles D. E., Hansman D., Paton J. C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989 Jul;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Paton J. C., Mitchell T. J., Andrew P. W. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991 Nov;5(11):2611–2616. doi: 10.1111/j.1365-2958.1991.tb01969.x. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Lester R. L., Hsu L. C. Characterization of the extracellular bactericidal factors of rat alveolar lining material. J Clin Invest. 1984 Oct;74(4):1269–1279. doi: 10.1172/JCI111537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane P. G., Rubins J. B., Weisel H. R., Janoff E. N. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun. 1993 Oct;61(10):4392–4397. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito A. L., Pennington J. E. Effects of aging on antibacterial mechanisms in experimental pneumonia. Am Rev Respir Dis. 1983 Oct;128(4):662–667. doi: 10.1164/arrd.1983.128.4.662. [DOI] [PubMed] [Google Scholar]
- Feldman C., Munro N. C., Jeffery P. K., Mitchell T. J., Andrew P. W., Boulnois G. J., Guerreiro D., Rohde J. A., Todd H. C., Cole P. J. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am J Respir Cell Mol Biol. 1991 Nov;5(5):416–423. doi: 10.1165/ajrcmb/5.5.416. [DOI] [PubMed] [Google Scholar]
- Houldsworth S., Andrew P. W., Mitchell T. J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect Immun. 1994 Apr;62(4):1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson W. G., Jr, Stephen J. J., Pierce A. K. Bacterial growth in vivo. An important determinant of the pulmonary clearance of Diplococcus pneumoniae in rats. J Clin Invest. 1974 May;53(5):1320–1325. doi: 10.1172/JCI107679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Geoffroy C., Alouf J. E. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980 Jan;27(1):97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. R., Rudensky B., Hadas-Halperin I., Isacsohn M., Melzer E. Pneumococcal bacteremia--no change in mortality in 30 years: analysis of 104 cases and review of the literature. Isr J Med Sci. 1987 Mar;23(3):174–180. [PubMed] [Google Scholar]
- Lock R. A., Hansman D., Paton J. C. Comparative efficacy of autolysin and pneumolysin as immunogens protecting mice against infection by Streptococcus pneumoniae. Microb Pathog. 1992 Feb;12(2):137–143. doi: 10.1016/0882-4010(92)90116-6. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Andrew P. W., Saunders F. K., Smith A. N., Boulnois G. J. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991 Aug;5(8):1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Walker J. A., Saunders F. K., Andrew P. W., Boulnois G. J. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim Biophys Acta. 1989 Jan 23;1007(1):67–72. doi: 10.1016/0167-4781(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Musher D. M. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1992 Apr;14(4):801–807. doi: 10.1093/clinids/14.4.801. [DOI] [PubMed] [Google Scholar]
- Nandoskar M., Ferrante A., Bates E. J., Hurst N., Paton J. C. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986 Dec;59(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Hansman D. J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983 May;40(2):548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Lee C. J., Li J. P., Berry A. M., Mitchell T. J., Andrew P. W., Hansman D., Boulnois G. J. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991 Jul;59(7):2297–2304. doi: 10.1128/iai.59.7.2297-2304.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Rowan-Kelly B., Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Mar;43(3):1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J. B., Duane P. G., Charboneau D., Janoff E. N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992 May;60(5):1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J. B., Duane P. G., Clawson D., Charboneau D., Young J., Niewoehner D. E. Toxicity of pneumolysin to pulmonary alveolar epithelial cells. Infect Immun. 1993 Apr;61(4):1352–1358. doi: 10.1128/iai.61.4.1352-1358.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J. B., Freiberg M. R. Anti-pneumolysin activity of commercially available alpha 1-antitrypsin is due to cholesterol impurities. Microb Pathog. 1994 Mar;16(3):221–228. doi: 10.1006/mpat.1994.1022. [DOI] [PubMed] [Google Scholar]
- Saunders F. K., Mitchell T. J., Walker J. A., Andrew P. W., Boulnois G. J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989 Aug;57(8):2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfort C., Wilson R., Mitchell T., Feldman C., Rutman A., Todd H., Sykes D., Walker J., Saunders K., Andrew P. W. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun. 1989 Jul;57(7):2006–2013. doi: 10.1128/iai.57.7.2006-2013.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G. B., Vial W. C. The role of C5 in polymorphonuclear leukocyte recruitment in response to Streptococcus pneumoniae. Am Rev Respir Dis. 1984 Jan;129(1):82–86. doi: 10.1164/arrd.1984.129.1.82. [DOI] [PubMed] [Google Scholar]
- Wangensteen O. D., Wittmers L. E., Jr, Johnson J. A. Permeability of the mammalian blood-gas barrier and its components. Am J Physiol. 1969 Apr;216(4):719–727. doi: 10.1152/ajplegacy.1969.216.4.719. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A. Complement and the host's defense against the pneumococcus. Crit Rev Microbiol. 1984;11(3):187–208. doi: 10.3109/10408418409105903. [DOI] [PubMed] [Google Scholar]



