Abstract
Sex steroids assist adult neural tissue in the protection from and repair of damage resulting from neural injury; some steroids may be synthesized in the brain. Songbirds are especially useful models to explore steroidal neuroprotection and repair. First, the full suite of cholesterol transporters and steroidogenic enzymes are expressed in the zebra finch (ZF) brain. Second, estrogens promote recovery of behavioral function after damage to the adult ZF cerebellum. Third, the estrogen synthetic enzyme aromatase is rapidly upregulated in reactive glia following neural injury, including in the ZF cerebellum. We hypothesized that cerebellar injury would locally upregulate steroidogenic factors upstream of aromatase, providing the requisite substrate for neuroestrogen synthesis. We tested this hypothesis by lesioning the cerebellum of adult songbirds using both males and females that peripherally synthesize steroids in different amounts. We then used quantitative PCR to examine expression of mRNAs for the neurosteroidogenic factors TSPO, StAR, SCC, 3β-HSD, CYP17, and aromatase, at 2 and 8 days post-lesion. Compared to sham lesions, cerebellar lesions significantly upregulated mRNA levels of TSPO and aromatase. Sex differences in response to the lesions were detected for TSPO, StAR, and aromatase. All birds responded to experimental conditions by showing time-dependent changes in the expression of TSPO, SCC, and aromatase, suggesting that acute trauma or stress may impact neurosteroidogensis for many days. These data suggest that the cerebellum is an active site of steroid synthesis in the brain, and each steroidogenic factor likely provides neuroprotection and promotes repair.
Key words: estradiol, neuroprotection, neurosteroids, songbirds
Introduction
Sex steroids are pluripotent signaling molecules that play a key role in neural protection and neural repair (Garcia-Segura and Balthazart, 2009; Saldanha et al., 2009). Estrogens, in particular, stand out for their capacity to protect against neural damage (Azcoitia et al., 2001; Garcia-Ovejero et al., 2005; Wise, 2002). The estrogen synthetic enzyme aromatase is naturally expressed in some neurons in the brain to synthesize estrogens from circulating androgens (Balthazart et al., 1990; MacLusky and Naftolin, 1981; Schlinger, 1997). After neurotrauma, however, expression of this enzyme is upregulated in reactive astrocytes adjacent to the injury site (Azcoitia et al., 2003; Garcia-Segura et al., 2003; Peterson et al., 2001; Saldanha et al., 2004), and locally-produced estrogens reduce neurodegeneration by suppressing apoptotic signaling pathways (Saldanha and Coomaralingam, 2005; Saldanha et al., 2005). Estrogens produced by injury-induced aromatase appear to be a conserved property of the vertebrate brain, offering neuroprotection to breeding and non-breeding males and females. However, estrogen synthesis requires androgens as substrates, and the availability of androgens in the periphery can be highly variable across different sexual and reproductive conditions. One possibility is that other enzymes in the steroidogenic pathway are also upregulated by neural injury, and that these provide substrates for astrocytic aromatization.
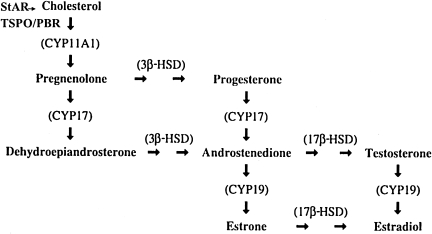
Sex steroidogenesis (Fig. 1) is initiated by transport of cholesterol into specialized mitochondria involving two proteins: translocator protein (TSPO) and steroidogenic acute regulatory protein (StAR). A series of mitochondrial and endoplasmic reticulum–bound enzymes (side chain cleavage enzyme [SCC], 3β-hydroxysteroid dehydrogenase/isomerase [3β-HSD], cytochrome P450 17α-hydroxylase/C17,20 lyase, [CYP17], and aromatase) catalyze the synthesis of pregnenolone, progesterone, androgens, and estrogens. Cholesterol transporters and enzymes of steroidogenesis are expressed in the vertebrate brain (Chung et al., 1986; Culty et al., 2002; Miller, 1988; Papadopoulos et al., 1997; Stocco and Clark, 1996), so the substrates for estrogen synthesis might be available for neuroandrogen synthesis (Baulieu et al., 1997; Corpechot et al., 1981, 1983; Tsutsui and Schlinger, 2001; Vockel et al., 1990). However, these factors are expressed in a regionally-specific manner and may not be locally available to serve in neuroprotection. Recently, we observed that estrogens promote recovery of function after cerebellar lesions in a female songbird with low levels of circulating androgenic substrates (Spence et al., 2009). Birds naturally show considerable recovery from damage to the cerebellum up to 8 days post-lesion, and this improvement is significantly impaired when the birds are deprived of estradiol. Furthermore, as has been shown in other brain regions, aromatase was upregulated in reactive astrocytes at 8 days post-injury, but also in Bergmann glia (Spence et al., 2009). The source of androgen for cerebellar aromatization is currently unknown. Whereas steroidogenic factors are expressed naturally in the songbird cerebellum (London and Schlinger, 2003; London et al., 2006), as well as in the cerebellum of other species (Fester et al., 2009; Lavaque et al., 2005; Tsutsui, 2008), we do not know how expression of these factors may be affected by injury in either male or female birds.
FIG. 1.
The major steroid synthesizing enzymes (in parentheses) that catalyze the conversion of cholesterol to the sex steroids (StAR, steroidogenic acute regulatory protein; TSPO/PBR, translocator protein/peripheral benzodiazepine receptor; CYP11A1, cytochrome P450 side-chain cleavage; 3β-HSD, 3β-hydroxysteroid dehydrogenase/isomerase; CYP17, cytochrome P450 17α-hydroxylase/C17,20 lyase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; CYP19, cytochrome P450 aromatase).
To assess the possibility that males and/or females synthesize androgens in the brain that serve as substrates for injury-induced estrogen synthesis, we examined expression of a full suite of steroidogenic factors in the songbird cerebellum in control and lesioned birds. We examined males and females, and examined expression at two time points (2 and 8 days) post-injury, the period during which estrogens enhance recovery of cerebellar function in these birds (Spence et al., 2009).
Methods
Animals and housing
Adult male and female (>100 days of age) zebra finches were obtained from our breeding colony located in the UCLA Life Science vivarium. The birds were housed under a 14/10-h light/dark cycle, with food and water available ad libitum in same-sex aviaries. All protocols were approved by the UCLA Chancellor's Committee on Animal Care and Use following National Institutes of Health Guidelines.
Cerebellum lesions
On day 1 the birds were deprived of food, but not water, for 2 h prior to surgery. Following food deprivation, anesthesia was accomplished with Equithesin (3.2 mL/kg IM). The birds were placed in a stereotaxic frame (Herb Adams Engineering, Glendora, CA) at 20° angle inferior to the horizontal, and placed beneath a binocular microscope. The feathers at the caudal region of the head were plucked to expose the skin covering the skull, and a small dorsal incision was made at the base of the skull. A craniotomy was then made over the cerebellum. All birds received bilateral lesions with a 26-gauge needle at coordinates lateral (±0.92 mm) and rostral (−2.25 mm) to the bifurcation of the Y-sinus, and at a depth of −4.9 mm from the surface of the brain. The incision was then carefully closed and sealed with ethyl cyanoacrylate. Cerebellar lesions produced in this manner in zebra finches have been shown to induce aromatase expression in reactive astrocytes and Bergmann glia (Spence et al., 2009). Sham-experimental birds underwent all of the same surgery procedures except for needle penetration. Following surgery, the birds recovered from anesthesia under a heating pad and were housed in same-sex cages until sacrifice.
Tissue collection and RNA preparation
The birds (n = 5 per sex/treatment) were decapitated and the cerebellum was rapidly dissected out and stored at −80° until processing. Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA) per the manufacturer's protocol. Total RNA quantity was determined spectrophotometrically. The integrity of the isolated RNA was determined by visualization of 28S and 18S ribosomal RNA bands after separation on a 1% agarose gel stained with ethidium bromide. Total RNA (1 μg) was treated with DNase and reverse transcribed using Superscript II (Invitrogen) on a thermal cycler (MJ Research, Waltham, MA) for 50 min at 42°C, followed by 15 min at 70°C. The resulting cDNA was amplified with SYBR Green PCR master mix (Applied Biosystems Inc., Carlsbad, CA) in 25 μL of total reaction volume. Primers for StAR (gene bank accession no. AY505123), SCC (accession no. AY633556), 3β-HSD (accession no. AY445525), CYP17 (accession no. AY313844), and aromatase (accession no. 001076691), were designed to span intron-exon borders based on the known zebra finch sequence for each gene (London and Schlinger, 2003; London et al., 2006, 2007; Shen et al., 1994), except TSPO. TSPO primers for rtPCR were designed initially based on the chicken sequence. Products amplified from brain tissues were sequenced and blasted against the zebra finch genome (ESTIMA and UCSC Genome Bioinformatics), confirming the TSPO sequence and identifying appropriate zebra finch–specific primers for quantitative PCR (accession no. DV952129). For the qPCR analysis varying concentrations of primers were determined by primer optimization: TSPO, forward CCTACCTGGTGTGGAAGGAA and reverse, AGAGTCACCAACCCCCATC (150-bp product); StAR, forward AGA AAT CCC TGC GAA TCC TG and reverse ACC GTC TCT GTC TTC CAG TCG T (52-bp product); SCC, forward GAC CGC GAG AAG ATG CTG AAA and reverse TCT CCT TGA TGG TGG CCT TGA G (55-bp product); 3β-HSD, forward CAG AGG ATT GTG TGC TTG CTG and reverse AAC TTT CCA AAT CTC CCG AGC (104-bp product); CYP-17, forward CAT CAA CCT CTG GTC TGT GCA C and reverse AAG CGG CCA GGA TTG AAC T (72-bp product); and aromatase (CYP19), forward GGATGAGCACATGGATT TTGC and reverse GCAGTCAGATCCCCTCTGTTCT (62-bp product). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control, with primers forward GACCTGCCGTCTGGAAAA and reverse CCATCAGCAGCAGCCTTCA (70-bp product) (accession no. AF-047874; Chen et al., 2005). Amplification was performed in an Applied Biosystems 7300 qPCR instrument. Dissociation curves of the PCR products were evaluated to verify the absence of DNA contamination. The assays were performed in 96-well optical plates and each sample was amplified in duplicate. In each qPCR run, wells without cDNA were included to verify the absence of external contamination. Standard curves with correlation coefficients of >0.99 were generated with known concentrations of cDNA for TSPO, StAR, SCC, 3β-HSD, CYP17, aromatase, and GAPDH, generating the slopes that were used to calculate amplification efficiency (E = 10-1/slope-1; Klein et al., 1999). The delta (Ct) method was used for quantification. Using this method, the relative abundance of each gene was calculated based on the threshold cycle number (CT) for the gene relative to the Ct for GAPDH: 2– (Ct, gene – Ct, GAPDH) × 1000.
Hormone levels
Plasma corticosterone (Cort) and testosterone (T) were quantified via enzyme-linked immunosorbent assay (ELISA; Cayman Chemical, Ann Arbor, MI), according to the manufacturer's protocols (Cort), or a protocol previously validated in our laboratory (T; Remage-Healey et al., 2008).
Statistical analysis
Data were analyzed via three-way analysis of variance (ANOVA) to compare across treatment (lesioned versus sham), sex, and time (survival time post-surgery), followed by post-hoc Fisher's protected least significant difference (PLSD) tests. For all analyses, delta Ct values were log-transformed to achieve normality (delta CT values are presented as means ± standard error of the mean [SEM]). Plasma steroid levels were analyzed by t-tests. The results were considered significant when p ≤ 0.05. All analyses were performed using STATVIEW 5.0 software (SAS Institute, Cary, NC).
Results
Translocator protein
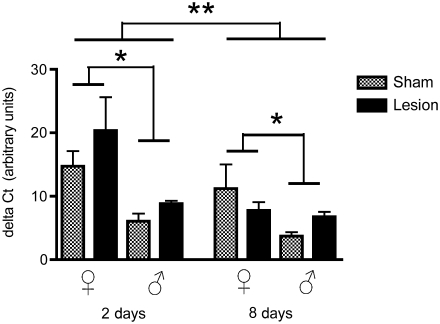
There were significant effects of sex (F1,32 = 19.31, p = 0.0001), time (F1,32 = 12.21, p = 0.001), and treatment (F1,32 = 4.02, p = 0.05), but no significant interactions for the other parameters (treatment × time: F1,32 = 0.22, p = 0.64; treatment × sex: F1,32 = 2.7, p = 0.10; time × sex: F1,32 = 1.05, p = 0.31; and treatment × time × sex: F1,32 = 0.97, p = 0.33; Fig. 2). TSPO was upregulated in all birds at 2 days post-surgery, an effect not detected at 8 days post-surgery. In general, expression levels were greater in females than in males, with levels higher in all birds at 2 days than at 8 days post-surgery. Even in sham-treated birds, TSPO expression levels were greater in females than in males (p = 0.002).
FIG. 2.
Levels of translocator protein (TSPO) mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; delta CT values) in adult male and female zebra finches at 2 and 8 days post-surgery. The lesions significantly influenced levels of TSPO mRNA (p ≤ 0.05). TSPO expression was significantly higher in females than in males, and TSPO expression was higher at 2 days than at 8 days post-surgery (*p = 0.001, **p = 0.0001).
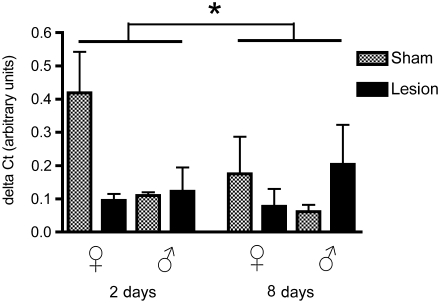
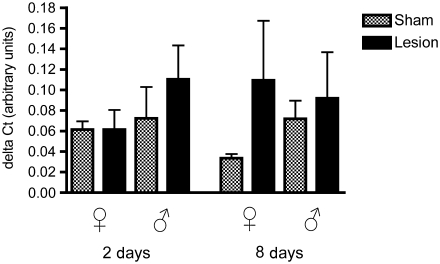
Steroidogenic acute regulatory protein
There was a significant effect of sex (F1,32 = 7.82, p = 0.008), but not of treatment (F1,32 = 0.68, p = 0.41) or time (F1,32 = 0.14, p = 0.71; Fig. 3). We found a significant interaction between treatment × sex (F1,32 = 5.27, p = 0.02), but no other significant interactions (treatment × time: F1,32 = 3.13, p = 0.86; time × sex: F1,32 = 2.85, p = 0.1; treatment × time × sex: F1,32 = 9.37, p =0.99). As was the case with TSPO, mRNA levels for StAR in sham-treated birds were greater in females than in males (p = 0.002). In contrast to TSPO, however, the trend was that mean mRNA levels for StAR were reduced by lesions in females and increased in males.
FIG. 3.
Levels of steroidogenic acute regulatory protein (StAR) mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; delta CT value), in both male and female zebra finches at 2 and 8 days post-surgery. StAR expression was significantly higher in females than males (p = 0.0087), and STAR mRNA levels were significantly higher in females than in males at 2 days and 8 days post-surgery (*p = 0.01, **p = 0.0004).
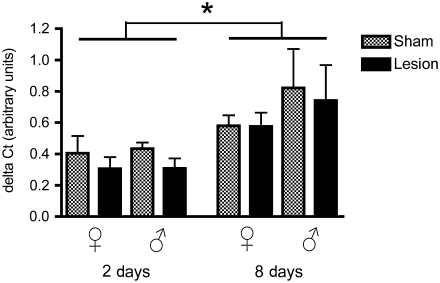
Side chain cleavage enzyme
We found a significant effect of time for SCC expression level (F1,32 = 4.72, p = 0.03), but not of treatment (F1,32 = 0.22, p = 0.64) or sex (F1,32 = 1.14, p = 0.29; Fig. 4). There were no significant interactions between any of the other parameters (treatment × time: F1,32 = 0.76, p = 0.38; treatment × sex: F1,32 = 1.85, p = 0.18; time × sex: F1,32 = 3.0, p = 0.09; treatment × time × sex: F1,32 = 0.91, p = 0.34). The effect of time seemed largely driven by a decreased expression of SCC in sham-treated birds at 8 days post-surgery as compared to 2 days post-surgery.
FIG. 4.
Levels of side chain cleavage enzyme (SCC) mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; delta CT value), in both male and female zebra finches at 2 and 8 days post-surgery. Levels of SCC mRNA were significantly greater at 2 days than at 8 days post surgery (*p = 0.03).
3β-Hydroxysteroid dehydrogenase/isomerase
There were no significant effects of treatment (F1,32 = 1.99, p = 0.16), time (F1,32 = 0.43, p = 0.51), or sex (F1,32 = 0.46, p = 0.5), and no significant interactions between the other parameters (treatment × time: F1,32 = 0.46, p = 0.50; treatment × sex: F1,32 = 0.19, p = 0.66; time × sex: F1,32 = 0.5, p = 0.48; treatment × time × sex: F1,32 = 1.41, p = 0.24; Fig. 5).
FIG. 5.
Levels of 3β-hydroxysteroid dehydrogenase/isomerase (3β-HSD) mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; delta CT value) in both male and female zebra finches at 2 and 8 days post-surgery.
Cytochrome P450 17α-hydroxylase/C17,20 lyase
We detected a significant effect of time (F1,32 = 15.69, p = 0.0004), but not of treatment (F1,32 = 1.81, p = 0.18), or sex (F1,32 = 0.69, p = 0.4). There were no significant interactions between the other parameters (treatment × time: F1,32 = 0.73, p = 0.39; treatment × sex: F1,32 = 0.24, p = 0.62; time × sex: F1,32 = 0.07, p = 0.78; treatment × time × sex: F1,32 = 0.01, p = 0.89; Fig. 6). The time effect was driven by the elevated levels of mRNA expression seen at 8 days, compared to those seen at 2 days, post-surgery.
FIG. 6.
Levels of cytochrome P450 17α-hydroxylase/C17,20 lyase (CYP17) mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; delta CT value) in both male and female zebra finches at 2 and 8 days post-surgery. Levels of CYP17 mRNA were significantly greater at 8 days than at 2 days post-surgery (*p = 0.0004).
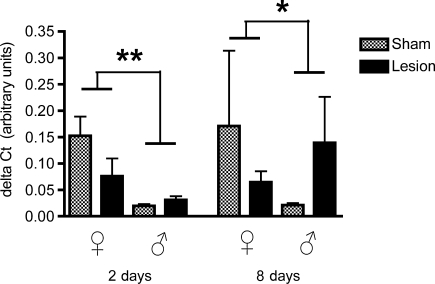
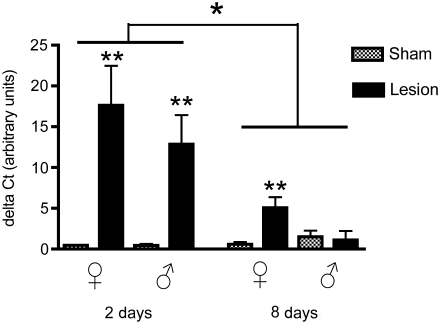
Aromatase
There were significant effects of treatment (F1,32 = 21.08, p < 0.0001), time (F1,32 = 11.35, p = 0.002), and sex (F1,32 = 6.9, p = 0.01; Fig. 7) on aromatase levels. We also found significant interactions for treatment × time (F1,32 = 20.2, p < 0.0001), treatment × sex (F1,32 = 8.65, p = 0.006), and treatment × time × sex (F1,32 = 10.07, p = 0.003), but not of time × sex (F1,32 = 2.48, p = 0.12). Aromatase mRNA was profoundly upregulated by lesions in both males and females, with expression levels lower in both sexes at 8 days compared to 2 days post-surgery. By 8 days post-surgery, aromatase was no longer upregulated in males, whereas it remained so in females.
FIG. 7.
Levels of aromatase mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; delta CT value) in both male and female zebra finches at 2 and 8 days post-surgery. Lesions significantly upregulated aromatase mRNA in both males (at 2 days), and females (at 2 and 8 days; **p < 0.001). There were significantly higher levels of aromatase mRNA at 2 days than at 8 days post-surgery (*p = 0.002). Finally, there was an overall effect of sex, with mRNA upregulated more in females than in males (p < 0.0001).
Circulating steroids
Levels of mRNA for TSPO, SCC, and CYP17 showed significant changes with time in all birds, including sham-treated birds, suggesting that injury-independent mechanisms regulate expression of these factors. We tested whether stress and/or sex steroid levels might have been altered during the post-surgery period before sacrifice, and if this might have impacted neurosteroidogenesis. To test this possibility, we measured Cort in sham-operated males and T, in lesioned males, at 2 and 8 days post-surgery. There were no significant differences seen in the amount of Cort measured at 2 days compared to 8 days post-surgery (79.97 ± 41.79 versus 91.16 ± 24.55 ng/mL; t = −0.017; p = 0.98), or the amount of T measured at 2 days compared to 8 days post-surgery (664 ± 344.68 pg/mL versus 760 ± 343 pg/mL; t = −0.19; p = 0.84).
Discussion
The results of this study provide evidence that injury regulates steroidogenic factors in the cerebellum. When superimposed upon the background of subject sex, as well as time post-surgery, a complex pattern emerges that likely produces variable levels of neurosteroids available to influence the repair of cerebellar tissue and recovery of behavioral function. Whereas we have previously identified expression of StAR, SCC, 3β-HSD, CYP17, and aromatase in the uninjured zebra finch cerebellum (London and Schlinger, 2003; London et al., 2006), and aromatase in the injured cerebellum (Spence et al., 2009), here we provide new data on TSPO expression in the uninjured cerebellum, and quantitative measures of all steroidogenic factors in the injured cerebellum.
Our results confirm previous observations that aromatase gene expression is upregulated following neuronal injury (Garcia-Segura et al., 2003; Peterson et al., 2001; Saldanha et al., 2004). Consistent with the low levels of expression seen in sham-lesioned birds, aromatase immunoreactivity is naturally weak in the songbird cerebellum, and is restricted to a few scattered Purkinje cells (Spence et al., 2009). Eight days after injury, however, we see a profound increase in aromatase immunoreactivity in astrocytes and Bergmann glia around cerebellar lesion sites (Spence et al., 2009). By this time aromatase expression has already decreased from the much higher levels seen 2 days post-injury. A rapid increase in aromatization likely affords a degree of neuroprotection that is required early after injury (Garcia Segura et al., 1999; Saldanha and Coomaralingam, 2005; Saldanha et al., 2005). The decrease in expression we see after 8 days may indicate that estrogens begin to lose their ability to repair the injured cerebellum by this time, especially in males. Another possibility is that substrates for aromatization are elevated to a greater degree in males than in females by 8 days, reducing the need for elevated aromatase at this time point in males. As we discuss below, our data provide some evidence for this possibility. It is also possible that injury-induced aromatase protein is particularly stable in males; in the adult male songbird telencephalon, glial aromatase immunoreactivity is detectable up to 6 weeks post-injury (Ryan et al., 2008).
In addition to aromatase, TSPO mRNA levels were elevated 2 days after cerebellar injury, replicating for TSPO what has been observed 3–14 days after injury to the rodent thalamus (Raghavendra Rao et al., 2000). These data indicate that TSPO, by transport of cholesterol into mitochondria expressing SCC, might initiate the process of steroidogenesis after neural injury, as has been proposed in previous models of brain injury (Azcoitia et al., 2001; Sierra et al., 2003). Our data indicate that StAR might perform a similar function as TSPO in males, but apparently not in females. It is possible that StAR in females is subject to more rapid and transient upregulation at times not captured in the present study (Kuhlmann and Guilarte, 2000; Lavaque et al., 2005; Sierra et al., 2003). In this set of studies, no other enzyme was influenced by neural injury, a result similar to those of several studies of rodent neural tissues (Lavaque et al., 2005; Labombarda et al., 2006), suggesting that cholesterol transport in the cerebellum may be a primary step in injury-induced steroidogenesis, with aromatization completing the synthesis of neuroestrogens. In the case of 3β-HSD, previous reports describe either up- or downregulation after neuroinjury (Hashimoto et al., 2003; Meffre et al., 2007). We were somewhat surprised that 3β-HSD showed little evidence for regulation, as we have evidence that this enzyme is subject to other forms of regulation in the songbird brain (Pradhan et al., 2010; Soma et al., 2005).
We detected several sex differences in cerebellar expression levels of steroidogenic factors that in some cases influenced the response to injury. In all cases where sex differences in expression were detected, TSPO, StAR, and aromatase, levels were higher in females than in males. For TSPO and StAR, expression levels were elevated in both sham-lesioned and lesioned birds, whereas for aromatase it was only seen in lesioned birds. Presumably sex differences in circulating substrates impacted these expression levels, as males have higher circulating levels of testosterone than females, but levels of estradiol are roughly similar (Adkinsregan et al., 1990; Schlinger and Arnold, 1993). We cannot exclude the possibility that there are sex chromosome–dependent mechanisms that produce the phenotypic sex differences we observed (Arnold and Burgoyne, 2004). In the case of TSPO, lesioned females had nearly threefold higher expression than did males 2 days after lesioning. Expression levels of StAR were nearly eightfold higher in sham-lesioned females than in sham-lesioned males, a difference that was eliminated by lesioning. Sex differences in aromatase levels of lesioned birds although significant, were of a smaller magnitude. Overall, these data indicate that the steroidal background within the cerebellum may be strikingly different between males and females, due to both differences in gonadal steroid secretion, and to local neural differences in expression of steroidogenic enzymes. Thus the sources of substrate for injury-induced neural aromatization may be complex; females may express higher levels of some factors to compensate for their lower levels of circulating androgenic substrates.
Finally, TSPO, SCC, and CYP17 demonstrated differences in expression between days 2 and 8 in sham-lesioned birds. In the case of SCC this was driven by high levels seen in sham-lesioned females on day 2 compared to day 8. For TSPO there seemed to be a general reduction in all birds, whereas for CYP17 there was a general increase in all birds. We cannot explain these differences based on levels of circulating Cort or T, so we can only speculate that some condition of housing post-surgery or the residual effects of the acute stress of surgery were causal. Whatever the case, these represent altogether different forms of regulation that may well be of import to our understanding of the control of the cerebellar neurosteroidal environment, with or without injury. We have evidence from an auditory processing center that social context and auditory input can locally alter the neurosteroidal environment (Remage-Healey et al., 2008). Future experiments could prove useful to determine if sensory stimulation or motor performance can themselves influence expression of neurosteroidogenic enzymes in the cerebellum.
We have previously documented that cerebellar lesions similar to those used here produce transient deficits in motor and cognitive function of small songbirds (Spence et al., 2009). Despite these behavioral losses, the birds show a marked recovery of both motor and cognitive function, and appear behaviorally undiminished within as little as 8 days post-injury. Understanding the natural mechanisms producing neural recovery is crucial for developing treatments that can be applied to improve recovery following traumatic brain injury in humans, with songbirds as useful models for tackling this problem (Morganti-Kossmann et al., 2010; Saldanha et al., 2009). Given the substantial evidence for the benefit of steroids in reducing the extent of damage seen after traumatic brain injury (Azcoitia et al., 2003; Garcia-Segura and Balthazart, 2009; Wise, 2002), insights into the natural steroidogenic potential of the brain may lead to the discovery of new treatment paradigms (Herson et al., 2009).
We have previously demonstrated that estrogens promote recovery of behavioral function following injury to the cerebellum (Spence et al., 2009). The natural source of neuroestrogens to promote this neural repair/protection is likely the cerebellum itself, because aromatase is locally upregulated. Our data indicate that the source of the androgenic substrate for neural aromatization may well be the cerebellum itself, given that all of the components of the steroidogenic pathway are expressed in the cerebellum. Moreover, upregulation of TSPO may initiate local steroidogenesis to a higher degree after injury, and thus contribute substrate for glial aromatization. The amount of substrate available likely differs between males and females, perhaps due to compensatory responses to the availability of circulating substrate. The cerebellum is an intriguing steroidogenic tissue and steroid target. These results contribute to a better understanding of the regulatory mechanisms at work to control the cerebellar steroidal environment.
Acknowledgments
We thank Dr. Amnon Katz, Dr. Xuqi Chen, and Bradley Walters for their technical assistance. We thank Dr. Stephanie White for providing the surgery room. Funding was provided by National Institute of Mental Health grant NIMH-061944, NS-042767 and LNE Training Grant HD07228-26.
Author Disclosure Statement
No competing financial interests exist.
References
- Adkinsregan E. Abdelnabi M. Mobarak M. Ottinger M.A. Sex steroid-levels in developing and adult male and female zebra finches (Poephila guttata) Gen. Comp. Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Arnold A.P. Burgoyne P.S. Are XX & XY brain cells intrinsically different? Trends Endocrinol. Metabol. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Azcoitia I. Sierra A. Veiga S. Garcia-Segura L.M. Aromatase expression by reactive astroglia is neuroprotective. Ann. NY Acad. Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- Azcoitia I. Sierra A. Veiga S. Honda S. Harada N. Garcia-Segura L.M. Brain aromatase is neuroprotective. J. Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Balthazart J. Foidart A. Surlemont C. Vockel A. Harada N. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: An immunocytochemical study. J. Comp. Neurol. 1990;301:276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- Baulieu E.E. Schumacher M. Neurosteroids, with special reference to the effect of progesterone on myelination in peripheral nerves. Mult. Scler. 1997;3:105–112. doi: 10.1177/135245859700300209. [DOI] [PubMed] [Google Scholar]
- Chen X. Agate R.J. Itoh Y. Arnold. A.P. Sexually dimorphic expression of trkB, a Z-linked gene, in early posthatch zebra finch brain. Proc. Natl. Acad. Sci. USA. 2005;102:7730–7735. doi: 10.1073/pnas.0408350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B.C. Matteson K.J. Voutilainen R. Mohandas T.K. Miller W.L. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc. Natl. Acad. Sci. USA. 1986;83:8962–8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C. Robel P. Axelson M. Sjövall J. Baulieu E.E. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C. Synguelakis M. Talha S. Axelson M. Sjövall J. Vihko R. Baulieu E.E. Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;27:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- Culty M. Luo L. Yao Z.-X. Chen H. Papadopoulos V. Zirkin B.R. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging leydig cells. J. Androl. 2002;23:439–447. [PubMed] [Google Scholar]
- Fester L. Zhou L. Butow A. Huber C. von Lossow R. Prange-Kiel J. Jarry H. Rune G.M. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus. 2009;19:692–705. doi: 10.1002/hipo.20548. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D. Azcoitia I. DonCarlos L.L. Melcangi R.C. Garcia-Segura L.M. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res. Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L.M. Balthazart J. Steroids and neuroprotection. Adv. Front. Neuroendocrin. 2009;30:5–9. doi: 10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura L.M. Veiga S. Sierra A. Melcangi R.C. Azcoitia I. Aromatase: a neuroprotective enzyme. Prog. Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L.M. Wozniak A. Azcoitia I. Rodriguez J.R. Hutchison R.E. Hutchison J.B. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto N. Yamanaka H. Mizushima T. Noguchi K. Increased expression of 3[beta]-hydroxysteroid dehydrogenase mRNA in dorsal root ganglion neurons of adult rats following peripheral nerve injury. Neurosci. Lett. 2003;340:45–48. doi: 10.1016/s0304-3940(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Herson P.S. Koerner I.P. Hurn P.D. Sex, sex steroid and brain injury. Semin. Reprod. Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. Janda P. Steinborn R. Müller M. Salmons B. Günzburg W.H. Proviral load determination of different feline immunodeficiency virus isolates using real-time polymerase chain reaction: influence of mismatches on quantification. Electrophoresis. 1999;20:291–299. doi: 10.1002/(SICI)1522-2683(19990201)20:2<291::AID-ELPS291>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kuhlmann A.C. Guilarte T.R. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J. Neurochem. 2000;74:1694–1704. doi: 10.1046/j.1471-4159.2000.0741694.x. [DOI] [PubMed] [Google Scholar]
- Labombarda F. Pianos A. Liere P. Eychenne B. Gonzalez S. Cambourg A. De Nicola A.F. Schumacher M. Guennoun R. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology. 2006;147:1847–1859. doi: 10.1210/en.2005-0955. [DOI] [PubMed] [Google Scholar]
- Lavaque E. Mayen A. Azcoitia I. Tena-Sempere M. Garcia-Segura L.M. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J. Neurobiol. 2005;66:308–318. doi: 10.1002/neu.20221. [DOI] [PubMed] [Google Scholar]
- London S.E. Schlinger B.A. Evidence for steroid synthesis in songbird brain: cloning and expression of 3β-HSD. Horm. Behav. 2003;44:62. [Google Scholar]
- London S.E. Schlinger B.A. Steroidogenic enzymes along the ventricular proliferative zone in the developing songbird brain. J. Comp. Neurol. 2007;502:507–521. doi: 10.1002/cne.21335. [DOI] [PubMed] [Google Scholar]
- London S.E. Monks D.A. Wade J. Schlinger B.A. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky N.J. Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Meffre D. Delespierre B. Gouézou M. Schumacher M. Stein D.G. Guennoun R. 3[beta]-Hydroxysteroid dehydrogenase/5-ene-4-ene isomerase mRNA expression in rat brain: Effect of pseudopregnancy and traumatic brain injury. J. Steroid Biochem. Mol. Biol. 2007;104:293–300. doi: 10.1016/j.jsbmb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Miller W.L. Molecular biology of steroid hormone synthesis. Endocrinol. Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann M.C. Yan E. Bye N. Animal models of traumatic brain injury: Is there an optimal model to reproduce human brain injury in the laboratory? Injury. 2010;41:S10–S13. doi: 10.1016/j.injury.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V. Amri H. Li H. Boujrad N. Vidic B. Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J. Biol. Chem. 1997;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Peterson R.S. Saldanha C.J. Schlinger B.A. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata) J. Neuroendocrinol. 2001;13:317–323. doi: 10.1046/j.1365-2826.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Pradhan D.S. Newman A.E. Wacker D.W. Wingfield J.C. Schlinger B.A. Soma K.K. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm. Behav. 2010;57:381–389. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra Rao V.L. Dogan A. Bowen K.K. Dempsey R.J. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp. Neurol. 2000;161:102–114. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L. Maidment N.T. Schlinger B.A. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D.W. Bradley J.W. David J.B. Saldanha C.J. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia. 2008;56:97–105. doi: 10.1002/glia.20594. [DOI] [PubMed] [Google Scholar]
- Saldanha C.J. Coomaralingam L. Overlap and co-expression of estrogen synthetic and responsive neurons in the songbird brain—a double-label immunocytochemical study. Gen. Comp. Endocrinol. 2005;141:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Saldanha C.J. Duncan K.A. Walters B.J. Neuroprotective actions of brain aromatase. Front. Neuroendocrin. 2009;30:106–118. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha C.J. Rohmann K.N. Coomaralingam L. Wynne R.D. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata) J. Neurobiol. 2005;64:192–201. doi: 10.1002/neu.20147. [DOI] [PubMed] [Google Scholar]
- Saldanha C.J. Schlinger B.A. Micevych P.E. Horvath T.L. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J. Comp. Neurol. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- Schlinger B.A. Arnold A.P. Estrogen synthesis in vivo in the adult zebra finch: additional evidence that circulating estrogens can originate in brain. Endocrinology. 1993;133:2610–2616. doi: 10.1210/endo.133.6.8243284. [DOI] [PubMed] [Google Scholar]
- Schlinger B.A. The activity and expression of aromatase in songbirds. Brain Res. Bull. 1997;44:359–364. doi: 10.1016/s0361-9230(97)00215-3. [DOI] [PubMed] [Google Scholar]
- Shen P. Campagnoni C.W. Kampf K. Schlinger B.A. Arnold A.P. Campagnoni A.T. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol. Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Sierra A. Lavaque E. Perez-Martin M. Azcoitia I. Hales D.B. Garcia-Segura L.M. Steroidogenic acute regulatory protein in the rat brain: cellular distribution, developmental regulation and overexpression after injury. Eur. J. Neurosci. 2003;18:1458–1467. doi: 10.1046/j.1460-9568.2003.02872.x. [DOI] [PubMed] [Google Scholar]
- Soma K.K. Sinchak K. Lakhter A. Schlinger B.A. Micevych P.E. Neurosteroids and female reproduction: Estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence R.D. Yin Z. White S. Schlinger B.A. Day L.B. Recovery of motor and cognitive function after cerebellar lesions in a songbird—role of estrogens. Eur. J. Neurosci. 2009;29:1225–1234. doi: 10.1111/j.1460-9568.2009.06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco D.M. Clark B.J. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem. Pharmacol. 1996;51:197–205. doi: 10.1016/0006-2952(95)02093-4. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. Schlinger B.A. Steroidogenesis in the avian brain. I. Avian Endocrinol. 2001:59–77. [Google Scholar]
- Tsutsui K. Progesterone biosynthesis and action in the developing neuron. Endocrinology. 2008;149:2757–2761. doi: 10.1210/en.2007-1592. [DOI] [PubMed] [Google Scholar]
- Vockel A. Prove E. Balthazart J. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J. Neurobiol. 1990;21:808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- Wise P.M. Estrogens and neuroprotection. Trends. Endocrinol. Metabol. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]