Abstract
Diagnosis and treatment of stroke and traumatic brain injury remain significant health care challenges to society. Patient care stands to benefit from an improved understanding of the interactive biochemistry underlying neurotrauma pathobiology. In this study, we assessed the power of neuroproteomics to contrast biochemical responses following ischemic and traumatic brain injuries in the rat. A middle cerebral artery occlusion (MCAO) model was employed in groups of 30-min and 2-h focal neocortical ischemia with reperfusion. Neuroproteomes were assessed via tandem cation-anion exchange chromatography–gel electrophoresis, followed by reversed-phase liquid chromatography–tandem mass spectrometry. MCAO results were compared with those from a previous study of focal contusional brain injury employing the same methodology to characterize homologous neocortical tissues at 2 days post-injury. The 30-min MCAO neuroproteome depicted abridged energy production involving pentose phosphate, modulated synaptic function and plasticity, and increased chaperone activity and cell survival factors. The 2-h MCAO data indicated near complete loss of ATP production, synaptic dysfunction with degraded cytoarchitecture, more conservative chaperone activity, and additional cell survival factors than those seen in the 30-min MCAO model. The TBI group exhibited disrupted metabolism, but with retained malate shuttle functionality. Synaptic dysfunction and cytoarchitectural degradation resembled the 2-h MCAO group; however, chaperone and cell survival factors were more depressed following TBI. These results underscore the utility of neuroproteomics for characterizing interactive biochemistry for profiling and contrasting the molecular aspects underlying the pathobiological differences between types of brain injuries.
Key words: controlled cortical impact; middle cerebral artery occlusion, ischemia; proteomics; traumatic brain injury
Introduction
Stroke and traumatic brain injury (TBI) represent significant burdens to society and remain challenging to diagnose, manage, and treat (Lynch et al., 2004; Prieto et al., 2008). Efforts continue to better understand their complex pathobiologies as required to further our ability to evaluate and care for brain injury patients. For example, rapid and accurate diagnosis of stroke will allow more timely administration of thrombolytic agents within the effective time window (Kent et al., 2003; Schellinger et al., 2003). Greater knowledge of injury mechanisms aids in enhancing diagnosis, and can help guide the conduct of clinical research to investigate novel drug targets and interventions (Dash et al., 2004; Lynch et al., 2004; Pike et al., 2004).
Research into biomarkers has produced diagnostic candidates for ischemic stroke and TBI, the most studied of which are putative markers for both injuries, including neuron-specific enolase (NSE) and S-100B (Berger et al., 2007, 2002; Buttner et al., 1997; Dash et al., 2010; Dauberschmidt et al., 1991; Hergenroeder et al., 2008b; Kleindienst et al., 2005; Kukacka et al., 2006; Martens et al., 1998; Pineda et al., 2004; Pleines et al., 2001; Roine et al., 1989; Wunderlich et al., 2006). Historically, such markers have been discovered through traditional biochemical methods, and in some instances have been tied with biochemical processes, including inflammation (e.g., interleukin-6), impaired hemostasis and thrombosis, and markers of glial activation such as S-100B (Kochanek et al., 2008; Montaner, 2006). Inflammatory markers have been shown to correlate well with ischemia and TBI, but lack brain specificity, appearing elevated by other conditions such as myocardial and inflammatory diseases (Berger et al., 2009; Hergenroeder et al., 2010; Lynch et al., 2004, 2007b; Minambres et al., 2003; Tzoulaki et al., 2007a). NSE is brain specific and has been clinically shown to predict infarct volume, but is less predictive of functional outcomes (Cunningham et al., 1991, 1996; Missler et al., 1997). S-100B protein also correlates well with infarct volume and may predict neurological outcome (Missler et al., 1997; Reynolds et al., 2003); however, S-100B alone lacks specificity as a diagnostic tool, as its elevation is common among many other pathologies, and even following physical activity (Buttner et al., 1997; Kochanek et al., 2008; Pike et al., 2004; Reynolds et al., 2003). Indeed, S-100B highlights the difficulty in applying individual markers to the diagnosis of brain injury. S-100B release from astrocytes may be modulated by neuronal uptake, and levels in peripheral fluid depend on factors that may exhibit entirely different temporal dynamics, such as blood–brain barrier integrity (Kleindienst and Ross Bullock, 2006; Kleindienst et al., 2007). Thus efforts to improve biochemical diagnostics continue toward more sensitive and specific evaluations of ischemic and traumatic brain injuries, and will likely involve a combination of markers (Baird, 2006; Dash et al., 2010; Kochanek et al., 2008; Laskowitz et al., 2009; Siman et al., 2009), from within and among biochemical processes.
Recently, neuroproteomics has become a prominent means to assess neurodegenerative conditions, and it has been found to be particularly useful for biomarker discovery (Ekegren et al., 2008; Hanrieder et al., 2009; Kochanek et al., 2008; Ottens et al., 2006, 2007; Zetterberg et al., 2008; Zhang et al., 2008). A number of proteomic studies have examined TBI (Agoston et al., 2009; Gao et al., 2007; Haqqani et al., 2007; Hanrieder et al., 2009; Hergenroeder et al., 2008a; Jenkins et al., 2002; Kochanek et al., 2006; Ottens et al., 2007; Prieto et al., 2008), as well as brain ischemia (Chen et al., 2007, 2006; Cid et al., 2007; Dhodda et al., 2004; Focking et al., 2006; Junker et al., 2007; Koh, 2009; Sung et al., 2009; Yao et al., 2008, 2009; Zhang et al., 2009), a common component of TBI. Such studies demonstrate the practicality of identifying brain injury neuroproteomes, and have provided insight into individual protein responses across modeled and clinical brain injury. Furthermore, these findings provide an ample reserve of biomarker candidates for subsequent evaluation. Yet proteins possess multiple functions, and when considered individually, any given protein is unlikely to provide sufficient information on the complex pathobiology of these injuries. Often there remains the question of what such arrays of protein dynamics mean in the context of the pathobiology of brain injury, and how else we might capitalize on this information.
In the present study, we report and examine neuroproteome dynamics following focal ischemic injury, and contrast the results with the neuroproteome dynamics seen following focal TBI obtained in an earlier, analogous study (Kobeissy et al., 2006). Indeed, the extent to which the biochemistry of focal ischemia and TBI overlap remains disputed, given that the first is present in the latter, and that they are known to share analogous pathophysiology (Khan et al., 2009). In this study we explore the utility of neuroproteomics as a means to biochemically differentiate the extent of brain injury seen following 30 min and 2 h focal ischemia using a middle cerebral artery occlusion model (MCAO), and the modality of injury by comparison it with a focal contusional TBI employing a controlled cortical impact (CCI) model at a common acute 2-day time point. We distinctively focus on the interrelationship among proteins within these neuroproteomes, and how they relate to common aspects of cell biology, rather than assessing individual protein elements as has been the norm. Through this assessment, we identify commonalities and differences among the injury groups that serve to differentiate their pathobiology.
Methods
Animal models
All experiments used male Sprague-Dawley rats (250–300 g). The animals were anesthetized with 5% isoflurane and maintained at 2% isoflurane in oxygen during all surgical manipulations, which were performed under sterile conditions. The Walter Reed Army Institute of Research or the University of Florida Animal Care and Use Committees approved all procedures, which were performed in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals from the National Research Council, and other statutes involving animals.
Middle cerebral artery occlusion model of focal ischemia
A transient MCAO model as previously characterized was used to induce focal ischemic injury in the neocortex (Williams et al., 2000; Yao et al., 2003, 2008, 2009). In this model, we first exposed the common carotid artery and placed microaneurysm clips on the common carotid and internal carotid arteries. After blocking blood flow, we transected the external carotid. We then inserted a 3-0 nylon suture into the external carotid stump. After removing the carotid microaneurysm clip, we advanced the suture into the internal carotid until resistance was met, occluding the MCA. A 4-0 nylon suture was then tightened around the external carotid stump to prevent bleeding. We removed the common carotid microaneurysm clip and closed the incision with wound clips. In this fashion, we prepared two MCAO groups (n = 8 in each), leaving the endovascular suture in place for 30 min and 2 h, before reopening the incision and withdrawing the suture to allow reperfusion. A sham control group (n = 8) was also prepared with the same surgical procedures, but without insertion of the suture into the MCA. The animals recovered for 2 days in normal housing with food and water access ad libitum, after which they were decapitated under anesthesia.
Controlled cortical impact model of focal traumatic brain injury
In a previous study (Kobeissy et al., 2006), we employed a CCI model for assessment of focal contusional TBI using the same neuroproteomic methodology employed in the present study. We again used the same CCI model in this study to generate additional immunoblot data for direct quantitative comparison with all MCAO immunoblot results. These new TBI results supplement our earlier published TBI neuroproteomic data, in which a homologous neocortical area was examined using the same CCI model. First, the animals were immobilized in a stereotactic frame. We performed a 6-mm ipsilateral craniotomy tangential to bregma and the sagittal suture leaving the underlying dura mater intact. We adjusted the 5-mm-wide impactor tip to just touch the exposed dura mater, thus defining the zero point. The control cortical impact device then impacted the neocortex at 3.5 m/sec with a 150 msec dwell time, compressing the tissue to a depth of 1.6 mm. The result was a focal injury to an area of neocortex corresponding with the caudal end of the infarct produced by the MCAO model, as previously characterized (Williams et al., 2000). The animals (n = 4) recovered for 2 days in normal housing with food and water access ad libitum, after which they and matched control animals (n = 4) were decapitated under anesthesia.
Triphenyltetrazolium chloride staining
The extent of injury was assessed 2 days following brain insult using 2,3,5-triphenyltetrazolium chloride (TTC) staining as detailed elsewhere (Britton et al., 1997). Briefly, immediately following dissection, the sections were soaked in pre-warmed 1% TTC solution for about 2–3 min until the tissue turned red. The reaction was then stopped by transferring the tissue into a dish containing formalin. The sections were then digitally imaged for qualitative assessment of the injured tissue.
Tissue processing
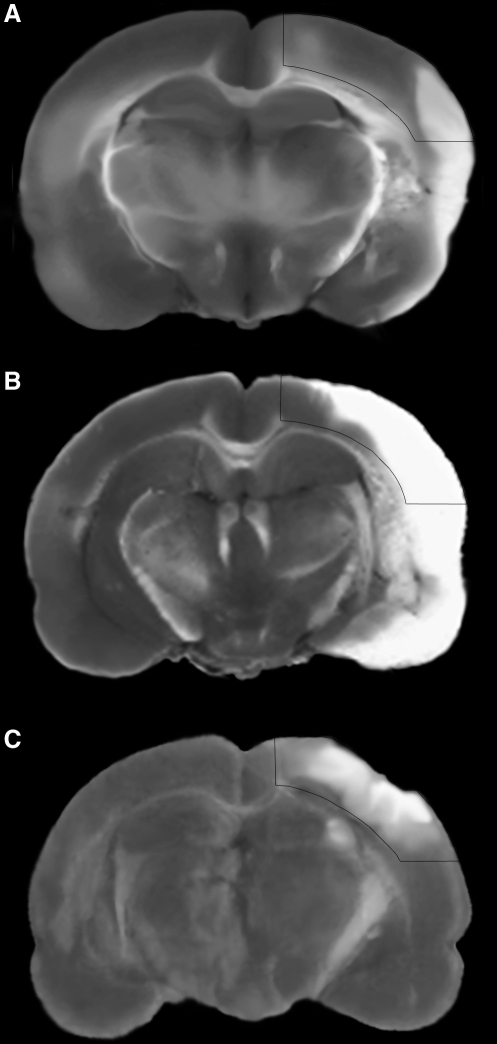
Immediately following decapitation, the brain was removed and immersed in ice-cold saline. Then 2-mm-thick sections were cut starting at approximately bregma −1.6 mm, and rapidly frozen in liquid nitrogen before storage at −80°C. The total time from decapitation to snap freezing of the tissue sections was about 3 min for all animals in injury and control groups. The first 2-mm section from each animal was used to prepare all lysates. The section was placed atop a dissection plate over dry ice. The ipsilateral neocortex was dissected from approximately 1 mm to 7 mm lateral as illustrated in Figure 1, and was comprised predominantly of somatosensory cortex. The removed ischemic and contusion-injured samples contained necrotic and perilesional tissue, with a similar ratio for the 2-h MCAO and TBI samples (Fig. 1). The dissected tissues were pulverized to a fine powder on dry ice, which was then suspended in lysis buffer (300 μL), containing protease inhibitors and 1 mM sodium vanadate. Tissue lysis continued for 90 min at 4°C, after which we removed insoluble material by centrifugation at 14,000g for 10 min. We then measured the protein concentration with a DC Protein Assay kit (Bio-Rad, Hercules, CA).
FIG. 1.
Histology of modeled injuries. Triphenyltetrazolium chloride (TTC)-stained coronal sections are shown to illustrate the extent of injury within the tissues prepared for the 30-min middle cerebral artery occlusion (MCAO) (A), 2-h MCAO (B), and 1.6-mm controlled cortical impact (CCI) (C) experimental groups. The presented sections are proximate to the rostral-caudal center of the dissected region prepared for proteomic and Western blot experiments (near bregma −2.6 mm). Histopathology was assessed 2 days after injury. An overlay of the target dissection area is shown.
Differential Proteomics
We prepared pooled lysates for the 30-min MCAO, 2-h MCAO, and sham control groups using an equal protein mass from individual animals as we had done in the earlier TBI neuroproteomic study (Kobeissy et al., 2006). A 1-mg sample of each pooled lysate was separated by tandem cation-anion exchange chromatography with fractions resolved by gel electrophoresis (CAX-PAGE; Kobeissy et al., 2006; Ottens et al., 2005). Densitometry with Phoretix 1D software (Nonlinear Dynamics, Durham, NC) was used to discriminate bands with a twofold or greater increased or decreased density in either MCAO group relative to controls. For each target identified using the Phoretix software, we excised adjacent gel bands for the 30-min MCAO, 2-h MCAO, and sham control groups, which were trypsinized and separated by reverse-phase chromatography using a Micro-pro pump (Eldex, Napa, CA) online with electrospray data-dependent tandem mass spectrometry with dynamic exclusion (RPLC-MSMS; LCQ Deca XP+; ThermoFisher, San Jose, CA), as in the previous TBI study (Kobeissy et al., 2006). Briefly, peptides were loaded onto a 100-μm × 5-cm reversed-phase capillary column packed in-house with 3-μm C18 particles (Grace, Deerfield, IL). The peptides were eluted via a linear gradient: 5–60% methanol in 0.4% acetic acid over 30 min at 500 nL/min, and electrosprayed through a pulled emitter with a 10-μm tip orifice (New Objectives, Woburn, MA). MSMS spectra were searched against an Integr8 rat database (Rel. 59) using Bioworks v3.3 (ThermoFisher) with the following filters: p < 0.001, two peptides per ID, and unique peptides. The search parameters considered 1 missed tryptic cleavage event, fixed carboxymethylation of cysteine residues, and variable modification of methionine. The results were used to identify those proteins present within the analyzed gel bands. Given the limited sample, the methodology pre-empted the use of biological replication; thus, to minimize false-positive differential events, a two-step quantification process was performed using orthogonal measures as performed in the previous TBI study. Relative protein quantities from both MCAO injury groups were assessed by densitometry for each gel band, and reported as a percentage of the control gel band density. The number of peptides identified per protein (peptide count) within a gel band then provided semiquantitative validation of the densitometric data, with correlation of the two measures employed as an inclusion criterion. Only unique peptides were included in the peptide count, with methionine-containing sequences counted once. The above inclusion criteria have previously been assessed via Western blot analysis to maintain the CAX-PAGE/RPLC-MSMS method false-discovery rate at a nominal 10% (Kobeissy et al., 2006; Ottens et al., 2007). In the present study, quantitative results were likewise verified by Western blot analysis over a 10% sampling of the MCAO neuroproteome across n = 4 biological replicates.
Western immunoblotting
Western blot analysis was performed comparing n = 4 neocortical lysates from the 30-min MCAO, 2-h MCAO, and control groups, as well as a newly prepared TBI group and its control. Lysates were resolved by SDS-PAGE and transferred to PVDF membranes via a semi-dry method. The blots were blocked in 5% milk, probed with primary antibody overnight, and then biotinylated with a secondary antibody. The primary antibodies were: amphiphysin (1:10,000; BD Biosciences, San Jose, CA), GAPDH (1:5000; EnCor Biotechnology, Alachua, FL), NSE (1:500; Aves Labs, Tigard, OR), PYGB (1:500; Research Diagnostics, Concord, MA), SNAP25 (1:500; Pierce, Rockford, IL), αII-spectrin (fodrin) (1:5000; BioMol, Plymouth Meeting, PA), synapsin (1:5000; BD Biosciences), and UCHL1 (1:500; BioMol). β-Actin (1:5000; Sigma-Aldrich, St. Louis, MO) was employed as the load control. The blots were visualized by streptavidin alkaline phosphatase conjugate and BCIP/NBT reagent. We scanned each blot as a 16-bit grayscale image, and acquired integrated band density values with Image-J software (National Institutes of Health, Bethesda, MD). Data for the 30-min and 2-h MCAO groups and the matched control group were analyzed by one-way analysis of variance (ANOVA), with a Holm-Sidak multiple comparisons test to evaluate statistical significance. An unpaired t-test was applied to determine statistical significance between the TBI group and its control group. The experiments were performed with n = 4 biological replicates per group, with results reported as mean ± standard deviation as a percentage of control.
Results
Neuroproteomic analysis
Neuroproteomic data included 73 proteins that exhibited a twofold or greater difference in abundance following either the 30-min or 2-h MCAO injury relative to sham controls, which we refer to hereafter as the MCAO neuroproteome (Table 1). In comparison, the previous TBI proteomic study revealed 46 proteins that exhibited a twofold or greater difference in abundance following focal contusion, referred to hereafter as the TBI neuroproteome (Kobeissy et al., 2006). Both neuroproteomic studies used the same methodology, analyzing a homologous neocortical region, with tissues collected 2 days following injury. The number of increased proteins was similar between the MCAO (23) and TBI (27) groups. The number of decreased proteins identified in the MCAO neuroproteome (50) was 2.5 times that identified in the TBI neuroproteome (19). Five protein products (transferrin, degraded phosphoglycerate kinase, albumin, degraded spectrin, and fetuin β) were similarly increased in the MCAO and TBI neuroproteomes (Fig. 2A). Seven proteins (pyruvate kinase M2, enolase α, GAPDH, aconitase 2, transgelin-3, aldolase, and MAP2) were similarly decreased in the MCAO and TBI neuroproteomes (Fig. 2B). The levels of four proteins diverged between injury models. Neuronal enolase and HSP70 were increased in the 30-min and 2-h MCAO groups, but were decreased after TBI. Conversely, UCHL1 and 14-3-3 proteins decreased in the 2-h MCAO group, but were increased after TBI. Within the MCAO neuroproteome, 32 of 73 measured differential proteins (44%) had previously been reported in association with brain ischemia in the literature, substantiating a pathobiological correlation (Fig. 2C).
Table 1.
MCAO Neuroproteome: Proteins with Greater Than a Twofold Increase/Decrease in the MCAO Groups
| Protein | Symbol | Accession no. | % Control 30 min | % Control 2 h | Enriched in CNS | Cell process |
|---|---|---|---|---|---|---|
| Increased abundance at 2-days post-MCAO insult | ||||||
| Aconatase 2Deg. | ACO2 | Q9ER34 | 70% | 260% | Metabolic | |
| Adaptor protein complex AP-2Deg. | AP2A2 | P18484 | 3200% | 800% | Synaptic | |
| Adenosylhomocysteinase | AHCY | P10760 | 3200% | 800% | Cell signaling | |
| Adenylate kinase 1 | AK1 | P39069 | 2800% | 3200% | Metabolic | |
| Albumin | ALB | P02770 | 350% | 180% | Blood factor | |
| Aldolase CDeg. | ALDOC | P09117 | 300% | 400% | Metabolic | |
| Fetuin-B | FETUB | Q9QX79 | 64% | 160% | Blood factor | |
| Heat shock protein 10 | HSPE1 | P26772 | 100% | 250% | Chaperone | |
| Heat shock protein 70 (HSP70) | HSPA8 | P63018 | 350% | 180% | Chaperone | |
| Macroglobulin (α1) | ALPHA1M | Q63041 | 140% | 490% | Blood factor | |
| Malate dehydrogenase, MitoDeg. | MDHM | P04636 | 100% | 400% | Metabolic | |
| Neuron-specific enolase (NSE) | ENO2 | P07323 | 280% | 530% | Yes | Metabolic |
| Neurogranin (Ng) | NRGN | Q04940 | 280% | 110% | Yes | Synaptic |
| Peroxiredoxin 2 | PRDX2 | P35704 | 2800% | 3200% | Cell survival | |
| Phosphoglycerate kinase 1Deg. | PGK1 | P16617 | 100% | 250% | Metabolic | |
| Protein disulfide-isomeraseDeg. | P4HB | P04785 | 210% | 230% | Chaperone | |
| Protein phosphatase 2ADeg. | PP2A | Q5MKL3 | 570% | 210% | Signal trans. | |
| Stress-induced phosphoprotein 1 ( – phospho) | STIP1 | O35814 | 350% | 180% | Chaperone | |
| Succinate semialdehyde dehydrogenase | SSADH | P51650 | 64% | 160% | Metabolic | |
| Synapsin 2Deg. | SYN2 | Q63537 | 64% | 160% | Yes | Synaptic |
| Transferrin | TF | P12346 | 130% | 370% | Blood factor | |
| Transketolase | TKT | P50137 | 200% | 230% | Metabolic | |
| Spectrin, α2Deg. | SPTAN1 | P16086 | 140% | 490% | Yes | Cytoskeletal |
| Decreased abundance at 2-days post-MCAO insult | ||||||
| 14-3-3 β/α | YWHAB | P35213 | 120% | 9% | Chaperone | |
| 14-3-3 ɛ | YWHAE | P62260 | 150% | 50% | Chaperone | |
| 14-3-3 ζ/δ | YWHAZ | P63102 | 100% | 47% | Chaperone | |
| 3-Hydroxyisobutyrate dehydrogenase, Mito | 3HIDH | P29266 | 3200% | 800% | Metabolic | |
| 4-Trimethylaminobutyraldehyde dehydrogenase | ALDH9A1 | Q9JLJ3 | 56% | 44% | Metabolic | |
| Aconitase 2 (mito precursor) | ACO2 | Q9ER34 | 51% | 35% | Metabolic | |
| Actin, α1 | ACTA1 | P68136 | 100% | 47% | Cytoskeletal | |
| ADP-ribosylation factor 1 | ARF1 | P84079 | 31% | 8% | Protein shuttle | |
| Alanine aminotransferase | GPT | P25409 | 56% | 44% | Metabolic | |
| Aldolase C | ALDOC | P09117 | 47% | 53% | Metabolic | |
| Amphiphysin | AMPH | O08838 | 120% | 38% | Yes | Synaptic |
| Aspartate Aminotransferase | AATM | P00507 | 12% | 22% | Metabolic | |
| ATPase V1, H+ transporting | ATP6V1E1 | Q6PCU2 | 47% | 65% | Synaptic | |
| Carbonyl reductase [NADPH] 1 | CBR1 | P47727 | 62% | 44% | Metabolic | |
| COP9 signalosome complex subunit 2 | CSN2 | P61203 | 88% | 44% | Differentiation | |
| Enolase α | ENOA | P04764 | 56% | 44% | Metabolic | |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | P04797 | 27% | 110% | Metabolic | |
| Glycyl-tRNA synthetase | GARS | Q5I0G4 | 51% | 35% | tRNA synthesis | |
| Glycogen phosphorylase (brain form) | PYGB | P53534 | 54% | 20% | Yes | Metabolic |
| Heat shock protein 105 | HSPH1 | Q66HA8 | 120% | 38% | Chaperone | |
| Heat shock protein 90 | HSPCB | P34058 | 110% | 41% | Chaperone | |
| Heterogeneous nuclear ribonucleoprotein | HNRPA1 | P04256 | 29% | 16% | mRNA transport | |
| Imprinted and ancient protein | IMPACT | Q5GFD9 | 150% | 71% | Yes | Signal trans. |
| Inositol monophosphatase | IMPA1 | P97697 | 93% | 41% | Metabolic | |
| Iron-responsive element binding protein 1 | IREB1 | Q63270 | 54% | 20% | Fe homeostasis | |
| Ischemia responsive 94-kDa protein | IRP94 | O88600 | 120% | 38% | Chaperone | |
| Malate dehydrogenase cytosolic | MDHC | O88989 | 31% | 8% | Metabolic | |
| Map-tau | TAU | P19332 | 88% | 44% | Yes | Cytoskeletal |
| Microtubule associated protein 1 | MAP1 | P15205 | 54% | 20% | Yes | Cytoskeletal |
| Microtubule associated protein 2 | MAP2 | P15146 | 89% | 20% | Yes | Cytoskeletal |
| Neurolysin (mito precursor) | NEUL | P42676 | 51% | 35% | Yes | Endopeptidase |
| Neuronal calcium binding protein | NECAB2 | Q9ESB4 | 150% | 71% | Yes | Cell signaling |
| N-myc downstream regulator 2 | NDRG2 | Q6JE36 | 38% | 23% | Yes | Differentiation |
| PEST-containing nuclear protein | PCNP | Q7TP40 | 27% | 51% | Cell survival | |
| Programmed cell death 6-interacting protein | PDC6I | Q9QZA2 | 54% | 20% | Cell survival | |
| Pyruvate dehydrogenase E1 | PDHB | P49432 | 29% | 16% | Metabolic | |
| Pyruvate kinase M2 | PKM2 | P11980 | 180% | 47% | Yes | Metabolic |
| Ras-related protein Rab-3C | RAB3C | P62824 | 100% | 50% | Synaptic | |
| Prolyl endopeptidase | PREP | O70196 | 51% | 35% | Endopeptidase | |
| Protein phosphatase 2B | PP2B | P63329 | 47% | 65% | Yes | Signal Trans |
| Small glutamine-rich tetratricopeptide-repeat containing protein A | SGTA | O70593 | 110% | 16% | Chaperone | |
| Stress-induced-phosphoprotein 1 (+phospho) | STIP1 | O35814 | 35% | 100% | Chaperone | |
| Superoxide dismutase 1 | SOD1 | P07632 | 12% | 22% | Cell survival | |
| Synapsin-2 | SYN2 | P09951 | 55% | 38% | Yes | Synaptic |
| Synaptosomal-associated protein 25 | SNAP25 | P60881 | 100% | 47% | Yes | Synaptic |
| Thimet oligopeptidase | THOP1 | P24155 | 51% | 35% | Yes | Endopeptidase |
| Transgelin-3 (NP25) - like NP22 | NP25 | P37805 | 31% | 8% | Yes | Cytoskeletal |
| Tubulin β | TUBB | P04691 | 88% | 44% | Yes | Cytoskeletal |
| Ubiquitin carboxyl-terminal hydrolase L1 | UCHL1 | Q00981 | 100% | 50% | Yes | Cell survival |
| Spectrin, α2 | SPTAN1 | P16086 | 89% | 20% | Yes | Cytoskeletal |
Protein product identified as degraded from gel migration.
MCAO, middle cerebral artery occlusion.
FIG. 2.
Comparison of middle cerebral artery occlusion (MCAO) and traumatic brain injury (TBI) neuroproteomes. Numbers of protein products with an increased (A) or decreased (B) abundance, and the overlap of the MCAO and TBI neuroproteomes at 2 days following neocortical insults. (C) Thirty-two proteins from the MCAO differential neuroproteome that have a reported pathobiological association with brain ischemia in previous literature. The proteins are illustrated a top their native cell compartment: membrane depiction defines extracellular, intermembrane, and intracellular proteins; nuclear, mitochondrial, and golgi-associated proteins appear atop their respective cartoons; the remaining intracellular proteins are common to the cytosol (SYN1, synaptin 1; TF, transferrin; PYGB, glycogen phosphorylase (brain form); SNAP25, synaptosomal-associated protein 25; P4HB, protein disulfide-isomerase; MAP2, microtubule-associated protein 2; HSPE1, heat shock protein 10; ALB, albumin; TKT, transketolase; HSPH1, heat shock protein 105; ALDH 9A1, 4-trimethylaminobutyraldehyde dehydrogenase; GPT, alanine aminotransferase; PPP3CA, calcineurin (PP2B); PGK1, phosphoglycerate kinase 1; MAP1B, microtubule-associated protein 1B; SPTAN1, spectrin α2; AHCY, adenosylhomocysteinase; THOP1, thimet oligopeptidase; gad, ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1); ARF1, ADP-ribosylation factor 1; ALDOC, aldolase C; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PKM2, pyruvate kinase M2; HSPCB, heat shock protein 90; ENO2, neuron-specific enolase; ACO2, aconatase 2; ACO1, aconatase 1; SOD1, superoxide dismutase 1; HSPA 8, heat shock protein 70; PREP, prolyl endopeptidase; STIP1, stress-induced phosphoprotein 1).
Immunoblot analysis
Quantitative proteomic measures were validated by complementary immunoblot analysis for a 10% sampling of the MCAO neuroproteome. The eight proteins were selected based on the availability of working antibodies, brain specificity, and relevance to the pathobiology. The immunoblot results are reported in Table 2, along with corresponding quantitative MCAO neuroproteomic data. We similarly probed TBI and matched control groups with the eight antibodies for direct comparison (Table 2). The differences between the MCAO and control groups were statistically significant for the eight proteins, and were consistent with the neuroproteomic data. Moreover, the opposing responses for NSE and UCHL1 between the MCAO and TBI groups were confirmed. The 2-h MCAO group exhibited protein differences of greater magnitude, than the 30-min MCAO group, relative to controls, for all proteins except GAPDH.
Table 2.
Validation of MCAO Differential Neuroproteomic Data and Immunoblot Comparison between Injury Models
| |
|
|
MCAO |
TBI |
|||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Gel banda |
Δ Peptide count |
Blot banda |
Blot banda |
|||
| Protein | Accession no. | Mr (kDa) | 30 min | 2 h | 30 min | 2 h | 30 min | 2 h | 1.6 mm |
| Amphiphysin | O08838 | 125 | – | – | – | – | 91% | 68%*** | 86% |
| 110 | 120% | 38% | 0 | −2 | 110% | 44%** | 18%** | ||
| GAPDH | P04797 | 36 | 27% | 110% | −2 | 3 | 93%* | 100% | 70%* |
| PYGB | P53534 | 97 | 54% | 20% | −10 | −14 | 86% | 50%*** | 65%* |
| 67b | – | – | – | – | 110% | 170%*** | 260%*** | ||
| NSE | P07323 | 47 | 280% | 530% | 2 | 4 | 130%* | 120%* | 67%* |
| SNAP25 | P60881 | 23 | 100% | 47% | −1 | −3 | 150% | 50%* | 25%* |
| Spectrin (α2) | P16086 | 280 | 89% | 20% | 1 | −2 | 86% | 44%* | 66%* |
| 150b | 140% | 460% | 0 | 2 | 120% | 300%** | 370%*** | ||
| 145b | – | – | – | – | 130% | 430%*** | 750%*** | ||
| Synapsin | P09951 | 74 | 55% | 38% | −2 | −2 | 94% | 44%*** | 27%*** |
| UCHL1 | Q00981 | 25 | 100% | 50% | 2 | −2 | 93% | 71%* | 130% |
Percent of control value.
Protein breakdown product.
p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significantly different from sham-operated animals.
MCAO, middle cerebral artery occlusion; TBI, traumatic brain injury; UCHL1, ubiquitin carboxyl-terminal hydrolase L1; SNAP25, synaptosomal-associated protein 25; NSE, neuron-specific enolase; PYGB, glycogen phosphorylase (brain form); GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
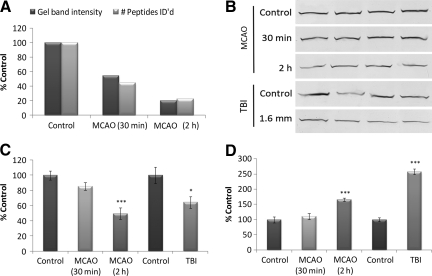
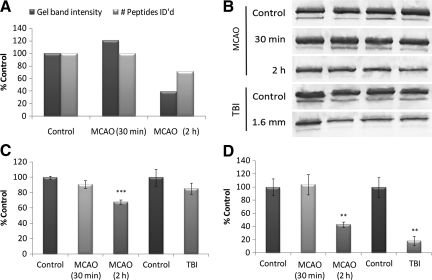
Figures 3 and 4 demonstrate the strong quantitative correlation between neuroproteomic and immunoblot data. The orthogonal neuroproteomic end-point gel band intensity and the peptide count per protein showed similar relative measures for the 30-min and 2-h MCAO groups relative to controls. These data were subsequently consistent with immunoblot results (Fig. 3C and D). The PYGB and amphiphysin immunoblots also exemplified two common confounds seen among neuroproteomic data. The PYGB immunoblots revealed a modest but increased 67-kDa band in the MCAO and TBI groups, suggestive of a breakdown product (Fig. 4D). Conversely, the amphiphysin immunoblots revealed altered expression of two known isoforms at 125 kDa and 110 kDa. Amphiphysin isoforms responded similarly to injury; however, translational products of other proteins may differ in function or localization, and as such may contrast in their response to injury.
FIG. 3.
Comparison of brain-specific glycogen phosphorylase (PYGB) neuroproteomic and immunoblot data. (A) Protein quantity as assessed by gel band density measurement and peptide count for PYGB. (B) PYGB immunoblot of MCAO and TBI neocortical tissue lysates and matched controls. (C) Histogram of densitometric data for the intact 97-kDa band of PYGB among the MCAO and TBI groups normalized to controls. (D) Histogram of densitometric data for degraded PYGB (67-kDa band). All samples were of neocortical tissue collected 2 days post-injury (n = 4 per group). Load was controlled by co-immunoblot with β-actin. Values are presented as mean ± standard deviation as a percentage of control. MCAO data were evaluated by one-way analysis of variance (Holm-Sidak test), and TBI data by t-test (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; TBI, traumatic brain injury; MCAO, middle cerebral artery occlusion).
FIG. 4.
Comparison of amphiphysin neuroproteomic and immunoblot data. (A) Protein quantity assessed by gel band density measurement and peptide count for amphiphysin. (B) Amphiphysin immunoblot of MCAO and TBI neocortical tissue lysates and matched controls. (C) Histogram of densitometric data for the 125-kDa isoform of amphiphysin among the MCAO and TBI groups normalized to controls. (D) Histogram of densitometric data for the 110-kDa isoform of amphiphysin. All samples were of neocortical tissue collected 2 days post-injury (n = 4 per group). Load was controlled by co-immunoblot with β-actin. Values are presented as mean ± standard deviation as a percentage of control. MCAO data were evaluated by one-way analysis of variance (Holm-Sidak test), and TBI data by t-test (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; TBI, traumatic brain injury; MCAO, middle cerebral artery occlusion).
Protein process analysis
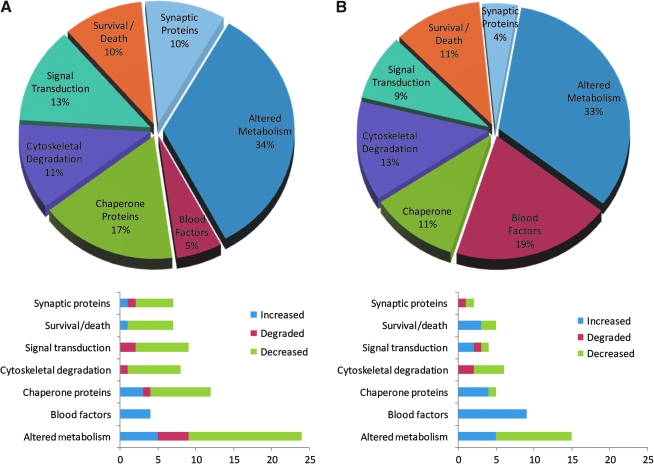
The MCAO and TBI neuroproteomes corresponded with similar sets of post-injury cellular processes (Fig. 5). The same percentage of each neuroproteome correlated with altered metabolism, cytoskeletal degradation, and cell survival/death processes. In particular, 30 proteins of the 2-h MCAO neuroproteome have a known relationship with apoptotic cell death, and most (>70%) were decreased at the 2-day time point. Those apoptosis-associated proteins that increased, peroxiredoxin 2 and transferrin, are known to play an antiapoptotic role.
FIG. 5.
Classification of neuroproteomic data by cellular process. Proportions of proteins identified in the MCAO neuroproteome (A) and the TBI neuroproteome (B) divided among the seven most prominent processes identified. The indicated percentages are out of the total number of differential proteins with known biological processes from the MCAO (54) and TBI (45) neuroproteomes. Bar graphs depict the proportion of increased, decreased, or degraded proteins associated with a given process (TBI, traumatic brain injury; MCAO, middle cerebral artery occlusion).
Blood-associated proteins were all increased in the MCAO and TBI neuroproteomes, and comprised a much greater percentage (fourfold) of the TBI data. The hemorrhage observed with the contusion model (TBI) was not prominant in the MCAO model. In contrast, a greater proportion of the MCAO neuroproteome consisted of increased heat-shock chaperone proteins, while HSP70 actually decreased in the TBI neuroproteome. Similarly, the MCAO neuroproteome contained a greater proportion of synaptic-associated proteins that were decreased or degraded in association with the synaptic vesicle cycle (Fig. 6). In all, the neuroproteomic data, as validated by representative immunoblot analyses, presented a wide view of the neurochemical differences between brain injury groups, which is discussed in relation to the differences among relevant the cellular processes (Fig. 5).
FIG. 6.
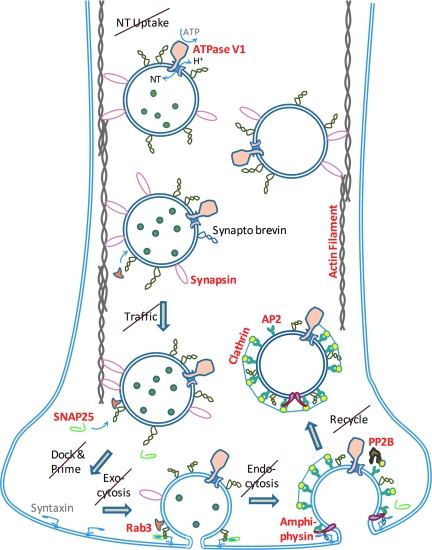
Loss of synaptic vesicle cycle elements following middle cerebral artery occlusion (MCAO). The 2-h MCAO neuroproteome included the loss of many key synaptic proteins (in bold red type) associated with the synaptic vesicle cycle, from neurotransmitter (NT) uptake on through docking, exocytosis, endocytosis, and vesicle recycling. The data correlated with the observed degradation of axonal cytoskeletal components: actin microfilaments, αII-spectrin membrane scaffold, and tau-supported tubulin microtubules were all reduced or degraded. The combined 2-h MCAO results depict prominent axonal degradation and loss of synaptic function in accord with the massive cell loss associated with the infarcted pathobiology. In contrast, only vesicle ATPase was decreased in the 30-min MCAO group (ATP, adenosine triphosphate; SNAP25, synaptosomal-associated protein 25; AP2, adapter protein complex AP-2; Rab3, ras-related protein-3; PP2B, protein phosphatase 2B).
Discussion
In this section we contrast the biochemistry revealed within the 30-min MCAO, 2-h MCAO, and contusional TBI neuroproteomes, and relate the results to the cell biology. From the data, it is apparent that fewer proteins are modulated in the 30-min than in the 2-h MCAO group, consistent with the extent of injury (Fig. 1) as discussed later. The MCAO and TBI neuroproteomes displayed similar but differing pathobiology. Transferrin and albumin are blood factors expected to be found in damaged tissue due to the deteriorated cerebrovasculature and blood–brain barrier disruption. Decreased transgelin-3 and microtubule-associated protein (MAP 2), and increased αII-spectrin proteolysis, were observed following each injury, and are suggestive of cytoskeletal derangement of the affected tissue, as reported previously (Minger et al., 1998; Pettigrew et al., 1996; Pike et al., 2004; Posmantur et al., 1996). However, key differences between the MCAO and TBI groups are discussed below and summarized in Table 3.
Table 3.
Divergent Biochemical Responses among Injury Groups Across Key Cellular Processes
| Cell process | 30-min MCAO | 2-h MCAO | TBI (CCI) |
|---|---|---|---|
| Energy metabolism Comparison: 30-min > 2-h MCAO 2-h MCAO ≠ TBI |
↑Transketolase (pentose phosphate to GAPDH) – Phosphoglycerate kinase (+ATP) ↑ Neuron-specific enolase ↑ Pyruvate kinase (+ATP) (ATP production) |
↑Transketolase (pentose phosphate to GAPDH) ↓Phosphoglycerate kinase (+ATP) ↑ Neuron-specific enolase ↓ Pyruvate kinase (+ATP) (degraded ATP production) |
N/A ↓Phosphoglycerate kinase (+ATP) ↓ Neuron-specific enolase ↓ Pyruvate kinase (+ATP) (degraded ATP production) |
| ↓Pyruvate dehydrogenase ↓ Malate dehydrogenase (lost aerobic respiration) |
↓Pyruvate dehydrogenase ↓ Malate dehydrogenase (lost aerobic respiration) |
↑Malate dehydrogenase (malate shuttle active; aerobic respiration possible) |
|
| Synaptic function Comparison: 30-min ≠ 2-h MCAO 2-h MCAO = TBI |
↓Vesicle ATPase (less ATP and less neurotransmitter) |
↓Neurotransmitter update ↓ Vesicle trafficking ↓ Vesicle docking/exocytosis ↓ Vesicle endocytosis ↓ Vesicle recycling (lost synaptic vesicle cycle) |
↓Neurotransmitter update ↓ Vesicle trafficking ↓ Vesicle docking/exocytosis ↓ Vesicle endocytosis ↓ Vesicle recycling (lost synaptic vesicle cycle) |
| ↓Transgelin 3 (signaling dysfunction) |
↓Map-tau, and spectrins ↓ Tubulins, actin, map 1 and 2 ↓ Transgelin 3 (degraded cytoarchitecture) |
↓Map-tau, and spectrins ↓ Tubulins, actin, map 1 and 2 ↓ Transgelin 3 (degraded cytoarchitecture) |
|
| ↑Neurogranin (synaptic plasticity) |
N/A | N/A | |
| Chaperone activity Comparison: 30-min > 2-h MCAO 2-h MCAO > TBI |
↑HSP70, ↓p-STIP1, ↑HSP90 (upregulated HSP70 and HSP90 activity) |
↑HSP70, – p-STIP1, – HSP90 (less HSP70 and HSP90 activity) |
↓HSP70 (lost HSP70 activity) |
| N/A | ↓SGTA, ↑HSP10 (efficient use of HSP60 complex) |
N/A | |
| Cell survival factors Comparison: 30-min < 2-h MCAO 2-h MCAO ≠ TBI |
↑PRDX (antioxidant) (to reduce TNF-induced apoptosis) |
↑PRDX (antioxidant) ↑ Fetuin-B (calpain/caspase inhibitor) (to reduce apoptosis) |
↑Fetuin-B (protease inhibitor) (to reduce apoptosis-associated calpain and caspase activity) |
| ↓Calcineurin (to reduce apoptosis) |
↓Calcineurin ↓ Free 14-3-3's (to reduce apoptosis) |
↑Free 14-3-3's (uninhibited apoptosis) |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ATP, adenosine triphosphate; MCAO, middle cerebral artery occlusion; TBI, traumatic brain injury; HSP70, heat shock protein 70; p-STIP1, phosphorylated stress-induced phosphoprotein 1; HSP90, heat shock protein 90; SGTA, small glutamine-rich tetratricopeptide-repeat containing protein A; HSP10, heat shock protein 10; PRDX, peroxiredoxin 2.
Injury-specific alterations to energy metabolism
Ischemia-induced injury is rapid in the brain, the organ most dependent on aerobic metabolism to maintain function. Enzymes associated with the entry (energy-consuming) stage of glycolysis were downregulated in the 30-min and 2-h MCAO groups. Glycogen phosphorylase (brain form) was also decreased, though to a lesser extent in the 30-min than in the 2-h MCAO group (Fig. 3), indicating a dwindled glycogen reserve. However, the substantial transketolase increase in both MCAO groups pointed to pentose-phosphate as an alternative sugar supply for intermediate entry into glycolysis via glyceraldehyde-3-phosphate (G3P) production. G3P and NAD+ are converted to 1,3-bisphosphoglycerate and NADH by GAPDH, which showed only a modest decrease in the 30-min MCAO group, and was unchanged in the 2-h MCAO group (Table 2). 1,3-Bisphosphoglycerate and ADP are then catalyzed to produce ATP by phosphoglycerate kinase, which was unchanged in the 30-min MCAO group, but was degraded in the 2-h MCAO group. In subsequent steps, enolase catalyzes the production of phosphoenolpyruvate, which with ADP is used to produce pyruvate and more ATP with pyruvate kinase (which was unchanged in the 30-min, but decreased in the 2-h, MCAO groups). The alpha enolase isoform found in astrocytes was decreased in both the 30-min and the 2-h MCAO groups; however, neuron-specific enolase was increased in both MCAO groups (Table 2). The difference between isoforms suggests preferential ATP production by neurons, which is essential for maintaining ion gradients.
Entry into the oxygen-dependent citric acid cycle was blocked by a substantial decrease in pyruvate dehydrogenase, pushing pyruvate toward lactic acid production by lactate dehydrogenase, and resulting in lactic acidosis. While detrimental in the long term, the production of lactic acid is necessary to convert NADH back to the NAD+ form required for continued function of GAPDH. Overall, the 30-min MCAO neuroproteome depicts the use of pentose-phosphate to convert 2 ADP to 2 ATP molecules by anaerobic glycolysis, culminating in lactic acid production. In contrast, the key enzymes phosphoglycerate kinase and pyruvate kinase appear to be degraded and decreased, respectively, suggesting that even glycolysis is ineffective for ATP production by 2 days following the 2-h MCAO injury (Table 3). The substantial increase in adenylate kinase in the 30-min and 2-h MCAO groups denoted one remaining opportunity to convert ADP to ATP, which is upregulated at low ATP levels. The observed suppression of aerobic metabolism in the 30-min and 2-h MCAO neuroproteomes was consistent with known ischemic pathobiology, in which despite early reperfusion of the tissue, a decline in oxygen metabolism is observed within a day of injury, as the infarct develops (Baron, 2001; Terada et al., 2001). Metabolic changes in the TBI group also displayed glycolytic dysfunction, though with differences from the MCAO groups. First, GAPDH decreased significantly in the TBI group (Table 2), and transketolase did not increase, which eliminated the use of pentose-phosphate. Next, NSE was also significantly reduced in the TBI group, further preventing the use of glycolysis for ATP production. One last difference between the MCAO and TBI groups was that malate dehydrogenase increased after TBI. Elevated malate dehydrogenase suggested the possible aerobic use of malate via the malate-aspartate shuttle as a source for oxaloacetate, which can be used to produce ATP via pyruvate carboxylase.
Extent of disrupted synaptic function
Proteins associated with all steps in the synaptic vesicle cycle were decreased/degraded within the 2-h MCAO neuroproteome, denoting pervasive axonal dysfunction (Fig. 6), which was confirmed by immunoblot analysis (Table 2). Synaptic dysfunction tied with the observed axonal degradation denoted by decreased αII-spectrin and map-tau axonal cytoarchitecture proteins in connection with reduced actin (microfilaments) and tubulin (microtubules). The cytoskeletal-associated protein transgelin 3 was also prominently decreased in the 2-h as well as the 30-min MCAO neuroproteomes. Transgelin 3 is known to be essential to the maintenance of process cytoarchitecture (Depaz and Wilce, 2006), and when decreased results in dysregulated neuronal signaling (Ito et al., 2005). Yet the levels of synaptic vesicle cycle proteins were generally unchanged in the 30-min MCAO neuroproteome. Only vesicle ATPase decreased, potentially an adaptive response to low ATP levels and reduced neurotransmission. Axonal cytoarchitecture proteins were also unchanged in the 30-min MCAO group. Thus the predominant synaptic structure and machinery appeared to be intact in the 30-min MCAO group, in contrast to the degradation observed in the 2-h MCAO group (Table 3), which corresponded with the extent of injury (Fig. 1). Interestingly, neurogranin levels were significantly increased in the 30-min MCAO neuroproteome, but not in the 2-h MCAO group. Neurogranin is known to induce synaptic plasticity and remodeling (Pak et al., 2000).
The TBI neuroproteome contained fewer synaptic proteins than the MCAO neuroproteome, but did include decreased transgelin 3 (see relevance above) and degraded synaptotagmin, another protein essential for vesicle endocytosis. The TBI immunoblot data in Table 2 indicate that other parts of the synaptic vesicle cycle were also dysfunctional, via decreased synapsin, SNAP25, and amphiphysin. In addition, SNAP25, synapsin, and other synaptic proteins (e.g., synaptotagmin and syntaxin) were found to be decreased 2 days following injury, in an orthogonal high-throughput immunoblot (HTPI) neuroproteomic TBI study using the same CCI model (Liu et al., 2006). Taken together, the results illustrate similar synaptic degradation within the 2-h MCAO and TBI neuroproteomes (Table 3). Ultimately, the HTPI and CAX-PAGE/RPLC-MSMS neuroproteomic methods are complementary, illustrating the tenet of proteomic science that no one method is comprehensive. The separation space of the CAX-PAGE/RPLC-MSMS method is finite, resulting in co-migration of proteins, a large portion of which are expressed at low concentrations. Our inclusion criteria necessitated a twofold change in gel band intensity, along with a corresponding quantitative difference in peptide count, to control the type I error rate; however, these criteria limited sensitivity and increased type II errors, which explains the missed differential synaptic proteins identified by immunoblot methods. In contrast, immunoblots exhibit high sensitivity and a low type II error rate, but at the cost of a greater type I error rate due to the limited number of specific protein targets (Hergenroeder et al., 2008a). As a consequence, fewer differential targets were identified by HTPI in the MCAO and TBI neuroproteomes (Liu et al., 2006; Yao et al., 2008), relative to the CAX-PAGE/RPLC-MSMS approach presented here.
Modulation of the heat shock chaperone system
Cellular stress invoked by ischemia has been shown to modulate heat shock proteins and their cofactors (Rajdev and Sharp, 2000). The most prominent chaperone is HSP70, which conforms damaged proteins, and is considered neuroprotective following brain ischemia (Giffard et al., 2004; Lee et al., 2001; Tsuchiya et al., 2003). The relationships among heat shock proteins after ischemia, however, have not been examined in detail. HSP90, for example, performs final adjustments on proteins refolded by HSP70, and verifies protein conformation. Before receiving a protein from HSP70, HSP90 must complex with dephosphorylated stress-induced phosphoprotein 1 (STIP1; Daniel et al., 2008). In the 30-min MCAO group, increased HSP70 was accompanied by a proportional increase in dephosphorylated STIP1 and an increase in HSP90, signifying upregulated and fully functional chaperone activity.
In contrast, HSP70 and dephosphorylated STIP1 increased only half as much in the 2-h MCAO group. HSP90 was found to be decreased in the 2-h MCAO group, and more inactive phosphorylated STIP1 was seen. In addition to the reduced chaperone response, the 2-h MCAO group was modulated for increased energy efficiency (at the expense of function). HSP90-STIP1 activity is ATP-intensive, and in this case was sacrificed. HSP70 operated more efficiently in the 2-h MCAO group due to a decrease in small glutamine-rich tetratricopeptide-repeat containing protein A (SGTA; Angeletti et al., 2002). Further, the co-chaperone HSP10, which is known to increase HSP60 efficiency, was increased in the 2-h MCAO and not the 30-min MCAO group (Dubaquie et al., 1997). In comparison, heat shock proteins were conspicuously absent from earlier neuroproteomic studies of TBI (Kobeissy et al., 2006; Liu et al., 2006). HSP70 was, in contrast, decreased in the TBI group. Chen and associates previously found HSP70 to be elevated after CCI out to 1 day post-injury, but levels returned to baseline by the next (3-day) time point (Chen et al., 1998). Ultimately, at the present 2-day time point, heat shock chaperone activity appeared to be suppressed in the TBI group, in contrast to the MCAO groups (Table 3).
Distinctive cell survival factor response
Neuroproteomic data indicated that pro-survival mechanisms were at play 2 days after brain injury. The pro-survival protein peroxiredoxin (PRDX) was substantially increased in both the 30-min and 2-h MCAO groups. PRDX is an antioxidant enzyme that reduces ROS, promotes growth factors, and inhibits tumor necrosis factor (TNF)-induced apoptosis. A pro-survival decrease in calcineurin (serine/threonine protein phosphatase 3) was also observed in the 30-min and 2-h MCAO groups. Decreased calcineurin inhibits the calcium-inducible apoptotic pathway through dephosphorylated Bad and Akt (Shibasaki et al., 1997; Wang et al., 1999). Indeed, the drugs cyclosporine-A and FK506 function by reducing calcineurin levels, and are reported to improve outcome after ischemia (Kang et al., 2008; Macleod et al., 2005), and contusional TBI (Singleton et al., 2001; Sullivan et al., 2005). In contrast, the only pro-survival response identified in the TBI neuroproteome was an increase in fetuin-B, a potent calpain/caspase inhibitor that was also found to be elevated in the 2-h MCAO group.
14-3-3 Protein binding also inhibits calcium-inducible apoptosis through binding the Akt substrates Bad and proline-rich Akt substrate (PRAS; Chan, 2004). The level of unbound 14-3-3's found at a molecular mass of ∼ 14 kDa were unchanged in the 30-min MCAO group, but was substantially decreased in the 2-h MCAO group. A decrease specifically in the unbound 14-3-3 epsilon isoform was identified in our earlier HTPI study of the 2-h MCAO neuroproteome (Yao et al., 2008). The same study found that 14-3-3 epsilon was also decreased following a model of penetrating ballistic-like brain injury. Less unbound 14-3-3 would suggest an increase in binding and potentially inhibition of apoptosis. In contrast, unbound 14-3-3's were increased in the TBI neuroproteome, suggesting less binding of 14-3-3 and potentially reduced inhibitory function. Together, these results demonstrate distinct differences in the modulations of cell survival factors seen between the MCAO and TBI groups (Table 3).
In conclusion, neuroproteomics provides a broad-spectrum means to assess the biochemical differences associated with the extent (30-min versus 2-h ischemia), and modality of focal injury (ischemic versus contusional), within a defined post-injury time point and anatomical region. An important caveat of this work is defined by what tissues were sampled. The present study required comparison between injury models and a relatively large amount of tissue, which restricted how the tissue was sampled. As such, the dissected area included a variable amount of lesioned and peri-lesional tissues (Fig. 1), which prevented making a distinction between the two. Nevertheless, the neuroproteomic data were reflective of the extent of injury. For example, synaptic cycle elements were largely preserved in the 30-min MCAO injury, for which we found less necrotic tissue, while the more severely lesioned 2-h MCAO and TBI tissues exhibited loss of synaptic cycle elements and cytoarchetecture. Future studies will ultimately capitalize on more advanced methods that require less starting material, and thus can be more discriminative in sampling. We also warn of the present methodology's far-from-exhaustive coverage of the neuroproteome; however, the results substantiate the utility of associating proteins modulated by injury with common, relevant cellular processes. In summary, the proteins found to be altered following the 30-min MCAO injury were largely metabolic, indicating energy dysfunction, but with otherwise intact cytoarchitecture, which was assisted by increased chaperon function and pro-survival elements. In contrast, the 2-h MCAO injury exhibited near-complete metabolic disruption, loss of synaptic function and structure, and less chaperon functionality, but with an elevated cell-survival response relative to the 30-min MCAO injury. The TBI group resembled the 2-h MCAO group in terms of lost synaptic function and cytoskeletal structure, in accord with the similar proportions of necrotic tissues; however, distinct differences in metabolism, chaperone activity, and cell survival factors underscored the dissimilar cell biology seen within the bulk tissues by 2 days post-injury. These results clearly support the finding that although ischemia may be a part of TBI, an ischemic model is not a surrogate for TBI. In the future, neuroproteomics will provide a useful means to delineate the biochemical profiles that differentiate types of brain injuries.
Acknowledgments
Our thanks go to Drs. Povlishock and McGinn Greer for their thoughtful feedback. This work was supported by Department of Defense grant DAMD1703-1-0066, and the National Institute of Neurological Disorders and Stroke grant NS055012. This article was reviewed by the Walter Reed Army Institute of Research, and there was no objection to its presentation and publication. The opinions and assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting the views of the Department of the Army or the Department of Defense.
Author Disclosure Statement
Drs. Hayes and Wang own equity in and are executive officers of Banyan Biomarkers, Inc., and may benefit financially from the results presented in this article.
References
- Agoston D.V. Gyorgy A. Eidelman O. Pollard H.B. Proteomic biomarkers for blast neurotrauma: targeting cerebral edema, inflammation, and neuronal death cascades. J. Neurotrauma. 2009;26:901–911. doi: 10.1089/neu.2008.0724. [DOI] [PubMed] [Google Scholar]
- Angeletti P.C. Walker D. Panganiban A.T. Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity. Cell Stress Chaperones. 2002;7:258–268. doi: 10.1379/1466-1268(2002)007<0258:sgrpvp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A.E. Blood biologic markers of stroke: improved management, reduced cost? Curr. Atheroscler. Rep. 2006;8:267–275. doi: 10.1007/s11883-006-0003-2. [DOI] [PubMed] [Google Scholar]
- Baron J.C. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc. Dis. 2001;11(Suppl. 1):2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- Berger R.P. Beers S.R. Richichi R. Wiesman D. Adelson P.D. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J. Neurotrauma. 2007;24:1793–1801. doi: 10.1089/neu.2007.0316. [DOI] [PubMed] [Google Scholar]
- Berger R.P. Ta'asan S. Rand A. Lokshin A. Kochanek P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr. Res. 2009;65:97–102. doi: 10.1203/PDR.0b013e31818c7e27. [DOI] [PubMed] [Google Scholar]
- Berger R. Pierce M. Wisniewski S. Adelson P.D. Clark R.S.B. Ruppel R. Kochanek P. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109:E31–E31. doi: 10.1542/peds.109.2.e31. [DOI] [PubMed] [Google Scholar]
- Britton P. May X.C. Laskosky M.S. Tortella F.C. Dextromethorphan protects against cerebral injury following transient, but not permanent, focal ischemia in rats. Life Sci. 1997;60:1729–1740. doi: 10.1016/s0024-3205(97)00132-x. [DOI] [PubMed] [Google Scholar]
- Buttner T. Weyers S. Postert T. Sprengelmeyer R. Kuhn W. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28:1961–1965. doi: 10.1161/01.str.28.10.1961. [DOI] [PubMed] [Google Scholar]
- Chan P.H. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem. Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Chen A. Liao W.P. Lu Q. Wong W.S. Wong P.T. Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats—a proteomics approach. Neurochem. Int. 2007;50:1078–1086. doi: 10.1016/j.neuint.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Chen D.M. Xiao L. Cai X. Zeng R. Zhu X.Z. Involvement of multitargets in paeoniflorin-induced preconditioning. J. Pharmacol. Exp. Ther. 2006;319:165–180. doi: 10.1124/jpet.106.104380. [DOI] [PubMed] [Google Scholar]
- Chen M. Clark R.S. Kochanek P.M. Chen J. Schiding J.K. Stetler R.A. Simon R.P. Graham S.H. 72-kDa heat shock protein and mRNA expression after controlled cortical impact injury with hypoxemia in rats. J. Neurotrauma. 1998;15:171–181. doi: 10.1089/neu.1998.15.171. [DOI] [PubMed] [Google Scholar]
- Cid C. Garcia-Bonilla L. Camafeita E. Burda J. Salinas M. Alcazar A. Proteomic characterization of protein phosphatase 1 complexes in ischemia-reperfusion and ischemic tolerance. Proteomics. 2007;7:3207–3218. doi: 10.1002/pmic.200700214. [DOI] [PubMed] [Google Scholar]
- Cunningham R.T. Watt M. Winder J. McKinstry S. Lawson J.T. Johnston C.F. Hawkins S.A. Buchanan K.D. Serum neurone-specific enolase as an indicator of stroke volume. Eur. J. Clin. Invest. 1996;26:298–303. doi: 10.1046/j.1365-2362.1996.129282.x. [DOI] [PubMed] [Google Scholar]
- Cunningham R.T. Young I.S. Winder J. O'Kane M.J. McKinstry S. Johnston C.F. Dolan O.M. Hawkins S.A. Buchanan K.D. Serum neurone specific enolase (NSE) levels as an indicator of neuronal damage in patients with cerebral infarction. Eur. J. Clin. Invest. 1991;21:497–500. doi: 10.1111/j.1365-2362.1991.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Daniel S. Bradley G. Longshaw V.M. Soti C. Csermely P. Blatch G.L. Nuclear translocation of the phosphoprotein Hop (Hsp70/Hsp90 organizing protein) occurs under heat shock, and its proposed nuclear localization signal is involved in Hsp90 binding. Biochim. Biophys. Acta. 2008;1783:1003–1014. doi: 10.1016/j.bbamcr.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Kobori N. Moore A.N. A molecular description of brain trauma pathophysiology using microarray technology: an overview. Neurochem. Res. 2004;29:1275–1286. doi: 10.1023/b:nere.0000023614.30084.eb. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Zhao J. Hergenroeder G. Moore A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7:100–114. doi: 10.1016/j.nurt.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauberschmidt R. Zinsmeyer J. Mrochen H. Meyer M. Changes of neuron-specific enolase concentration in plasma after cardiac arrest and resuscitation. Mol. Chem. Neuropathol. 1991;14:237–245. doi: 10.1007/BF03159939. [DOI] [PubMed] [Google Scholar]
- Depaz I.M. Wilce P.A. The novel cytoskeleton-associated protein neuronal protein 22: elevated expression in the developing rat brain. Brain Res. 2006;1081:59–64. doi: 10.1016/j.brainres.2006.01.126. [DOI] [PubMed] [Google Scholar]
- Dhodda V. Sailor K. Bowen K. Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J. Neurochem. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Dubaquie Y. Looser R. Rospert S. Significance of chaperonin 10-mediated inhibition of ATP hydrolysis by chaperonin 60. Proc. Natl. Acad. Sci. USA. 1997;94:9011–9016. doi: 10.1073/pnas.94.17.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekegren T. Hanrieder J. Bergquist J. Clinical perspectives of high-resolution mass spectrometry-based proteomics in neuroscience: exemplified in amyotrophic lateral sclerosis biomarker discovery research. J/Mass Spectrom. 2008;43:559–571. doi: 10.1002/jms.1409. [DOI] [PubMed] [Google Scholar]
- Focking M. Besselmann M. Trapp T. Proteomics of experimental stroke in mice. Acta Neurobiol. Exp. (Wars.) 2006;66:273–278. doi: 10.55782/ane-2006-1616. [DOI] [PubMed] [Google Scholar]
- Gao W. Chadha M. Berger R. Omenn G. Allen D. Pisano M. Adelson P.D. Clark R.S.B. Jenkins L. Kochanek P. A gel-based proteomic comparison of human cerebrospinal fluid between inflicted and non-inflicted pediatric traumatic brain injury. J. Neurotrauma. 2007;24:43–53. doi: 10.1089/neu.2006.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard R.G. Xu L. Zhao H. Carrico W. Ouyang Y. Qiao Y. Sapolsky R. Steinberg G. Hu B. Yenari M.A. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J. Exp. Biol. 2004;207:3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- Hanrieder J. Wetterhall M. Enblad P. Hillered L. Bergquist J. Temporally resolved differential proteomic analysis of human ventricular CSF for monitoring traumatic brain injury biomarker candidates. J. Neurosci. Meth. 2009;177:469–478. doi: 10.1016/j.jneumeth.2008.10.038. [DOI] [PubMed] [Google Scholar]
- Haqqani A. Hutchison J. Ward R. Stanimirovic D. Biomarkers and diagnosis protein biomarkers in serum of pediatric patients with severe traumatic brain injury identified by ICAT-LC-MS/MS. J. Neurotrauma. 2007;24:54–74. doi: 10.1089/neu.2006.0079. [DOI] [PubMed] [Google Scholar]
- Hergenroeder G. Redell J.B. Moore A.N. Dubinsky W.P. Funk R.T. Crommett J. Clifton G.L. Levine R. Valadka A. Dash P.K. Identification of serum biomarkers in brain-injured adults: potential for predicting elevated intracranial pressure. J. Neurotrauma. 2008a;25:79–93. doi: 10.1089/neu.2007.0386. [DOI] [PubMed] [Google Scholar]
- Hergenroeder G.W. Moore A.N. McCoy J.P., Jr. Samsel L. Ward N.H., 3rd Clifton G.L. Dash P.K. Serum IL-6: a candidate biomarker for intracranial pressure elevation following isolated traumatic brain injury. J. Neuroinflammation. 2010;7:19. doi: 10.1186/1742-2094-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenroeder G.W. Redell J.B. Moore A.N. Dash P.K. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol. Diagn. Ther. 2008b;12:345–358. doi: 10.1007/BF03256301. [DOI] [PubMed] [Google Scholar]
- Ito M. Depaz I. Wilce P. Suzuki T. Niwa S. Matsumoto I. Expression of human neuronal protein 22, a novel cytoskeleton-associated protein, was decreased in the anterior cingulate cortex of schizophrenia. Neurosci. Lett. 2005;378:125–130. doi: 10.1016/j.neulet.2004.12.079. [DOI] [PubMed] [Google Scholar]
- Jenkins L.W. Peters G.W. Dixon C.E. Zhang X. Clark R.S.B. Skinner J.C. Marion D.W. Adelson P.D. Kochanek P.M. Conventional and functional proteomics using large format two-dimensional gel electrophoresis 24 hours after controlled cortical impact in postnatal day 17 rats. J. Neurotrauma. 2002;19:715–740. doi: 10.1089/08977150260139101. [DOI] [PubMed] [Google Scholar]
- Junker H. Suofu Y. Venz S. Sascau M. Herndon J.G. Kessler C. Walther R. Popa-Wagner A. Proteomic identification of an upregulated isoform of annexin A3 in the rat brain following reversible cerebral ischemia. Glia. 2007;55:1630–1637. doi: 10.1002/glia.20581. [DOI] [PubMed] [Google Scholar]
- Kang C.B. Hong Y. Dhe-Paganon S. Yoon H.S. FKBP family proteins: immunophilins with versatile biological functions. Neurosignals. 2008;16:318–325. doi: 10.1159/000123041. [DOI] [PubMed] [Google Scholar]
- Kent D.M. Ruthazer R. Selker H.P. Are some patients likely to benefit from recombinant tissue-type plasminogen activator for acute ischemic stroke even beyond 3 hours from symptom onset? Stroke. 2003;34:464–467. doi: 10.1161/01.str.0000051506.43212.8b. [DOI] [PubMed] [Google Scholar]
- Khan M. Im Y.B. Shunmugavel A. Gilg A.G. Dhindsa R.K. Singh A.K. Singh I. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J. Neuroinflammation. 2009;6:32. doi: 10.1186/1742-2094-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A. Hesse F. Bullock M.R. Buchfelder M. The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog. Brain Res. 2007;161:317–325. doi: 10.1016/S0079-6123(06)61022-4. [DOI] [PubMed] [Google Scholar]
- Kleindienst A. Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J. Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- Kleindienst A. Tolias C.M. Corwin F.D. Muller C. Marmarou A. Fatouros P. Bullock M.R. Assessment of cerebral S100B levels by proton magnetic resonance spectroscopy after lateral fluid-percussion injury in the rat. J. Neurosurg. 2005;102:1115–1121. doi: 10.3171/jns.2005.102.6.1115. [DOI] [PubMed] [Google Scholar]
- Kobeissy F.H. Ottens A.K. Zhang Z. Liu M.C. Denslow N.D. Dave J.R. Tortella F.C. Hayes R.L. Wang K.K. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol. Cell Proteomics. 2006;5:1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- Kochanek A. Kline A. Gao W. Chadha M. Lai Y. Clark R.S.B. Dixon C.E. Jenkins L. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev. Neurosci. 2006;28:410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek P. Berger R. Bayir H. Wagner A. Jenkins L. Clark R.S.B. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- Koh P.O. Proteomic analysis of focal cerebral ischemic injury in male rats. J. Vet. Med. Sci. 2009;72:181–185. doi: 10.1292/jvms.09-0364. [DOI] [PubMed] [Google Scholar]
- Kukacka J. Vajtr D. Huska D. Prusa R. Houstava L. Samal F. Diopan V. Kotaska K. Kizek R. Blood metallothionein, neuron specific enolase, and protein S100B in patients with traumatic brain injury. Neuro. Endocrinol. Lett. 2006;27(Suppl. 2):116–120. [PubMed] [Google Scholar]
- Laskowitz D.T. Kasner S.E. Saver J. Remmel K.S. Jauch E.C. BRAIN Study Group. Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke. 2009;40:77–85. doi: 10.1161/STROKEAHA.108.516377. [DOI] [PubMed] [Google Scholar]
- Lee J.E. Yenari M.A. Sun G.H. Xu L. Emond M.R. Cheng D. Steinberg G.K. Giffard R.G. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp. Neurol. 2001;170:129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- Liu M.C. Akle V. Zheng W. Dave J.R. Tortella F.C. Hayes R.L. Wang K.K. Comparing calpain- and caspase-3-mediated degradation patterns in traumatic brain injury by differential proteome analysis. Biochem. J. 2006;394:715–725. doi: 10.1042/BJ20050905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.R. Blessing R. White W.D. Grocott H.P. Newman M.F. Laskowitz D.T. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. [DOI] [PubMed] [Google Scholar]
- Macleod M.R. O'Collins T. Horky L.L. Howells D.W. Donnan G.A. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J. Cereb. Blood Flow Metab. 2005;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- Martens P. Raabe A. Johnsson P. Serum S-100 and neuron-specific enolase for prediction of regaining consciousness after global cerebral ischemia. Stroke. 1998;29:2363–2366. doi: 10.1161/01.str.29.11.2363. [DOI] [PubMed] [Google Scholar]
- Minambres E. Cemborain A. Sanchez-Velasco P. Gandarillas M. Diaz-Reganon G. Sanchez-Gonzalez U. Leyva-Cobian F. Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit. Care Med. 2003;31:933–938. doi: 10.1097/01.CCM.0000055370.66389.59. [DOI] [PubMed] [Google Scholar]
- Minger S.L. Geddes J.W. Holtz M.L. Craddock S.D. Whiteheart S.W. Siman R.G. Pettigrew L.C. Glutamate receptor antagonists inhibit calpain-mediated cytoskeletal proteolysis in focal cerebral ischemia. Brain Res. 1998;810:181–199. doi: 10.1016/s0006-8993(98)00921-4. [DOI] [PubMed] [Google Scholar]
- Missler U. Wiesmann M. Friedrich C. Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- Montaner J. Stroke biomarkers: Can they help us to guide stroke thrombolysis? Drug News Perspect. 2006;19:523–532. doi: 10.1358/dnp.2006.19.9.1050422. [DOI] [PubMed] [Google Scholar]
- Ottens A.K. Kobeissy F.H. Fuller B.F. Liu M.C. Oli M.W. Hayes R.L. Wang K.K. Novel neuroproteomic approaches to studying traumatic brain injury. Prog. Brain Res. 2007;161:401–418. doi: 10.1016/S0079-6123(06)61029-7. [DOI] [PubMed] [Google Scholar]
- Ottens A.K. Kobeissy F.H. Golden E.C. Zhang Z. Haskins W.E. Chen S.S. Hayes R.L. Wang K.K. Denslow N.D. Neuroproteomics in neurotrauma. Mass Spectrom. Rev. 2006;25:380–408. doi: 10.1002/mas.20073. [DOI] [PubMed] [Google Scholar]
- Ottens A.K. Kobeissy F.H. Wolper R.A. Haskins W.E. Hayes R.L. Denslow N.D. Wang K.K. A multidimensional differential proteomic platform using dual-phase ion-exchange chromatography-polyacrylamide gel electrophoresis/reversed-phase liquid chromatography tandem mass spectrometry. Anal. Chem. 2005;77:4836–4845. doi: 10.1021/ac050478r. [DOI] [PubMed] [Google Scholar]
- Pak J.H. Huang F.L. Li J. Balschun D. Reymann K.G. Chiang C. Westphal H. Huang K.P. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc. Natl. Acad. Sci. USA. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew L.C. Holtz M.L. Craddock S.D. Minger S.L. Hall N. Geddes J.W. Microtubular proteolysis in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1996;16:1189–1202. doi: 10.1097/00004647-199611000-00013. [DOI] [PubMed] [Google Scholar]
- Pike B.R. Flint J. Dave J.R. Lu X.C. Wang K.K. Tortella F.C. Hayes R.L. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 2004;24:98–106. doi: 10.1097/01.WCB.0000098520.11962.37. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. Wang K.K. Hayes R.L. Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 2004;14:202–209. doi: 10.1111/j.1750-3639.2004.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleines U.E. Morganti-Kossmann M.C. Rancan M. Joller H. Trentz O. Kossmann T. S-100 beta reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J. Neurotrauma. 2001;18:491–498. doi: 10.1089/089771501300227297. [DOI] [PubMed] [Google Scholar]
- Posmantur R.M. Kampfl A. Taft W.C. Bhattacharjee M. Dixon C.E. Bao J. Hayes R.L. Diminished microtubule-associated protein 2 (MAP2) immunoreactivity following cortical impact brain injury. J. Neurotrauma. 1996;13:125–137. doi: 10.1089/neu.1996.13.125. [DOI] [PubMed] [Google Scholar]
- Prieto D.A. Ye X. Veenstra T.D. Proteomic analysis of traumatic brain injury: the search for biomarkers. Expert Rev. Proteomics. 2008;5:283–291. doi: 10.1586/14789450.5.2.283. [DOI] [PubMed] [Google Scholar]
- Rajdev S. Sharp F.R. Stress proteins as molecular markers of neurotoxicity. Toxicol. Pathol. 2000;28:105–112. doi: 10.1177/019262330002800113. [DOI] [PubMed] [Google Scholar]
- Reynolds M.A. Kirchick H.J. Dahlen J.R. Anderberg J.M. McPherson P.H. Nakamura K.K. Laskowitz D.T. Valkirs G.E. Buechler K.F. Early biomarkers of stroke. Clin. Chem. 2003;49:1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- Roine R.O. Somer H. Kaste M. Viinikka L. Karonen S.L. Neurological outcome after out-of-hospital cardiac arrest. Prediction by cerebrospinal fluid enzyme analysis. Arch. Neurol. 1989;46:753–756. doi: 10.1001/archneur.1989.00520430047015. [DOI] [PubMed] [Google Scholar]
- Schellinger P.D. Fiebach J.B. Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: present status. Stroke. 2003;34:575–583. [PubMed] [Google Scholar]
- Shibasaki F. Kondo E. Akagi T. McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- Siman R. Toraskar N. Dang A. McNeil E. McGarvey M. Plaum J. Maloney E. Grady M.S. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma. 2009;26:1867–1877. doi: 10.1089/neu.2009.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton R.H. Stone J.R. Okonkwo D.O. Pellicane A.J. Povlishock J.T. The immunophilin ligand FK506 attenuates axonal injury in an impact-acceleration model of traumatic brain injury. J. Neurotrauma. 2001;18:607–614. doi: 10.1089/089771501750291846. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Waldmeier P.C. Springer J.E. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Sung J.H. Cho E.H. Kim M.O. Koh P.O. Identification of proteins differentially expressed by melatonin treatment in cerebral ischemic injury—a proteomics approach. J. Pineal Res. 2009;46:300–306. doi: 10.1111/j.1600-079X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Terada K. Inao S. Mizutani N. Tsukada H. Yoshida J. Cerebral blood flow, glucose metabolism and tunel-positive cells in the development of ischemia. Cerebrovasc. Dis. 2001;11:9–19. doi: 10.1159/000047606. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D. Hong S. Matsumori Y. Kayama T. Swanson R.A. Dillman W.H. Liu J. Panter S.S. Weinstein P.R. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery. 2003;53:1179–1187. doi: 10.1227/01.neu.0000090341.38659.cf. discussion 1187–1188. [DOI] [PubMed] [Google Scholar]
- Tzoulaki I. Murray G.D. Lee A.J. Rumley A. Lowe G.D. Fowkes F.G. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur. Heart J. 2007a;28:354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- Tzoulaki I. Murray G.D. Lee A.J. Rumley A. Lowe G.D. Fowkes F.G. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007b;115:2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- Wang H.G. Pathan N. Ethell I.M. Krajewski S. Yamaguchi Y. Shibasaki F. McKeon F. Bobo T. Franke T.F. Reed J.C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Williams A.J. Dave J.R. Phillips J.B. Lin Y. McCabe R.T. Tortella F.C. Neuroprotective efficacy and therapeutic window of the high-affinity N-methyl-D-aspartate antagonist conantokin-G: in vitro (primary cerebellar neurons) and in vivo (rat model of transient focal brain ischemia) studies. J. Pharmacol. Exp. Ther. 2000;294:378–386. [PubMed] [Google Scholar]
- Wunderlich M.T. Lins H. Skalej M. Wallesch C.W. Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin. Neurol. Neurosurg. 2006;108:558–563. doi: 10.1016/j.clineuro.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yao C. Williams A.J. Lu X.C. Price R.A. Cunningham B.S. Berti R. Tortella F.C. Dave J.R. The sodium channel blocker RS100642 reverses down-regulation of the sodium channel alpha-subunit Na(v) 1.1 expression caused by transient ischemic brain injury in rats. Neurotox. Res. 2003;5:245–253. doi: 10.1007/BF03033382. [DOI] [PubMed] [Google Scholar]
- Yao C. Williams A.J. Ottens A. Lu X.C. Liu M.C. Hayes R.L. Wang K.K. Tortella F.C. Dave J.R. P43/pro-EMAP-II: A potential biomarker for discriminating traumatic versus ischemic brain injury. J. Neurotrauma. 2009;26:1295–1305. doi: 10.1089/neu.2008.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C. Williams A.J. Ottens A.K. May Lu X.C. Chen R. Wang K.K. Hayes R.L. Tortella F.C. Dave J.R. Detection of protein biomarkers using high-throughput immunoblotting following focal ischemic or penetrating ballistic-like brain injuries in rats. Brain Inj. 2008;22:723–732. doi: 10.1080/02699050802304706. [DOI] [PubMed] [Google Scholar]
- Yao H. Nakahara T. Nakagawa N. Hashimoto K. Kuroki T. Regional and temporal changes in proteomic profile after middle cerebral artery occlusion with or without reperfusion in rats. Neurochem. Res. 2009;34:1999–2007. doi: 10.1007/s11064-009-9988-6. [DOI] [PubMed] [Google Scholar]
- Zetterberg H. Ruetschi U. Portelius E. Brinkmalm G. Andreasson U. Blennow K. Brinkmalm A. Clinical proteomics in neurodegenerative disorders. Acta Neurol. Scand. 2008;118:1–11. doi: 10.1111/j.1600-0404.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- Zhang J. Keene C.D. Pan C. Montine K.S. Montine T.J. Proteomics of human neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2008;67:923–932. doi: 10.1097/NEN.0b013e318187a832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Wu R. Li P. Liu F. Zhang W. Zhang P. Li P. Wang Y. Baicalin administration is effective in positive regulation of twenty-four ischemia/reperfusion-related proteins identified by a proteomic study. Neurochem. Int. 2009;54:488–496. doi: 10.1016/j.neuint.2009.02.005. [DOI] [PubMed] [Google Scholar]