Abstract
The purpose of this study was to examine the effects of hyperventilation or hyperoxia on cerebral hemodynamic parameters over time in patients with severe traumatic brain injury (TBI). We prospectively studied 186 patients with severe TBI. CO2 and O2 reactivity tests were conducted twice a day on days 1–5 and once daily on days 6–10 after injury. During hyperventilation there was a significant decrease in intracranial pressure (ICP), mean arterial pressure (MAP), jugular venous oxygen saturation (Sjvo2), brain tissue Po2 (Pbto2), and flow velocity (FV). During hyperoxia there was an increase in Sjvo2 and Pbto2, and a small but consistent decrease in ICP, end-tidal carbon dioxide (etco2), partial arterial carbon dioxide pressure (Paco2), and FV. Brain tissue oxygen reactivity during the first 12 h after injury averaged 19.7 ± 3.0%, and slowly decreased over the next 7 days. The autoregulatory index (ARI; normal = 5.3 ± 1.3) averaged 2.2 ± 1.5 on day 1 post-injury, and gradually improved over the 10 days of monitoring. The ARI significantly improved during hyperoxia, by an average of 0.4 ± 1.8 on the left, and by 0.5 ± 1.8 on the right. However, the change in ARI with hyperoxia was much smaller than that observed with hyperventilation. Hyperventilation increased ARI by an average of 1.3 ± 1.9 on the left, and 1.5 ± 2.0 on the right. Pressure autoregulation, as assessed by dynamic testing, was impaired in these head-injured patients. Acute hyperoxia significantly improved pressure autoregulation, although the effect was smaller than that induced by hyperventilation. The very small change in Paco2 induced by hyperoxia does not appear to explain this finding. Rather, the vasoconstriction induced by acute hyperoxia may allow the cerebral vessels to respond better to transient hypotension. Further studies are needed to define the clinical significance of these observations.
Key words: cerebral autoregulation, hyperoxia, hyperventilation, traumatic brain injury
Introduction
Probes to monitor brain tissue Po2 (Pbto2) have been commercially available since 1987 (Schultheiss et al., 1987), and are becoming more widely used in the management of patients with TBI, subarachnoid hemorrhage, and other acute neurological problems. Pbto2 monitoring may be useful to assess the efficacy of treatments based on their ability to restore adequate Pbto2 levels, but there are several technical issues involved with monitoring Pbto2 that remain controversial.
Arterial Po2 (Pao2) strongly influences the Pbto2 measured with these probes. The Pbto2 response to hyperoxia measured with the available probes varies widely in different patients, and it may also vary considerably over time. The measured effect of hyperoxia on cerebral metabolism after TBI has also been variable, with some studies suggesting an improvement in cerebral metabolic rate of oxygen (Cmro2) in at-risk brain tissue (Nortje et al., 2008), with an improved microdialysate lactate:pyruvate ratio (Nortje et al., 2008; Tolias et al., 2004). Other studies have found no change in Cmro2 and no change in the microdialysate lactate:pyruvate ratio with hyperoxia (Diringer et al., 2007; Magnoni et al., 2003).
Since raising the Pao2 when hemoglobin is fully saturated does not increase overall oxygen content very much, and since the studies performed to date have had variable results, the effectiveness of this practice remains controversial, and more information about the cerebral hemodynamic effects of hyperoxia would be clinically useful.
In normal subjects, breathing 100% oxygen results in vasoconstriction of cerebral blood vessels, and a subsequent decrease in cerebral blood flow (CBF). Breathing 100% oxygen also increases Pbto2. The vascular and tissue response to hyperoxia may change in disease states such as TBI. Two oxygen reactivity variables have been used to describe these two different effects of hyperoxia. Cerebral oxygen vasoreactivity (Covr) assesses the vasoconstriction induced by hyperoxia. Brain tissue oxygen reactivity (Btor) is defined as the increase in Pbto2 relative to the increase in Pao2 induced by hyperoxia. These derived variables might provide insights about regulation of CBF to maintain adequate cerebral oxygenation following injury.
Relatively little information is available about the changes that occur in these oxygen reactivity variables in disease states. In a few studies researchers have reported that Covr can be reduced in cerebrovascular disease and after TBI (Amano et al., 1983; Menzel et al., 1999; Nakajima et al., 1983). Some authors have found that the increase in Pbto2 with hyperoxia is much greater in pathological tissue than in normal brain tissue (Meixensberger et al., 1993). Others have found that Btor is directly related to the baseline Pbto2 (Longhi et al., 2002) or regional CBF (Hlatky et al., 2008), or that Btor is inversely related to outcome (Menzel et al., 1999). The purpose of this study was to examine the effects of hyperventilation and hyperoxia on cerebral hemodynamics after TBI.
Methods
Patient selection and clinical management
A total of 186 patients with severe TBI (motor component of the Glasgow Coma Scale score ≤5), who were admitted to Ben Taub General Hospital in Houston, Texas, were studied (Table 1). All were enrolled in a prospective study measuring cerebral hemodynamics serially during the first 10 days after injury. Thirty-five control subjects of approximately the same age as the patient population were also recruited to obtain normal baseline values for the transcranial Doppler (TCD) parameters. The study was approved by the Institutional Review Board of the Baylor College of Medicine, and informed consent was obtained from the patients' families and from the control subjects.
Table 1.
Demographic Characteristics of 186 patients with Traumatic Brain Injury
| Mean ± SE or number (%) | Median (range) | |
|---|---|---|
| Total number of patients | 186 | |
| Age (y) | 34.0 ± 1.0 | 33 (15–73) |
| Gender | ||
| Male | 165 (88.7%) | |
| Female | 21 (11.3%) | |
| GCS score in the emergency department | 6.1 ± 0.2 | 6 (3–14) |
| AIS score | 27.9 ± 0.5 | 25 (9–50) |
| Prehospital hypoxia | ||
| Unknown | 2 (1.1%) | |
| No | 127 (68.3%) | |
| Yes | 57 (30.6%) | |
| Prehospital hypotension | ||
| Unknown | 2 (1.1%) | |
| No | 164 (88.2%) | |
| Yes | 20 (10.7%) | |
| Emergency department CT scan codea | ||
| Diffuse injury 1 or 2 | 60 (32.2%) | |
| Diffuse injury 3 or 4 | 29 (15.6%) | |
| Mass lesion | 97 (52.2%) | |
Score based on the CT categories of Marshall and associates (Marshall et al., 1991).
SE, standard error; GCS, Glasgow Coma Scale; AIS, Abbreviated Injury Scale; CT, computed tomography.
Upon arrival in the emergency department, the patients immediately underwent intubation, hemodynamic stabilization, and resuscitation when necessary. Patients in whom an intracranial mass lesion was demonstrated on the initial computed tomography (CT) scan underwent an immediate neurosurgical procedure. ICP, blood pressure, etco2, Sjvo2, and Pbto2 were monitored in all patients during the acute post-injury period. The goals of early management were ICP <20 mm Hg and cerebral perfusion pressure (CPP) >60 mm Hg, unless the Sjvo2 and/or Pbto2 values indicated that a higher level of CPP was needed (Rangel-Castilla and Robertson, 2006).
Measurement of brain tissue Po2
The Pbto2 was measured continuously in all patients with a miniaturized Clark-type electrode Pbto2 probe (LICOX; Integra Neurocare LLC, San Diego, CA). An area of brain that appeared injured but that was not clearly necrotic was targeted for probe placement when possible. Otherwise, the probe was placed in the right frontal white matter. The Pbto2 values were temperature-compensated, either from a temperature probe incorporated into the Pbto2 probe, or from a separate temperature probe implanted near the Pbto2 probe. The position of the Pbto2 probe was confirmed on follow-up CT scan. In 38 of the patients, the probe was placed in normal-appearing tissue. In the remaining patients, the probe was placed either near contused brain tissue, or was placed at surgery in brain tissue that had been previously compressed by the hematoma that was evacuated.
The Pbto2 values were continuously collected at 30-sec intervals and stored in a computer. At the end of the monitoring period the Pbto2 probes were removed and calibration drift was determined by measuring stable Po2 in a 0% solution, in a Level 1 arterial blood gas control solution (Ciba-Corning Diagnostics Corp., Cambridge, MA), and in room air. Only data from Pbto2 probes that remained correctly calibrated were used in this study. Pbto2 data from five patients were excluded from the analysis for this reason.
Study protocol for the TBI patients
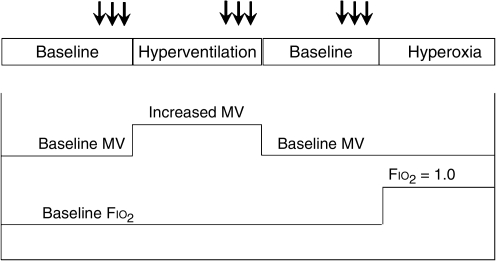
Testing of cerebral hemodynamics was performed serially during the first 10 days after injury (twice a day for the first 5 days, then once a day for 5 additional days). A diagram of the testing protocol is shown in Figure 1. Measurements of dynamic pressure autoregulation were obtained during four physiological conditions: baseline, hyperventilation, baseline, and hyperoxia. For the hyperventilation phase, the minute ventilation was increased, targeting a decrease in Pco2 of approximately 5 mm Hg. For the hyperoxia phase, the inspired oxygen concentration was increased from the baseline level (Fio2 usually 0.4) to an Fio2 of 1.0. CO2 reactivity (co2r) and Covr were calculated from the steady-state changes between the baseline and the hyperventilation and hyperoxia phases, respectively. On days when a Pbto2 probe was in place (usually the first 5–7 days), Btor was also calculated from the steady-state changes in Pao2 and Pbto2 at the baseline and hyperoxia conditions. Hemodynamic conditions were allowed to return to baseline between the hyperventilation and hyperoxia tests. This stabilization period usually lasted 10–15 min.
FIG. 1.
Protocol for the experiment. Dynamic pressure autoregulation was tested by the cuff deflation method in triplicate (tests are indicated by the arrows), after equilibrating for 10–15 min in four test conditions: baseline, hyperventilation, baseline, and hyperoxia (MV, minute ventilation, Fio2, fraction of inspired oxygen).
Dynamic pressure autoregulation was measured by the cuff deflation method of Aaslid and colleagues (Aaslid et al., 1989). Mean blood pressure and bilateral middle cerebral artery flow velocity were recorded continuously before, during, and after a step drop in blood pressure. Blood pressure was recorded from a radial artery catheter. Middle cerebral artery flow velocity was monitored bilaterally with a transcranial Doppler ultrasonography system equipped with dual 2-MHz transducers (Multi-Flow; DWL Electronic Systems GmbH, Sipplingen, Germany). The thigh cuff deflation technique was used to induce the drop in blood pressure. Large bilateral thigh cuffs were inflated to 20 mm Hg above the systolic arterial pressure for a period of 2 min. A transient blood pressure drop was then induced by rapid deflation of the thigh cuffs. A drop in blood pressure of at least 15 mm Hg was required to be considered a valid cuff deflation test (Hlatky et al., 2006).
Study protocol for control subjects
The control subjects had measurements of cerebral hemodynamics (baseline autoregulatory index [ARI] and co2r) using the same protocol and equipment as the TBI patients, except that hyperventilation was induced by having the subject increase his or her respiratory rate and depth of breathing, rather than by adjusting the ventilator settings as in the TBI patients.
Calculation of CO2 reactivity and O2 reactivity
co2r was calculated using the following formula: co2r = (ΔFV * 100%/baseline FV)/ΔpCO2.
Covr was calculated using the following formula: Covr = −(ΔFV * 100%)/baseline FV. Covr has been shown to vary in normal subjects depending on the method used for measuring the change in cerebral blood flow. When middle cerebral artery FV was measured to assess Covr, a 20% decrease in FV (from 65 ± 15 to 52 ± 16 cm/sec) was observed in normal subjects as oxygen concentration was increased from 21% to 100% (Omae et al., 1998).
Btor was calculated using the following formula: Btor = ΔPbto2 * 100%/ΔPao2 (van Santbrink et al., 1996). A normal Btor is not available since Pbto2 is measured with an invasive monitor that has limited use in normal subjects.
Calculation of autoregulatory index
An ARI ranging from 0–9 was calculated by the method proposed by Tiecks and associates (Tiecks et al., 1995), and implemented in the DWL MultiDop transcranial Doppler software (Compumedics DWL). Briefly, the recorded flow velocity response to the cuff deflation was compared with 10 models constructed using the mean arterial pressure as the input function. Each model is generated from the arterial tracing by a specific combination of time constant, damping factor, and autoregulatory dynamic gain. The closest match was selected based on the highest correlation coefficient. A normal ARI using this method is 5.0 ± 1.1 (mean ± standard deviation; Tiecks et al., 1995).
Statistical analysis
Summary data in the text, tables, and figures are shown as mean ± standard error unless otherwise noted. Physiological parameters before and during hyperventilation, and before and during hyperoxia, were analyzed using a general linear mixed model. The analysis method was chosen because the data were from multiple assessments made over time. This model provides unbiased estimates of the random effect, flexibility in the choice of the variance-covariance structures of the model, and maximum likelihood estimation. There were two observations per day across the first 5 days, and then one observation per day across 5 additional days. There was an effect of day, and an effect of hyperventilation or hyperoxia, and an interaction term that tested whether the difference with hyperventilation or hyperoxia varied significantly by day.
Results
Patient demographics
The characteristics of the 186 patients who were studied are summarized in Table 1. There was a predominance of males (88.7%), with a mean age of 34.0 ± 1.0 years, and an initial GCS score of 6.1 ± 0.2. Prehospital hypoxia and hypotension was present in 30.6% and 10.7% of patients, respectively. Initial head CT scans revealed mass lesions that required surgical evacuation in 97 (52.2%) patients, type 1 and 2 diffuse injury in 60 (32.2%) patients, and type 3 and 4 diffuse injury in 29 (15.6%) patients.
The 35 control subjects recruited to obtain normal baseline values for the TCD parameters consisted of 13 males and 22 females. The average age of the control subjects was 30.5 ± 1.4 years (median 30 years, range 19–51 years).
Normal baseline values for autoregulatory index and co2r
The average value for ARI in the 35 normal subjects was 5.3 ± 1.3 (mean ± standard deviation). The average value for co2r in the 35 normal subjects was 3.4 ± 1.9%/mm Hg (mean ± standard deviation). There was not a significant age or gender effect for these TCD parameters.
Cerebral hemodynamics and flow velocity during hyperventilation and hyperoxia
A total of 1460 assessments of co2r (average 7.8/patient), and 922 assessments of o2r (average 4.9/patient) were done in these 186 patients during the 10-day period of study. Tables 2 and 3 show the changes in ICP, MAP, Pbto2, Sjvo2, etco2, arterial Po2 (Pao2) and Pco2 (Paco2), middle cerebral artery flow velocity (FV), and ARI that occurred after hyperventilation induced by increasing minute ventilation (Table 2), and after hyperoxia induced by breathing 100% oxygen (Table 3). The statistics in each table give the p values for the main effects of the test (hyperventilation or hyperoxia, respectively), and the time of the exam after injury, and also for the interaction of test and time. When the interaction of test and time was significant, indicating that the change in the physiological parameter during the hyperventilation or hyperoxia test varied over time, p values are also given for comparisons of the change during exams 1–5 (admission through day 2.5) to the change during exams 6–10 (days 3–5), for exams 1–5 compared to exams 11–15 (days 6–10), and for exams 6–10 compared to exams 11–15. Since Pbto2 and Sjvo2 were usually not monitored for the full 10 days, the comparisons were done for exams 1–4 (admission though day 2), exams 5–8 (days 2.5–4), and exams 9–12 (days 4.5–7).
Table 2.
Physiological Variables During the Hyperventilation (HV) Test
| |
Steady-state changes with hyperventilation |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
|
|
p Value |
|||||
| Baseline values (mean ± standard error) | HV values (mean ± standard error) | HV test (baseline versus HV values) | Exam time | Interaction (HV test × time) | HV test for exams 1–5 versus 6–10a | HV test for exams 6–10 versus 11–15a | HV test for exams 1–5 versus 11–15a | |
| Intracranial pressure (mm Hg) | 19.3 ± 0.5 | 13.2 ± 0.4 | <0.0001 | .0520 | <0.0001 | <0.0001 | .7649 | .0002 |
| Mean arterial pressure (mm Hg) | 91.9 ± 0.6 | 89.1 ± 0.6 | <0.0001 | <0.0001 | .0051 | .0002 | .1988 | .1902 |
| Jugular venous o2 saturation (%) | 72.8 ± 0.6 | 68.5 ± 0.7 | <0.0001 | .1471 | .1965 | |||
| Brain tissue Po2 (mm Hg) | 34.2 ± 3.1 | 31.5 ± 3.3 | .0003 | .6881 | .0188 | .0143 | .2010 | .0074 |
| End-tidal co2 (mm Hg) | 35.7 ± 0.3 | 29.6 ± 0.3 | <0.0001 | <0.0001 | .0725 | |||
| Inspired o2 fraction | .44 ± .29 | .44 ± .25 | ||||||
| Arterial Po2 (mm Hg) | 152 ± 3 | 143 ± 3 | .0001 | <0.0001 | .6108 | |||
| Arterial Pco2 (mm Hg) | 37.1 ± 0.3 | 30.7 ± 0.3 | <0.0001 | .0274 | .0268 | .0198 | .6859 | .2949 |
| Flow velocity (cm/sec): Left | 75.5 ± 1.4 | 61.7 ± 1.2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | .0242 | <0.0001 |
| Flow velocity (cm/sec): Right | 73.4 ± 1.4 | 59.6 ± 1.2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | .9491 | <0.0001 |
| Autoregulatory index: Left | 2.7 ± 0.1 | 3.9 ± 0.1 | <0.0001 | <0.0001 | .7721 | |||
| Autoregulatory index: Right | 2.5 ± 0.1 | 4.0 ± 0.1 | <0.0001 | <0.0001 | .0842 | |||
For Pbto2, the comparisons are between exams 1–4, exams 5–8, and exams 9–12.
Table 3.
Physiological Variables During the Hyperoxia (HO) Test
| |
Steady-state changes with HO |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
|
|
p Value |
|||||
| Baseline (mean ± standard error) | HO (mean ± standard error) | HO test (baseline versus HO) | Exam time | Interaction (HO test × exam) | HO test for exams 1–5 versus 6–10a | HO test for exams 6–10 versus 11–15a | HO test for exams 1–5 versus 11–15a | |
| Intracranial pressure (mm Hg) | 19.6 ± 0.5 | 18.0 ± 0.4 | <0.0001 | .0472 | .3260 | |||
| Mean arterial pressure (mm Hg) | 92.7 ± 0.6 | 93.4 ± 0.7 | .1018 | <0.0001 | .0277 | .0004 | .2695 | .1431 |
| Jugular venous o2 saturation (%) | 71.9 ± 0.6 | 78.2 ± 0.6 | <0.0001 | .1217 | .3423 | |||
| Jugular venous Po2 (mm Hg) | 44 ± 1 | 54 ± 1 | <0.0001 | .5604 | .3940 | |||
| Brain tissue Po2 (mm Hg) | 27.8 ± 2.5 | 60.2 ± 4.2 | <0.0001 | .2745 | .0448 | .6949 | .7313 | .5563 |
| End-tidal co2 (mm Hg) | 34.7 ± 0.3 | 32.6 ± 0.3 | <0.0001 | <0.0001 | .3748 | |||
| Inspired o2 fraction | .41 ± .12 | .99 ± .10 | ||||||
| Arterial Po2 (mm Hg) | 151 ± 3 | 401 ± 7 | <0.0001 | <0.0001 | .0323 | <0.0001 | .8614 | .0001 |
| Arterial Pco2 (mm Hg) | 37.0 ± 0.3 | 36.1 ± 0.2 | .0024 | .2749 | .3054 | |||
| Flow velocity (cm/sec): Left | 74.5 ± 1.5 | 71.8 ± 1.4 | <0.0001 | <0.0001 | .0845 | |||
| Flow velocity (cm/sec): Right | 72.3 ± 1.3 | 69.6 ± 1.3 | <0.0001 | <0.0001 | .4769 | |||
| Autoregulatory index: Left | 2.6 ± 0.8 | 3.0 ± 0.9 | <0.0001 | .0001 | .4745 | |||
| Autoregulatory index: Right | 2.6 ± 0.8 | 3.1 ± 1.0 | <0.0001 | .0015 | .2615 | |||
For Pbto2, the comparisons are between exams 1–4, exams 5–8, and exams 9–12.
The pre-hyperventilation baseline values for MAP and FV were lowest for the first five exams (the first 2.5 days after injury), and significantly increased over time after injury (the time effect shown in Table 2). During the hyperventilation test, there was a significant decrease in ICP, Pbto2, Sjvo2, and FV (the test effect shown in Table 2). There was also a small but significant decrease in MAP with hyperventilation. The decrease induced by hyperventilation in all of these parameters except for Sjvo2 was smallest during the first five exams, and significantly increased over time after injury (the time by test interaction shown in Table 2).
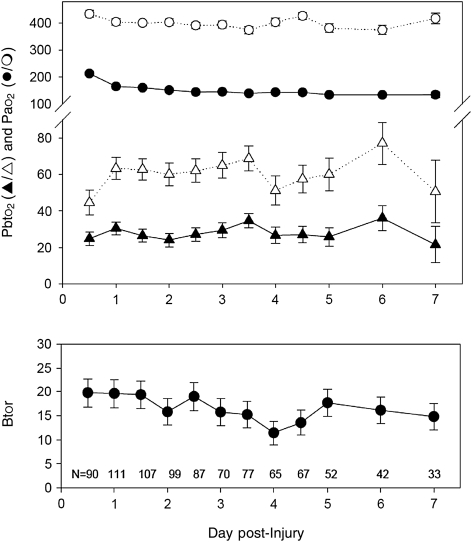
During hyperoxia there was an increase in SjvO2 and Pbto2, and a small but consistent decrease in ICP, etco2, Paco2, and FV (the test effect shown in Table 3). Except for Pbto2 and MAP, the physiological changes seen with hyperoxia did not significantly differ over time (the time by test interaction shown in Table 3). For Pbto2, both the baseline Pbto2 and the increase in Pbto2 with hyperoxia were significantly smaller during the first exam, which occurred within 12 h of injury, than during any subsequent exam. As shown in Figure 2 (upper graph), this smaller change in Pao2 during hyperoxia at the first exam may have contributed to the result of a smaller change in Pbto2 during that o2r test, since the baseline Pao2 was consistently higher for that exam.
FIG. 2.
Graphs of changes in arterial Po2 (Pao2; the circles in the upper graph), brain tissue Po2 (Pbto2; the triangles in the upper graph), and brain tissue oxygen reactivity (Btor; lower graph), over the first 7 days post-injury (mean ± standard error). The values represent 900 sets of o2r tests in 186 patients over the 7 days, and the number of data points for each time period are shown at the bottom of the graph. For the Po2 values, the closed symbols are the baseline pre-hyperoxia values, and the open symbols are the values during hyperoxia (the p values are given in Table 3). The changes in Pao2 and in Pbto2 during the first exam were significantly smaller than all of the other exams. The average values for Btor during the first six exams (admission through day 3) were significantly greater than the average Btor during the last six exams (days 3.5–7; p = 0.043).
Although MAP was not significantly altered by hyperoxia overall (test effect; p = 0.1018), there was a significant interaction of the hyperoxia test results for MAP by time after injury (p = 0.0277). During the first five exams (the first 2.5 days after injury), and the last five exams (days 6–10 after injury), the MAP was not altered by hyperoxia, but during the middle five exams (the second 2.5 days after injury), there was an increase in MAP during hyperoxia.
Vascular reactivity tests
Btor, as calculated from the results of the o2r test, was greatest during the first 36 h after injury, averaging 19.7 ±3.0% at the first exam, and then decreasing gradually over the first 7 days post-injury (Fig. 2). The average Btor for exams 1–6 (admission to day 3) was significantly greater than the average Btor during exams 7–12 (days 3.5–7; p = 0.043).
The change in Pao2 induced by increasing the inspired oxygen concentration to 100% was relatively constant over all the days of the study, except for the first exam, for which the baseline Pao2 was higher. The average Btor increased again after day 5, but the number of patients who still had Pbto2 catheter probes was too small to draw definitive conclusions.
Covr, also calculated from the results of the o2r test, during the first 12 h after injury averaged 3.6 ± 4.3 on the right and 3.7 ± 4.3 on the left. Covr tended to decrease, reaching a nadir at 7 days post-injury, but these differences over time were not significant.
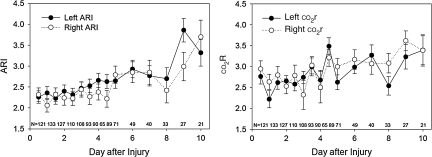
co2r, calculated from the results of the hyperventilation tests, was lowest initially and increased over time (Fig. 3; time effect, p = 0.0003 for the left side, p = 0.0342 for the right side). On the left, co2r averaged 2.6 ± 0.2%/mm Hg during the first five exams, compared to 2.9 ± 0.2%/mm Hg for exams 6–10, and 3.1 ± 0.3%/mm Hg for exams 11–15. On the right, co2r averaged 2.7 ± 0.2%/mm Hg for the first five exams, compared to 2.9 ± 0.2%/mm Hg for exams 6–10, and 3.3 ± 0.4%/mm Hg for exams 11–15.
FIG. 3.
Graphs of baseline (pre-hyperventilation) autoregulatory index (ARI) and co2 reactivity (co2r) over time after injury (mean ± standard error). The p values for these changes in ARI and co2r over time are given in Table 2.
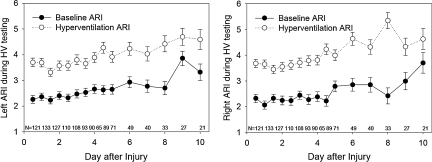
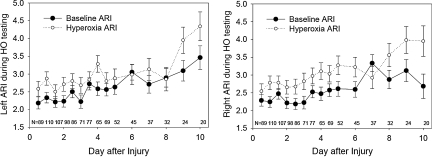
The ARI values during the two baseline conditions (baseline before hyperventilation, and baseline before hyperoxia) were not significantly different, and both averaged significantly less than the control value of 5.3 ± 1.3 obtained in the normal control subjects. The increases in ARI with hyperoxia were small, averaging 0.4 on the left and 0.5 on the right (Table 3). In contrast, the increase in ARI with hyperventilation was three times greater, averaging 1.2 on the left and 1.5 on the right (Table 2). Although the baseline ARI was lowest during the first exam, performed within 12 h of injury, and increased toward normal over the 10-day study period (time effect p < 0.0001; Fig. 3), there was not a significant interaction between the test-induced change in ARI and the exam time. This result indicates that the change in ARI induced by both hyperventilation and by hyperoxia did not significantly differ over time (Figs. 4 and 5).
FIG. 4.
Graphs of the baseline (closed circles) and hyperventilation (HV, open circles) autoregulatory index (ARI) over time after injury (mean ± standard error). The p values for these changes in ARI with hyperventilation over time are given in Table 2.
FIG. 5.
Graphs of the baseline (closed circles) and hyperoxia (HO, open circles) autoregulatory index (ARI) over time after injury (mean ± standard error). The p values for these changes in ARI with hyperoxia over time are given in Table 3.
Discussion
Cerebral hemodynamic effects of hyperventilation
Hyperventilation is sometimes used during anesthesia and intensive care to achieve a rapid reduction in intracranial blood volume and ICP. This immediate vascular effect is caused by a pH-dependent constriction of precapillary resistance vessels (Kontos et al., 1977). However, because the perivascular increase in pH induced by hyperventilation is compensated for metabolically within a few hours, the reductions in CBF and cerebral blood volume with chronic hyperventilation is transient despite persistent hypocapnia (Albrecht et al., 1987).
Gender and age have been reported in previous studies to alter co2r in normal subjects. Normal co2r is 3.7 ± 0.08%/mm Hg in women and 3.0 ± 0.10%/mm Hg in men (Kastrup et al., 1997). co2r tends to decrease with age in women, but not in men (Kastrup et al., 1998).
Cerebral hemodynamic effects of hyperoxia
In normal subjects, the change in CBF seen during hyperoxia varies from 13–27%, depending on the method used for assessing CBF (Kety and Schmidt, 1948; Nakajima et al., 1983; Omae et al., 1998; Watson et al., 2000). With transcranial Doppler techniques similar to those used in the present study, the Covr was 20% in normal volunteers (Omae et al., 1998). With cerebrovascular disease, advancing age, and TBI, Covr is generally reduced compared to normal subjects (Amano et al., 1983; Menzel et al., 1999; Nakajima et al., 1983). In a previous study of TBI patients in which CBF was measured with the stable xenon CT technique, the Covr for global changes was reduced to 9%, and the Covr response was related to the baseline (pre-hyperoxia) baseline CBF level (Menzel et al., 1999).
In the present study, both hyperventilation and hyperoxia caused vasoconstriction. However, the magnitude of the change was much smaller with hyperoxia. Both manipulations resulted in a significant decrease in ICP, as well as a decrease in FV. In contrast, hyperventilation caused a decrease in Pbto2 and Sjvo2, while hyperoxia caused a significant increase in Pbto2 and Sjvo2. Presumably the increase in oxygen-carrying capacity induced by hyperoxia more than offset the small reduction in blood flow; and as a result, total oxygen delivery increased with hyperoxia. With hyperventilation, a decrease in Paco2 occurs as a result of the study design. A small (average 0.9 mm Hg) decrease in Paco2 also occurred with hyperoxia (Table 3).
Since hyperoxia consistently results in a small decrease in Paco2, it is difficult to separate the direct effects of increased Pao2 from the effects of the decrease in Paco2. This hypocapnia has been attributed to hyperventilation induced by co2 retention in respiratory control centers, that is in turn caused by a reduced co2-carrying capacity of oxyhemoglobin in association with high concentrations of physically dissolved oxygen (the Haldane effect). Because the patients in this study were mechanically ventilated during most of the period of testing, only a very small reduction in Paco2 occurred with hyperoxia.
A direct vasoconstricting effect of hyperoxia has been demonstrated in pial window preparations for which Pco2 can be more easily controlled (Kawamura and Yasui, 1996). Floyd and colleagues (Floyd et al., 2003) demonstrated an independent vasoconstriction with hyperoxia by measuring CBF using an MRI technique at varying Paco2 values while breathing room air and 100% oxygen. The slope of the relationship between Paco2 and CBF was nearly parallel while breathing room air and 100% oxygen, but the slope was offset by a 30% decrease in CBF while breathing 100% oxygen.
Tissue oxygen reactivity
Factors that could alter the Btor might include variability in the Pao2 response to breathing 100% oxygen, a change in the diffusion of oxygen through tissues, changes in CBF, and changes in cerebral oxygen consumption, usually called the cerebral metabolic rate of oxygen (Cmro2). In addition, insertion of the Pbto2 probe could cause local injury to the brain, and transiently alter Btor during the early post-injury period.
The o2r test requires placing the patient on 100% oxygen to induce a transient increase in Pao2. Therefore the calculation of Btor could be influenced in two ways by the presence of pulmonary disease, which is common in the post-injury period. First, if the patient requires a high Fio2 to maintain oxygenation at baseline, then it would not be possible to conduct the test. Second, the increase in Pao2 induced by placing the patient on 100% oxygen might be variable, depending on the amount of shunting present in the lungs.
Expressing the Pbto2 response as a percentage of the Pao2 changes observed may help compensate for this problem, but this approach assumes that the Pbto2 response to changing Pao2 is linear throughout the range of Pao2 studied. A study carried out by Menzel and associates (Menzel et al., 1999) suggested that this is the case. Except for the first o2r test, the Pao2 response to 100% oxygen was relatively constant over time for the entire group of patients, suggesting that although the phenomenon may be important in interpreting individual patient responses, this is not the main factor causing Btor to decrease over time for the group.
Changes in CBF and also in Cmro2 could be other factors that might influence Btor. CBF determines oxygen delivery to the tissues, and was estimated in the present study by FV. Hlatky and colleagues observed that a reduction in regional CBF at the site of the Pbto2 probe may limit the increase in Pbto2 seen with hyperoxia, especially when regional CBF is very low (Hlatky et al., 2008). Although FV evolved over time in the present study, there was not a clear relationship between FV and Btor, and Btor was greatest in the first 36 h post-injury, when FV was lowest. Cmro2 was not measured in the current study. However, in PET studies specifically measuring Cmro2 before and during hyperoxia, the researchers did not find a significant change in Cmro2, except possibly in the penumbral region (Diringer et al., 2007; Nortje et al., 2008).
One limitation of commercially available Pbto2 probes for assessing the effect of hyperoxia on brain tissue oxygenation is that the Pbto2 value represents the average oxygen concentration in all tissue components (including arteries, capillaries, and veins), in the area surrounding the Pbto2 probe. As a consequence of the arterial contribution to the Pbto2 value, increasing Pao2 has a greater influence on Pbto2 than tissue oxygenation measured using microelectrodes. Alterations in the relative representation of arterial components in tissue surrounding the Pbto2 probe could also contribute to variability in the Btor.
The increase in Pbto2 after hyperoxia varied by patient and over time for the whole group of patients. The smallest increase in Pbto2 occurred during the first 12 h after injury, but this was probably because the increase in Pao2 was also smallest, and the Btor calculated for that time point was similar to that seen at 24 and 36 h after injury. Some authors have found that the increase in Pbto2 with hyperoxia is much greater in pathological tissue than in normal brain tissue (Longhi et al., 2002; Meixensberger et al., 1993; Sarrafzadeh et al., 1998). Recovery of more normal tissue characteristics over time may account for the gradual decrease in Btor observed over time.
Effects of hyperventilation and hyperoxia on cerebral pressure autoregulation
Pressure autoregulation is impaired in most patients after TBI (Lee et al., 2001), and this impairment can persist for up to 2 weeks post-injury (Sviri et al., 2009). In a study with serial measurements of dynamic autoregulation in 122 TBI patients, the lowest ARI value was seen at 48 h post-injury, when 87% of the patients had a value less than the lower limit of normal (Hlatky et al., 2002). Thereafter the ARI gradually returned toward normal. The same trend was seen in the current study, with the lowest ARI values occurring during the first 48 h post-injury. This vascular pathophysiology in part explains the vulnerability of the injured brain to hypotension.
Hypocapnia is known to improve, and hypercapnia impairs, cerebral pressure autoregulation. After severe TBI, dynamic pressure autoregulation significantly improves during transient hyperventilation (Newell et al., 1996). Hyperventilation has been used clinically to counteract the impairment in pressure autoregulation induced by inhaled anesthetics such as isoflurane (McCulloch et al., 2005). It is thought that vasodilation caused by isoflurane impairs pressure autoregulation by making it more difficult for vessels to further vasodilate to compensate for hypotension. Vasoconstriction caused by hypocapnia restores the ability to pressure autoregulate in this circumstance.
In normal subjects, hyperoxia does not alter dynamic pressure autoregulation (Nishimura et al., 2007). The present study shows that unlike normal subjects, hyperoxia results in a small but consistent improvement in ARI in TBI patients. As with hypocapnia, the mechanism of this improvement in ARI is likely to be the vasoconstriction induced by hyperoxia. The small improvement in cerebral perfusion pressure seen during hyperoxia may have also contributed to the improvement in dynamic pressure autoregulation.
One potential limitation of this study's method used to assess pressure autoregulation is that flow velocity does not reflect cerebral blood flow under all conditions. However, studies have demonstrated that under certain conditions, such as with blood pressure changes, and with hyperventilation, that flow velocity accurately reflects changes in blood flow. Newell and colleagues (Newell et al., 1994) simultaneously measured internal carotid artery flow and middle cerebral artery flow velocity in normal subjects. During the limited changes in blood pressure that occurred during dynamic autoregulatory testing, the responses of blood flow and flow velocity were similar. A number of studies using MRI, angiographic, and Doppler sonographic methods to measure the diameter of the middle cerebral artery during hyperventilation have failed to demonstrate a measurable decrease in diameter (Djurberg et al., 1998; Valdueza et al., 1997). Nevertheless, since middle cerebral arterial diameter was not measured in this study, a confounding effect of vasoconstriction on the assessments of ARI cannot be excluded.
Additionally, the cuff deflation method we used for assessing pressure autoregulation only tests for the ability of the cerebral circulation to respond to drops in cerebral perfusion pressure. The results might be different for autoregulatory constriction used to increase cerebral perfusion pressure.
From the design of this study, in which the hyperoxia was only transiently introduced, it is not possible to determine how long this potentially beneficial effect on autoregulation would persist. More studies are needed to determine the clinical significance of this finding.
Summary
When administered acutely, both hyperventilation and hyperoxia resulted in cerebral vasoconstriction and significantly decreased FV in these severely-injured TBI patients. Associated with this vascular response, there was also a significant decrease in ICP, and an improvement in ARI. The improvement in ARI suggests that vasoconstriction induced by acute hyperventilation and hyperoxia may allow the cerebral vessels to respond better to transient hypotension. Further studies are needed to define the clinical significance of these observations.
Acknowledgments
This work was funded by National Institutes of Health grant P01-NS38660.
Author Disclosure Statement
No competing financial interests exist.
References
- Aaslid R. Lindegaard K.F. Sorteberg W. Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Albrecht R.F. Miletich D.J. Ruttle M. Cerebral effects of extended hyperventilation in unanesthetized goats. Stroke. 1987;18:649–655. doi: 10.1161/01.str.18.3.649. [DOI] [PubMed] [Google Scholar]
- Amano T. Meyer J.S. Okabe T. Shaw T. Mortel K.F. Cerebral vasomotor responses during oxygen inhalation. Results in normal aging and dementia. Arch. Neurol. 1983;40:277–282. doi: 10.1001/archneur.1983.04050050045005. [DOI] [PubMed] [Google Scholar]
- Diringer M.N. Aiyagari V. Zazulia A.R. Videen T.O. Powers W.J. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J. Neurosurg. 2007;106:526–529. doi: 10.3171/jns.2007.106.4.526. [DOI] [PubMed] [Google Scholar]
- Djurberg H.G. Seed R.F. Evans D.A. Brohi F.A. Pyper D.L. Tjan G.T. al Moutaery K.R. Lack of effect of CO2 on cerebral arterial diameter in man. J. Clin. Anesth. 1998;10:646–651. doi: 10.1016/s0952-8180(98)00107-x. [DOI] [PubMed] [Google Scholar]
- Floyd T.F. Clark J.M. Gelfand R. Detre J.A. Ratcliffe S. Guvakov D. Lambertsen C.J. Eckenhoff R.G. Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J. Appl. Physiol. 2003;95:2453–2461. doi: 10.1152/japplphysiol.00303.2003. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Furuya Y. Valadka A.B. Gonzalez J. Chacko A. Mizutani Y. Contant C.F. Robertson C.S. Dynamic autoregulatory response after severe head injury. J. Neurosurg. 2002;97:1054–1061. doi: 10.3171/jns.2002.97.5.1054. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Gopinath S.P. Robertson C.S. Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J. Neurosurg. 2008;108:53–58. doi: 10.3171/JNS/2008/108/01/0053. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Robertson C.S. Analysis of dynamic autoregulation assessed by the cuff deflation method. Neurocrit. Care. 2006;4:127–132. doi: 10.1385/NCC:4:2:127. [DOI] [PubMed] [Google Scholar]
- Kastrup A. Dichgans J. Niemeier M. Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke. 1998;29:1311–1314. doi: 10.1161/01.str.29.7.1311. [DOI] [PubMed] [Google Scholar]
- Kastrup A. Thomas C. Hartmann C. Schabet M. Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke. 1997;28:2353–2356. doi: 10.1161/01.str.28.12.2353. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Yasui N. Vascular response to hyperoxemia in rat brain surface microvessels. Neurol. Med. Chir. (Tokyo) 1996;36:156–161. doi: 10.2176/nmc.36.156. [DOI] [PubMed] [Google Scholar]
- Kety S. Schmidt C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral oxygen consumption of normal young man. J. Clin. Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos H.A. Raper A.J. Patterson J.L., Jr. Analysis of vasoactivity of local pH, pCO2, and bicarbonate on pial vessels. Stroke. 1977;8:358–360. doi: 10.1161/01.str.8.3.358. [DOI] [PubMed] [Google Scholar]
- Lee J.H. Kelly D.F. Oertel M. McArthur D.L. Glenn T.C. Vespa P. Boscardin W.J. Martin N.A. Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J. Neurosurg. 2001;95:222–232. doi: 10.3171/jns.2001.95.2.0222. [DOI] [PubMed] [Google Scholar]
- Longhi L. Valeriani V. Rossi S. De M.M. Egidi M. Stocchetti N. Effects of hyperoxia on brain tissue oxygen tension in cerebral focal lesions. Acta Neurochir. Suppl. 2002;81:315–317. doi: 10.1007/978-3-7091-6738-0_80. [DOI] [PubMed] [Google Scholar]
- Magnoni S. Ghisoni L. Locatelli M. Caimi M. Colombo A. Valeriani V. Stocchetti N. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: a microdialysis study. J. Neurosurg. 2003;98:952–958. doi: 10.3171/jns.2003.98.5.0952. [DOI] [PubMed] [Google Scholar]
- Marshall L.F. Marshall S.B. Klauber M.R. van Berkum Clark M. Eisenberg H.M. Jane J.A. Luerssen T.G. Marmarou A. Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991;75:S14–S20. [Google Scholar]
- McCulloch T.J. Boesel T.W. Lam A.M. The effect of hypocapnia on the autoregulation of cerebral blood flow during administration of isoflurane. Anesth. Analg. 2005;100:1463–1467. doi: 10.1213/01.ANE.0000148623.06596.7E. [DOI] [PubMed] [Google Scholar]
- Meixensberger J. Dings J. Kuhnigk H. Roosen K. Studies of tissue PO2 in normal and pathological human brain cortex. Acta Neurochir. Suppl. (Wien.) 1993;59:58–63. doi: 10.1007/978-3-7091-9302-0_10. [DOI] [PubMed] [Google Scholar]
- Menzel M. Doppenberg E.M. Zauner A. Soukup J. Reinert M.M. Clausen T. Brockenbrough P.B. Bullock R. Cerebral oxygenation in patients after severe head injury: monitoring and effects of arterial hyperoxia on cerebral blood flow, metabolism and intracranial pressure. J. Neurosurg. Anesthesiol. 1999;11:240–251. doi: 10.1097/00008506-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Nakajima S. Meyer J.S. Amano T. Shaw T. Okabe T. Mortel K.F. Cerebral vasomotor responsiveness during 100% oxygen inhalation in cerebral ischemia. Arch. Neurol. 1983;40:271–276. doi: 10.1001/archneur.1983.04050050039004. [DOI] [PubMed] [Google Scholar]
- Newell D.W. Aaslid R. Lam A. Mayberg T.S. Winn H.R. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- Newell D.W. Weber J.P. Watson R. Aaslid R. Winn H.R. Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery. 1996;39:35–44. doi: 10.1097/00006123-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Nishimura N. Iwasaki K. Ogawa Y. Shibata S. Oxygen administration, cerebral blood flow velocity, and dynamic cerebral autoregulation. Aviat. Space Environ. Med. 2007;78:1121–1127. doi: 10.3357/asem.2177.2007. [DOI] [PubMed] [Google Scholar]
- Nortje J. Coles J.P. Timofeev I. Fryer T.D. Aigbirhio F.I. Smielewski P. Outtrim J.G. Chatfield D.A. Pickard J.D. Hutchinson P.J. Gupta A.K. Menon D.K. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit. Care Med. 2008;36:273–281. doi: 10.1097/01.CCM.0000292014.60835.15. [DOI] [PubMed] [Google Scholar]
- Omae T. Ibayashi S. Kusuda K. Nakamura H. Yagi H. Fujishima M. Effects of high atmospheric pressure and oxygen on middle cerebral blood flow velocity in humans measured by transcranial Doppler. Stroke. 1998;29:94–97. doi: 10.1161/01.str.29.1.94. [DOI] [PubMed] [Google Scholar]
- Rangel-Castilla L. Robertson C.S. Management of intracranial hypertension. Crit. Care Clin. 2006;22:713–732. doi: 10.1016/j.ccc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A.S. Kiening K.L. Bardt T.F. Schneider G.H. Unterberg A.W. Lanksch W.R. Cerebral oxygenation in contusioned vs. nonlesioned brain tissue: monitoring of PtiO2 with Licox and Paratrend. Acta Neurochir. Suppl. 1998;71:186–189. doi: 10.1007/978-3-7091-6475-4_54. [DOI] [PubMed] [Google Scholar]
- Schultheiss R. Leuwer R. Leniger-Follert E. Wassmann H. Wullenweber R. Tissue pO2 of human brain cortex—method, basic results and effects of pentoxifylline. Angiology. 1987;38:221–225. doi: 10.1177/000331978703800303. [DOI] [PubMed] [Google Scholar]
- Sviri G.E. Aaslid R. Douville C.M. Moore A. Newell D.W. Time course for autoregulation recovery following severe traumatic brain injury. J. Neurosurg. 2009;111:695–700. doi: 10.3171/2008.10.17686. [DOI] [PubMed] [Google Scholar]
- Tiecks F.P. Lam A.M. Aaslid R. Newell D.W. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- Tolias C.M. Reinert M. Seiler R. Gilman C. Scharf A. Bullock M.R. Normobaric hyperoxia-induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J. Neurosurg. 2004;101:435–444. doi: 10.3171/jns.2004.101.3.0435. [DOI] [PubMed] [Google Scholar]
- van Santbrink H. Mass A.I.R. Avezaat C.J.J. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996;38:21–31. doi: 10.1097/00006123-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Valdueza J.M. Balzer J.O. Villringer A. Vogl T.J. Kutter R. Einhaupl K.M. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. Am. J. Neuroradiol. 1997;18:1929–1934. [PMC free article] [PubMed] [Google Scholar]
- Watson N.A. Beards S.C. Altaf N. Kassner A. Jackson A. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur. J. Anaesthesiol. 2000;17:152–159. doi: 10.1046/j.1365-2346.2000.00640.x. [DOI] [PubMed] [Google Scholar]