Abstract
Severe pediatric traumatic brain injury (TBI) is associated with unfavorable outcomes secondary to injury from activation of the inflammatory cascade, the release of excitotoxic neurotransmitters, and changes in the reactivity of cerebral vessels, causing ischemia. Hypoperfusion of injured brain tissues after TBI is also associated with unfavorable outcomes. Therapeutic hypothermia is an investigational treatment strategy for use in patients with severe TBI that has shown differential effects on various cerebrospinal fluid (CSF) mediators in pediatric patients. Endothelin-1 (ET-1) is a powerful vasoconstrictor that exerts its effects on the cerebrovascular endothelium for sustained periods after TBI. The purpose of this study was to determine if CSF concentrations of ET-1 are increased after severe TBI in children, and if they are associated with demographics and outcomes that are affected by therapeutic hypothermia. This was an ancillary study to a prospective, randomized-controlled trial of early hypothermia in a tertiary care pediatric intensive care unit. Children (n = 34, age 3 months–15 years) suffering from severe TBI were randomized to hypothermia (n = 19) and normothermia (n = 15) as part of the efficacy study. Children undergoing diagnostic lumbar puncture (n = 11) to rule out infection were used as controls. Patients received either mild to moderate hypothermia (32–33°C) or normothermia as part of their treatment protocol. CSF was serially collected during the first 5 days after TBI. ET-1 concentrations were quantitated in patient and control CSF samples by a validated ELISA in duplicate with a limit of quantification of 0.195 pg/mL. CSF ET-1 concentrations were increased by two- to threefold in children after TBI compared to controls, and the increase was sustained for up to 5 days post-TBI. This relationship was not affected by hypothermia, and there were no differences in ET-1 response between children with inflicted and accidental TBI. Group-based trajectory analysis revealed two distinct groups with similar ET-1 levels over time. Univariate analysis showed a significant association between ET-1 levels and Glasgow Outcome Scale (GOS) scores, for which higher ET-1 levels over time were associated with unfavorable outcomes. ET-1 is increased in children with severe TBI and is associated with unfavorable outcomes. This increase in ET-1 may mediate the hypoperfusion or cerebrovascular dysfunction accompanying severe TBI in children. Importantly, hypothermia does not affect the brain's ET-1 response as measured in the CSF.
Key words: abusive head trauma, cerebral blood flow, endothelin, hypoperfusion, inflicted childhood neurotrauma, pediatric brain injury, vasospasm
Introduction
Disturbances in cerebral blood flow (CBF) are common after traumatic brain injury (TBI) in children. However, both hypoperfusion and hyperemia have been reported (Adelson et al., 2005; Muizelaar, 1992; Kochanek, 2006), making it difficult to make specific clinical recommendations. Reduced CBF has been strongly associated with unfavorable outcomes in these patients (Adelson et al., 2005), implicating a critical role for ischemia or hypoperfusion. Blood pressure autoregulation of CBF is commonly disturbed after pediatric TBI, and this loss of autoregulation is also associated with unfavorable outcomes (Vavilala et al., 2008). These associations are also observed in children who are victims of inflicted TBI (iTBI; Adelson et al., 2005; Vavilala et al., 2007). Vasospasm after TBI has also emerged as having significant importance in some cases, although reports in pediatric TBI are limited (Nabika et al., 2007; Ojha et al., 2005). However, biochemical or molecular mediators of post-traumatic CBF reductions in infants and children remain to be defined.
The cerebrovascular endothelium has a profound influence on cerebral blood vessels and CBF. The endothelium contributes to the resting tone of the cerebral arteries by maintaining a balance between vasodilation and vasoconstriction, thought to be mediated by nitric oxide (NO) and endothelin-1 (ET-1), among other mediators. Basal release of NO by endothelial nitric oxide synthase (eNOS) is believed to be responsible for the homeostatic vasodilation that regulates normal blood flow (Andresen et al., 2006). Various physiological and pathological conditions such as TBI, subarachnoid hemorrhage (SAH), and stroke cause disturbances in endothelial function that lead to disturbances in vasomotor tone, vasoconstriction, and ultimately decreased oxygen and substrate delivery (Maier et al., 2007).
ET-1 is a small (21-amino acid), potent, vasoconstrictor peptide implicated as a mediator of ischemia, hypoperfusion, and vasospasm. ET-1 is one of a family of vasoactive peptides (including ET-2, ET-3, and ET-4), and acts through binding to one of two transmembrane receptors, ET-A and ET-B. ET-B is concentrated within the CNS, particularly in the hippocampus, caudate, putamen, and cerebellum (Hama et al., 1997). Under homeostatic conditions, the role of ET-1 in regulating CBF appears minimal; however, under pathological conditions, ET-1 synthesis is increased in the cerebrovascular endothelium, and ET-B receptors are upregulated after injury (Andresen et al., 2006). Endothelial-derived ET-1 reduces CBF and is associated with clinical vasospasm and secondary neurological injury due to its high potency and long duration of action (Gorlach et al., 2001). In animal models of TBI, including pediatric models, brain ET-1 concentrations increase two- to threefold (Steiner et al., 2004), and are associated with prolonged vasoconstriction (Armstead, 2004). Moreover, antagonism of ET-1 partially reversed this vasoconstrictor response (Ho et al., 2001).
We have utilized CSF to evaluate a number of compounds after TBI, including adenosine, caffeine, vascular endothelial growth factor (VEGF), glutamate, nitrite, nitrate, and quinolinic acid (Bell et al., 1999, 2001; Clark et al., 1996; Ruppel et al., 2001; Shore et al., 2004), among others. Based on these previous studies, the purpose of this study was to examine the role of ET-1 in the CSF of pediatric patients after TBI. We hypothesized that ET-1 would be increased in CSF after severe TBI. In our prior studies, we have observed that hypothermia has had variable effects on various mediator levels that have depended on the specific mediator. It has been reported in the setting of hypothermic circulatory arrest that ET-1 levels may be increased by hypothermia. Thus we further hypothesized that ET-1 concentrations would be associated with injury mechanism and outcome, and increased by systemic hypothermia. The use of hypothermia as a therapeutic strategy in patients after severe TBI is currently under investigation. Thus our clinical study would provide the unique opportunity not only to examine the role of ET-1 in the CSF of pediatric patients after TBI, but could also yield insight into the effects of therapeutic hypothermia on a potent vasoconstrictor to help us better understand the pathophysiology of TBI and the effects of temperature at the vascular level. We utilized children enrolled in an ongoing randomized controlled trial (RCT) of hypothermia as a neuroprotectant to explore these hypotheses.
Methods
Informed consent was obtained from the parents of children suffering from severe TBI, in accordance with the regulations of the Institutional Review Board at the University of Pittsburgh. Specifically, parents consented to the intervention of hypothermia, as well as to the collection of CSF for analysis.
Patient sample
This was an ancillary study to a prospective RCT of early hypothermia in a tertiary care pediatric intensive care unit. Eligible children had Glasgow Coma Scale (GCS) scores ≤8 after resuscitation or after secondary deterioration, and had an externalized ventricular drain (EVD) placed as part of their routine clinical care. CSF was drained continuously at 3 cm above the midbrain, and all children received standard neurocritical care for intracranial hypertension (ICH) based on published guidelines (Adelson et al., 2003). Specifically, step-wise therapies were administered, including sedation, neuromuscular blockade, hyperosmolar therapies, and barbiturates. Children randomized to hypothermia were systemically cooled to 32–33°C for 48 h, followed by a slow, passive rewarming period as previously described (Adelson et al., 2005).
CSF collection and data analysis
CSF was collected daily for up to 5 days after TBI. The samples were immediately centrifuged, aliquotted, and stored at −80°C until analysis. CSF specimens from children undergoing diagnostic lumbar puncture (n = 11) to rule out infection were used as controls for this analysis. Ultimately, all control CSF cultures were negative for viral or bacterial pathogens. ET-1 levels were quantified using the QuantiGlo® Human Endothelin-1 Immunoassay kit (R&D Systems, Minneapolis, MN), with a modified standard curve range of 0.195–12.5 pg/mL, yielding interday and intraday reproducibility (<15% CV) across all concentrations. ET-1 chemiluminescence detection was performed using a Wallac Victor-2 1420 MultiLabel Counter (Perkin Elmer Wallac, Gaithersburg, MD). Each sample was analyzed separately. A repeated-measures model test showed that ET-1 levels did not differ significantly between two samples per day, so the mean value was used.
Demographic data including age, gender, mechanism of injury, GCS scores, and Glasgow Outcome Scale (GOS) scores (at 6 months) were also obtained (Table 1). Mixed-effects regression models were used to compare mean ET-1 levels between TBI cases and controls. Within the cases, a mixed-effects regression model was used to determine if there was a difference in ET-1 levels over 5 days between hypothermic and normothermic subjects. Mixed-effect models were required to control for the within-subject correlation in the TBI cases, due to repeated measures taken over 5 days. The Student's t-test was used to compare mean peak levels between hypothermic and normothermic subjects, as well as each group to controls, and between iTBI and non-iTBI subjects. A logistic regression model was used to estimate the association between peak ET-1 levels and outcome.
Table 1.
Demographic and Clinical Characteristics of the Total Study Sample
| Age (years) | Gender | Mechanism | Hypo/Normo | Initial GCS score | GOS score | |
|---|---|---|---|---|---|---|
| Patient 1 | 10 | Female | Bike versus car | Hypo | 5 | SD |
| Patient 2 | 7 | Male | Fall on floor | Hypo | 7 | D |
| Patient 3 | 5 | Male | Abuse | Hypo | 6 | SD |
| Patient 4 | 6 | Male | MVA | Hypo | 7 | G |
| Patient 5 | 3 | Female | Television fell on | Hypo | 10 | G |
| Patient 6 | 2 | Male | 3-Story fall | Hypo | 9 | MD |
| Patient 7 | 15 | Male | Pedestrian versus car | Hypo | 6 | D |
| Patient 8 | 13 | Male | Motorcycle versus car | Hypo | 7 | SD |
| Patient 9 | 12 | Female | 20-Foot fall | Hypo | 5 | G |
| Patient 10 | 0.7 | Male | Abuse | Hypo | 13 | D |
| Patient 11 | 7 | Female | MVA | Hypo | 7 | MD |
| Patient 12 | 8 | Male | Pedestrian versus car | Hypo | 10 | G |
| Patient 13 | 12 | Male | MVA | Hypo | 3 | MD |
| Patient 14 | 8 | Male | MVA | Hypo | 7 | G |
| Patient 15 | 15 | Male | Skateboard versus pole | Hypo | 7 | G |
| Patient 16 | 7 | Male | Pedestrian versus car | Hypo | 6 | SD |
| Patient 17 | 0.7 | Female | Abuse | Hypo | 3 | G |
| Patient 18 | 4 | Male | 2-Story fall | Hypo | 8 | G |
| Patient 19 | 0.16 | Female | Abuse | Hypo | 7 | SD |
| Patient 20 | 3 | Male | Pedestrian versus car | Normo | 7 | G |
| Patient 21 | 2 | Male | 2-Story fall | Normo | 8 | SD |
| Patient 22 | 10 | Male | MVA | Normo | 3 | G |
| Patient 23 | 9 | Male | Fall off bike | Normo | 15 | G |
| Patient 24 | 0.16 | Female | Abuse | Normo | 11 | SD |
| Patient 25 | 10 | Female | MVA | Normo | 4 | MD |
| Patient 26 | 8 | Female | MVA | Normo | 14 | G |
| Patient 27 | 9 | Female | Pedestrian versus auto | Normo | 8 | G |
| Patient 28 | 7 | Male | MVA | Normo | 6 | G |
| Patient 29 | 13 | Female | Pedestrian versus auto | Normo | 8 | MD |
| Patient 30 | 0.13 | Female | Abuse | Normo | 6 | SD |
| Patient 31 | 0.2 | Female | Abuse | Normo | 3 | MD |
| Patient 32 | 7 | Male | Pedestrian versus car | Normo | 9 | MD |
| Patient 33 | 11 | Male | MVA | Normo | 12 | MD |
| Patient 34 | 10 | Male | Arrow to head | Normo | 7 | SD |
MVA, motor vehicle accident, GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; G, good; MD, moderate disability; SD, severe disability; D, dead; Normo, normothermia; Hypo, hypothermia.
To further examine the time course of ET-1 over the entire 5-day study period, group-based trajectory analysis was performed with the PROC TRAJ macro in SAS version 9.1, using an algorithm adapted from Nagin (2005). ET-1 data were log transformed before the trajectory analysis. Chi-square analyses were completed to determine differences between trajectory group membership and demographic and clinical data, specifically age, gender, temperature, mechanism, GCS, and GOS. GOS scores were dichotomized into two groups: GOS score 1–3 (unfavorable outcome), and GOS 4–5 (favorable outcome) for analysis. A p value <0.05 was considered significant in all analyses.
Results
A total of 34 children with TBI were enrolled in this study (n = 19 hypothermic and n = 15 normothermic), and a total of 136 samples were obtained. Median age was 7 years (range 0.16–15 years), and median admission GCS score was 7 (range 3–14; Table 1). There were no significant differences in baseline characteristics between study groups.
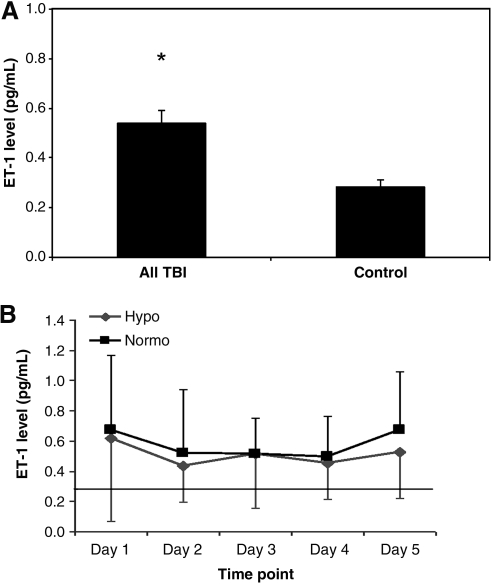
CSF ET-1 concentrations, averaged over the first week post-injury, were significantly increased in infants and children after TBI compared to controls. A two to threefold increase in ET-1 levels was maintained for up to 5 days post-TBI in all patients, and did not return to control values throughout the duration of sampling (Fig. 1A). In those with TBI, the mean CSF ET-1 concentrations over 5 days did not differ between those treated with hypothermia versus those with normothermia (Fig. 1B).
FIG. 1.
(A) Average CSF ET-1 levels in all patients with TBI and controls. Values represent mean with standard error of the mean. (*p < 0.05 for TBI patients versus controls). (B) Time course of daily mean CSF ET-1 levels in hypothermic (Hypo) and normothermic (Normo) patients after TBI. The solid black line indicates the mean ET-1 level for control patients on day 1. Values are represented as mean with standard deviation. No statistical significance was noted between these two groups (CSF, cerebrospinal fluid; TBI, traumatic brain injury; ET, endothelin).
Peak CSF levels of ET-1 were also similar in both hypothermic and normothermic patients, though the mean peak level of ET-1 was significantly elevated in both hypothermic and normothermic patients post-severe TBI (Fig. 2A). iTBI (abusive head trauma) patients demonstrated similar sustained increases in ET-1 concentrations to those seen in non-abused TBI patients. Peak ET-1 values for iTBI patients and non-iTBI patients were similar and increased (p < 0.05) compared to controls (Fig. 2B). No statistically significant associations between either peak or initial ET-1 levels and GOS scores were identified.
FIG. 2.
(A) Peak CSF ET-1 levels for hypothermic (Hypo), normothermic (Normo), and control patients were 0.74, 0.86, and 0.28 pg/mL, respectively (*p < 0.05 for hypothermic and normothermic patients versus controls). (B) Mean CSF ET-1 levels in TBI and iTBI patients, and peak CSF ET-1 levels in control patients, TBI, and iTBI patients. The peak ET-1 levels in iTBI and non-iTBI patients were similar (0.90 and 0.81 pg/mL, respectively), and were statistically significantly increased versus controls (*p < 0.05); their mean values were 0.59 and 0.51 pg/mL, respectively (CSF, cerebrospinal fluid; TBI, traumatic brain injury; iTBI, inflicted traumatic brain injury; ET, endothelin).
The trajectory analysis revealed two distinct groups based on the time course of ET-1 levels (Fig. 3): group 1, with lower ET-1 levels (54%), and group 2, with higher ET-1 levels (46%). Univariate analyses of these two distinct groups did not reveal any significant differences based on temperature, age, gender, GCS score, or injury mechanism (iTBI versus TBI). However, the results did show that GOS scores significantly differed between ET-1 trajectory groups (p = 0.03). Subjects with persistently higher ET-1 levels over time (group 2) had unfavorable outcomes compared to subjects with lower ET-1 levels (group 1; Fig. 4).
FIG. 3.
Log ET-1 concentrations versus time from insult (days) in the high ET-1 level (group 2) and the low ET-1 level (group 1) groups as identified by trajectory analysis. Data are presented as mean log ET-1 values measured per day. The solid line represents the actual value, and the dashed line represents the predicted value. The patients in the high-level group (n = 15; 45.8%) had higher ET-1 values than those in the low-level group (n = 18; 54.2%; ET, endothelin).
FIG. 4.
Glasgow Outcome Scale (GOS) stratification based on trajectory group assignment. Those in trajectory group 1 were associated with lower ET-1 levels and favorable outcomes, while those in trajectory group 2 were associated with higher ET-1 levels and unfavorable outcomes (MD, moderate disability; SD, severe disability; ET, endothelin).
Discussion
We have found that ET-1, a potent vasoconstrictor, is increased in CSF after severe TBI in children. Moreover, we have found by using trajectory analysis that those children with sustained increases in CSF ET-1 concentrations over time are associated with unfavorable neurological outcomes.
Severe pediatric TBI is associated with unfavorable outcomes due to secondary injury by a variety of mechanisms. A mismatch between CBF and metabolism has been suggested with early hypoperfusion after injury and superimposed hypermetabolism (Armstead et al., 2004; Kochanek et al., 2006; Robertson et al., 1992). Hypoperfusion of injured brain tissue after severe TBI in children is associated with unfavorable outcomes (Adelson et al., 2005). ET-1 is a powerful vasoconstrictor that exerts its effects on the cerebrovascular endothelium for sustained periods after TBI, causing vasoconstriction and decreased CBF, and this could represent a potential target for CBF-promoting therapies.
Our results demonstrate a sustained increase in CSF ET-1 levels in pediatric patients after severe TBI. This is a somewhat surprising finding in children. In the early 1980s, a commonly-accepted paradigm was that hyperemia resulted from increased cerebral blood volume after pediatric TBI, and caused diffuse swelling and worse outcomes in pediatric TBI patients (Kochanek, 2006). More recent work on CBF in pediatric TBI patients revealed that hyperemia occurred in a minority of patients, and that it was correlated with decreased, not increased, CBF, and unfavorable outcomes (Adelson et al., 2005). The sustained elevation of ET-1 seen in the CSF of patients after TBI could potentially result in persistent hypoperfusion or vasoconstriction, with resultant cerebral ischemia, and thus may prove to be a potential target for future therapies directed at optimizing CBF to injured brain tissue.
Increases in CSF ET-1 levels were observed consistently in our pediatric patients, and these increases were remarkably sustained. This sustained increase in ET-1 may result in microcirculatory changes and vasoconstriction, with the potential for vasospasm and decreased CBF. Alternatively, in some cases we could not rule out the possibility that ET-1 may protect against the detrimental effects of hyperemic flow that have been reported in some patients (Bruce et al., 1981), and that this may be linked to increased cerebral blood volume and ICH. Further work is needed to directly examine the association between ET-1 levels and CBF in pediatric TBI patients.
Our data did not reveal any association between initial GCS score and initial or peak ET-1 levels up to 5 days post-injury. However, the GCS scores used for analysis were the scores assigned at the time of initial presentation. The initial GCS score did not reflect the degree of neurological deterioration that occurred post-resuscitation that subsequently qualified patients for study enrollment.
Recent studies in adults have revealed increased CSF ET-1 levels up to 2 weeks post-TBI (Maier, 2007), and up to 5 days post-SAH (Kastner et al., 2005). In studies of experimental animal models researchers have also reported a two- to threefold increase in ET-1 after TBI alone (Steiner, 2004). Data collected by Maier and associates (2007) suggest that adults with higher ET-1 levels in the CSF after TBI had unfavorable clinical outcomes, including death, permanent vegetative state, or severe disability. We observed a similar association in the pediatric population when evaluating GOS based on trajectory group assignment.
The trajectory analysis identified two distinct groups in the study population based on ET-1 levels. Traditionally, studies of TBI have used single-point estimates, such as the mean or maximum biomarker levels, taken over a period of time to predict outcome after TBI. However, due to the variance of certain biomarker levels over time, traditional point estimates do not necessarily reflect all of the information present in time series biomarker data. Trajectory analysis incorporates all data collected over time by identifying subpopulations that follow specific paths of change in their biomarker levels. Once different subpopulations have been identified, a subject's outcome may be predicted based on his or her subpopulation assignment. Trajectory group membership appears to be a more sensitive measure for evaluating associations with outcome than peak or initial ET-1 values. The fact that trajectory group membership is more effective in predicting outcome than point estimates has been demonstrated by other TBI biomarker analyses (Amin et al., 2009; Niyonkuru et al., 2009; Ozawa et al., 2009).
The use of therapeutic hypothermia instituted early as a neuro-protectant for the treatment of pediatric TBI remains controversial (Adelson et al., 2005; Hutchison et al., 2008), and is currently being investigated in a RCT. This study took advantage of CSF samples from infants and children who were randomized to hypothermia versus normothermia treatment in the safety and feasibility study that served as the basis for an ongoing RCT. The feasibility trial revealed a reduction in intracranial pressure (ICP) with hypothermic treatment, and a trend toward improved neurological outcomes at 3 and 6 months post-injury (Adelson et al., 2005). A more recent pediatric study revealed no benefit of therapeutic hypothermia, and possibly an increase in mortality in those who received the treatment (Hutchison et al., 2008). Important differences in study protocols exist across these various trials.
The use of therapeutic hypothermia is currently an investigational therapy, and therefore it is important to understand the effects of this therapy on various anatomical and physiological processes. In our study population, therapeutic hypothermia did not appear to influence CSF ET-1 levels over the 5-day study period. We hypothesized that hypothermia would increase ET-1 levels after TBI, given prior reports of increases in ET-1 seen in patients treated with deep hypothermic circulatory arrest (Pasaoglu et al., 1993). Although in theory, hypothermia might be anticipated to produce nonspecific reductions in levels of all biochemical mediators through a temperature-dependent reduction in chemical reaction rates, we previously reported differential effects of mild therapeutic hypothermia on various CSF mediators in infants and children after severe TBI. Hypothermia attenuated increases in CSF markers of oxidative stress and loss of antioxidants (Bayir et al., 2009); however, no effect was reported on CSF levels of a battery of cytokines and chemokines (Buttram et al., 2007).
Despite the two- to threefold increases in ET-1 seen after severe TBI, these levels appear not to be attenuated or increased by mild hypothermia, consistent with the differential effects of hypothermia discussed above. Although this is a negative finding in our study, it is important to utilize this valuable patient population treated with therapeutic hypothermia, to examine for the first time the effects of hypothermia and re-warming on vascular mediators of CBF. There is concern for vascular dysfunction and disturbances in autoregulation, both early and late after severe TBI, and it is imperative to understand the effects of a potential treatment strategy at the vascular level. However, additional exploration might be indicated, for example with paired CSF levels in patients both before and after cooling, and before and after re-warming.
Although this work identified ET-1 as a potential mediator of sustained vasoconstriction and decreased CBF post-TBI, there were several limitations of this study. First, assessment of CBF was not documented in this patient population. Future work investigating levels of ET-1 after severe TBI, and correlation with CBF as measured by transcranial Doppler (TCD) and ICP, would provide more insight into the impact of increased levels of ET-1 on CBF after TBI. Second, other local mediators acting at the vascular level may also contribute to alterations in CBF after injury to the brain, and these also need to be explored. A recent study in adult patients with SAH revealed increased levels of arachidonic acid (AA) metabolites post-insult, with evidence of symptomatic vasospasm as measured by increased velocity via TCD (Poloyac et al., 2005). We are currently carrying out prospective studies examining TCD measurements and a number of vascular mediators, including ET-1 and AA metabolites, specifically epoxyeicosatrienoic acids and hydroxyeicosatetraenoic acids, to determine the relevance of concentrations of these mediators to CBF assessments. Finally, patients suffering from TBI typically also sustain other traumatic injuries (e.g., fractures, lacerations, and internal injuries) that may augment production of ET-1. Studies in experimental models have demonstrated increased ET-1 levels in the serum of rats after trauma-hemorrhage with isolated gastrointestinal injury (Ba et al., 2005). Therefore measurement of both local (CSF) and systemic (serum) levels of ET-1 may be helpful.
In summary, this is the first study to demonstrate a sustained increase in ET-1 levels in the CSF of pediatric patients after severe TBI, and that these increases are associated with unfavorable outcomes. Although the role of ET-1 in the pathogenesis of secondary injury after TBI is unclear, the profound vaso-constrictive properties of this protein make it an attractive focus of further studies to elucidate a possible relationship between its persistent elevation post-TBI and the effect on CBF, and may allow the development of successful CBF-improving therapies.
Acknowledgments
This work was supported by National Institutes of Health grants 5T32HD040686-09 (to R.S. and P.M.K.), NS30318 (to P.M.K.), GM 073031 (to S.M.P.),and National Heart, Lung, and Blood Institute T32HL007820 (to Michael R. Pinsky).
Author Disclosure Statement
No competing financial interests exist.
References
- Adelson D. Bratton S. Carney N. Chestnut R. du Coudray H. Goldstein B. Kochanek P. Miller H. Partington M. Selden N. Warden C. Wright D. Guidelines for acute medical management of severe traumatic brain injury in infants, children and adolescents. Pediatr. Crit. Care Med. 2003;4:S1–S75. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- Adelson P. Ragheb J. Kanev P. Brockmeyer D. Beers S. Brown S.D. Cassidy L. Chang Y. Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- Amin K. Postal B. Ozawa H. Fabio A. Dixon C. Rogers E. Wagner A. Bcl-2 and cytochrome C biomarker relationships to outcome. J. Neurotrauma. 2009;26:A-71. [Google Scholar]
- Andresen J. Shafi N. Bryan R., Jr. Endothelial Influences on cerebrovascular tone. J. Appl. Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Armstead W. Endothelins and the role of endothelin antagonists in the management of posttraumatic vasospasm. Curr. Pharm. Design. 2004;10:2185–2192. doi: 10.2174/1381612043384178. [DOI] [PubMed] [Google Scholar]
- Ba S. Shimizu T. Szalay L. Bland K. Chaudry I. Gender differences in small intestinal perfusion following trauma hemorrhage: The role of endothelin-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G860–G865. doi: 10.1152/ajpgi.00437.2004. [DOI] [PubMed] [Google Scholar]
- Bayir H. Adelson P. Wisniewski S. Shore P. Lai Y. Brown D. Janesko-Feldman K. Kagan V. Kochanek P. Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Crit. Care Med. 2009;37:689–695. doi: 10.1097/CCM.0b013e318194abf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M. Kochanek P. Heyes M. Wisniewski S. Sinz E. Clark R. Blight A. Marion D. Adelson P. Quinolinic acid in the cerebrospinal fluid of children after traumatic brain injury. Crit. Care Med. 1999;27:493–497. doi: 10.1097/00003246-199903000-00023. [DOI] [PubMed] [Google Scholar]
- Bell M. Robertson C. Kochanek P. Goodman J. Gopinath S. Carcillo J. Clark R. Marion D. Mi Z. Jackson E. Interstitial brain adenosine and xanthine increase during jugular venous oxygen desaturation in humans after traumatic brain injury. Crit. Care Med. 2001;29:399–404. doi: 10.1097/00003246-200102000-00033. [DOI] [PubMed] [Google Scholar]
- Bruce D. Alavi A. Bilaniuk L. Dolinskas C. Obrist W. Uzzell B. Diffuse cerebral swelling following head injuries in children: the syndrome of “malignant brain edema.”. J. Neurosurgery. 1981;54:170–178. doi: 10.3171/jns.1981.54.2.0170. [DOI] [PubMed] [Google Scholar]
- Buttram S. Wisniewski S. Jackson E. Adelson P. Feldman K. Bayır H. Berger R. Clark R. Kochanek P. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: Effects of moderate hypothermia. J. Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- Clark R. Kochanek P. Obrist W. Wong H. Billiar T. Wisniewski S. Marion D. Cerebrospinal fluid and plasma nitrite and nitrate concentrations after head injury in humans. Crit. Care Med. 1996;24:1243–1251. doi: 10.1097/00003246-199607000-00030. [DOI] [PubMed] [Google Scholar]
- Gorlach C. Hortobagyi T. Hortobagyi S. Benyo Z. Wahl M. Inhibition of endothelin-1 by the competitive ETA receptor antagonist Ro 61-1790 reduces lesion volume after cold injury in the rat. Eur. J. Physiol. 2001;441:844–849. doi: 10.1007/s004240000495. [DOI] [PubMed] [Google Scholar]
- Hama H. Kasuya Y. Sakurai T. Yarnanda G. Suzuki N. Masaki T. Goto K. Role of endothelin-1 in astrocyte responses after acute brain damage. J. Neurosci. Res. 1997;47:590–602. doi: 10.1002/(sici)1097-4547(19970315)47:6<590::aid-jnr4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ho M. Lo A. Kurihara H. Yu A. Chung S. Chung S. Endothelin-1 protects astrocytes from hypoxic/ischemic injury. FASEB J. 2001;15:618–626. doi: 10.1096/fj.99-1022com. [DOI] [PubMed] [Google Scholar]
- Hutchison J. Ward R. Lacroix J. Hebert P. Barnes M. Bohn D. Dirks P. Doucette S. Fergusson D. Gottesman R. Joffe A. Kirpalani H. Meyer P. Morris K. Moher D. Singh R. Skippen P. Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 2008;358:2447–2457. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- Kastner S. Oertel M. Scharbrodt W. Krause M. Boker D. Deinsberger W. Endothelin-1 in plasma, cisternal CSF and microdialysate following aneurysmal SAH. Acta Neurochir. 2005;147:1271–1279. doi: 10.1007/s00701-005-0633-0. [DOI] [PubMed] [Google Scholar]
- Kochanek P. Pediatric traumatic brain injury: Quo vadis? Dev. Neurosci. 2006;28:244–255. doi: 10.1159/000094151. [DOI] [PubMed] [Google Scholar]
- Maier B. Lehner M. Laurer H. Marzi I. Biphasic elevation in cerebrospinal fluid and plasma concentrations of endothelin 1 after traumatic brain injury in human patients. Shock. 2007;27:610–614. doi: 10.1097/shk.0b013e31802f9eaf. [DOI] [PubMed] [Google Scholar]
- Muizelaar J. Cerebral blood flow, cerebral blood volume and cerebrovascular reactivity after severe head injury. J. Neurotrauma. 1992;9:S333–S348. [PubMed] [Google Scholar]
- Nabika S. Kiya K. Satoh H. Mizoue T. Oshita J. Kondo H. Ischemia of the internal capsule due to mild head injury in a child. Pediatr. Neurosurg. 2007;43:312–315. doi: 10.1159/000103313. [DOI] [PubMed] [Google Scholar]
- Nagin D.S. Group Based Modeling of Development. Harvard University Press; Cambridge: 2005. [Google Scholar]
- Niyonkuru C. Rogers H. Ozawa H. Fabio A. Dixon C. Loucks T. Berga S. Wagner A. Serum hormones as prognostic indicators of recovery after severe TBI. J. Neurotrauma. 2009;26:A16. [Google Scholar]
- Ojha B. Jha D. Kale S. Mehta V. Transcranial Doppler in severe head injury: evaluation of pattern changes in cerebral blood flow velocity and its impact on outcome. Surg. Neurol. 2005;64:174–179. doi: 10.1016/j.surneu.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Ozawa H. A practical example of trajectory analysis for defining temporal CSF biomarker patterns to predict outcome after traumatic brain injury. Military Health Research Forum 2009; Aug 31 3;Sep 31 3;2009 ; Kansas City, MO. 2009. [Google Scholar]
- Ozawa H. Fabio A. Rogers E. Niyonkuru C. Loucks T. Dixon C. Wagner A. Modeling trajectory patterns of serum hormone production in comparison to clinical variables in severe TBI. J. Neurotrauma. 2009;26:A71. [Google Scholar]
- Pasaoglu I. Erbas B. Varoglu E. Yorgancioglu C. Hazan E. Koray Z. Bekdik C. Bozer Y.A. Changes in circulating endothelin and atrial natriuretic peptide levels during coronary artery bypass surgery. Japanese Heart J. 1993;34:693–706. doi: 10.1536/ihj.34.693. [DOI] [PubMed] [Google Scholar]
- Poloyac S. Reynolds R. Yonas H. Kerr M. Identification and quantification of the hydroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J. Neurosci. Methods. 2005;144:257–263. doi: 10.1016/j.jneumeth.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Robertson C. Goodman J. Grossman R. Blood flow and metabolic therapy in CNS injury. J. Neurotrauma. 1992;9:S579–S594. [PubMed] [Google Scholar]
- Ruppel R. Kochanek P. Adelson P. Rose M. Wisniewski S. Bell M. Clark R. Marion D. Graham S. Excitatory amino acid concentrations in ventricular cerebrospinal fluid after severe traumatic brain injury in infants and children: the role of child abuse. J. Pediatr. 2001;138:18–25. doi: 10.1067/mpd.2001.110979. [DOI] [PubMed] [Google Scholar]
- Shore P. Jackson E. Wisniewski S. Clark R. Adelson P. Kochanek P. Vascular endothelial growth factor is increased in cerebrospinal fluid after traumatic brain injury in infants and children. Neurosurgery. 2004;54:605–611. doi: 10.1227/01.neu.0000108642.88724.db. [DOI] [PubMed] [Google Scholar]
- Steiner J. Rafols D. Park H. Katar M.S. Rafols J. Petrov T. Attenuation of iNOS mRNA exacerbates hypoperfusion and upregulates endothelin-1 expression in hippocampus and cortex after brain trauma. Nitric Oxide. 2004;10:162–169. doi: 10.1016/j.niox.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Vavilala M. Muangman S. Waitayawinyu P. Roscigno C. Jaffe K. Mitchell P. Kirkness C. Zimmerman J. Ellenbogen R. Lam A. Neurointensive care: Impaired cerebral autoregulation in infants and young children early after inflicted traumatic brain injury: A preliminary report. J. Neurotrauma. 2007;24:87–96. doi: 10.1089/neu.2006.0058. [DOI] [PubMed] [Google Scholar]
- Vavilala M. Tontisirin N. Udomphorn Y. Armstead W. Zimmerman J. Chestnut R. Larn A. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit. Care. 2008;9:45–54. doi: 10.1007/s12028-007-9036-9. [DOI] [PubMed] [Google Scholar]