Abstract
Objective
The etiology of breast arterial calcification (BAC) is not well understood. We examined reproductive history and cardiovascular disease (CVD) risk factor associations with the presence of detectable BAC in asymptomatic postmenopausal women.
Methods
Reproductive history and CVD risk factors were obtained in 240 asymptomatic postmenopausal women from a community-based research study who had a screening mammogram within 2 years of their participation in the study. The mammograms were reviewed for the presence of detectable BAC. Age-adjusted logistic regression models were fit to assess the association between each risk factor and the presence of BAC. Multiple variable logistic regression models were used to identify the most parsimonious model for the presence of BAC.
Results
The prevalence of BAC increased with increased age (p < 0.0001). The most parsimonious logistic regression model for BAC presence included age at time of examination, increased parity (p = 0.01), earlier age at first birth (p = 0.002), weight, and an age-by-weight interaction term (p = 0.004). Older women with a smaller body size had a higher probability of having BAC than women of the same age with a larger body size.
Conclusions
The presence or absence of BAC at mammography may provide an assessment of a postmenopausal woman's lifetime estrogen exposure and indicate women who could be at risk for hormonally related conditions.
Introduction
Studies in women have suggested an association between the presence of breast arterial calcification (BAC) identified at mammography and increased risk for future cardiovascular disease (CVD) events.1–3 The etiology of BAC, however, is not well understood. The prevalence of BAC increases with increased age in women. Importantly, after adjusting for age, BAC is not consistently reported to be significantly associated with traditional CVD risk factors.4,5 Several studies have reported BAC to be positively associated with increased parity3–6 and lactation.5 Additionally, hormonal therapy has been associated with a decreased prevalence of BAC.7,8 These findings suggest that BAC may be associated with estrogen levels across the life course.
Dystrophic calcification occurs in soft tissues as a response to injury.9 There are two types of dystrophic calcification that occur within the vascular wall. One is intimal calcification, which is found in atherosclerotic plaque. The other is medial calcification, which occurs in nonatheromatous forms of arteriosclerosis, such as Monckeberg's sclerosis.10,11 BAC is localized to the media.12 Medial calcification can exist independently of atherosclerosis and may be triggered by a different mechanism than atherosclerosis.11,13–15 Regardless of its location, vascular calcification is recognized as an actively regulated process and is controlled, in part, by a variety of proteins, including promoters and inhibitors of bone mineralization.
The purpose of our study was to (1) examine the presence of detectable BAC at mammography and (2) investigate CVD risk factor and reproductive history associations with the presence of detectable BAC at mammography in asymptomatic, nonreferred women from a community-based research study.
Materials and Methods
Study groups
We obtained all available screening mammograms that were performed during or between 1992 and 2005 for women in the Epidemiology of Breast Arterial Calcification Study (EBAC). These women had also participated in the Epidemiology of Coronary Artery Calcification (ECAC) Study, a community-based study of nonreferred individuals that included 636 women 20–88 years of age. The ECAC Study women had baseline CVD risk factor assessment between 1991 and 1998; 393 women also had follow-up assessments between December 2000 and February 2005. Many women had multiple mammograms available for review. For temporal comparability between mammography and risk factor measurement, we restricted analyses to women who had a mammogram within 2 years of the ECAC Study examination. Only one set of data points, based on the least amount of time between mammography and study examination, was used for each woman.
A total of 316 women had a mammogram and collection of complete risk factor data from the ECAC Study examination, including information about reproductive history, within at most 2 years of one another. Analyses for the current study were restricted to postmenopausal women at least 45 years of age; 1 woman with a history of a CVD event and 1 nulliparous woman were excluded. The final sample consisted of 240 non-Hispanic white women with no history of CVD.
Women had previously provided general authorization for the research use of their medical record; therefore, review of their mammograms did not require additional informed consent and was compliant with Health Insurance Portability and Accountability Act (HIPAA) requirements. The study protocols were approved by the appropriate Institutional Review Boards. All women provided informed written consent for their participation in the ECAC Study.
Risk factor assessment in women
CVD risk factor information was collected at the time of the examination as part of the ECAC Study. Height and weight were measured and body mass index (BMI, kg/m2) was calculated. Waist circumference was measured at the umbilicus and hips at the level of maximal circumference. Systolic blood pressure (SBP, mm Hg) and diastolic blood pressure (DBP, mm Hg) were measured in the right arm using a random-zero sphygmomanometer (Hawksley and Sons). Three measurements at least 2 minutes apart were taken, and the average of the second and third measurements was used.
Standard enzymatic methods were used to measure total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, and glucose after an overnight fast. Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald equation.16 Cholesterol/HDL-C ratio and non-HDL-C were computed.
Self-reported history of physician-diagnosed diabetes and hypertension, as well as smoking history, education history, and menopause status, also were recorded. Use of blood pressure-lowering, diabetic, and lipid-lowering medications was recorded. Information about the number of live births and age at first live birth was collected at the time of mammography. Data on lactation history was incomplete for many women and was not considered in this study. Women were considered hypertensive if they reported a prior diagnosis of hypertension and use of prescription BP-lowering medication or if average SBP or DBP was ≥140 mm Hg or ≥90 mm Hg, respectively. Participants were considered diabetic if they reported using insulin or oral hypoglycemic agents or if they reported a physician diagnosis of diabetes. Impaired fasting glucose was defined as glucose levels of 110–125 mg/dL.
Mammograms
All mammograms were previously obtained during the course of the usual healthcare of the women and were not specifically obtained for research purposes. Only the standard mediolateral oblique and craniocaudal views (routine screening views) of each breast were reviewed. Any additional special mammogram views were not reviewed. BAC was judged to be present if detected with the unaided eye (without magnification) under standard mammogram viewing conditions (dedicated view box with American College of Radiology approved brightness in a darkened room). Arterial calcifications in the breast (BAC) are located in the media and are seen as parallel calcifications with fine, relatively uniform nodular thickening of the linear density. The dense areas are due to calcification in the tangential portion of the arterial wall, which is better visualized than the en face calcium. This appearance has been described as a train or tram track pattern. Only calcifications that clearly had this arterial appearance were counted as BAC; all calcifications that did not have this specific appearance were considered to represent other etiologies (nonarterial). BAC identified on either or both views of the breast was considered present in that breast.
Statistical analyses
A significance level of 0.05 was used for all analyses, and all tests were two-sided. The number of live births and age at first live birth were natural log-transformed to reduce skewness; pack-years of smoking was natural log-transformed after adding 1 to its value to reduce skewness. Means and standard deviations (SD) were computed for continuous variables, and frequencies and percentages were computed for discrete variables. The prevalence of detectable BAC was modeled as a nonparametric function of age by fitting a local regression smoother.17 This approach has the advantage in that age is considered as a continuous variable.
Age-adjusted logistic regression models were fit to assess the association between each risk factor and each measure of reproductive history and the presence of detectable BAC. Parameter estimates from these models were interpreted as the estimated odds ratio (OR) (95% confidence interval [CI]) associated with a 1-unit increase in the SD of a quantitative risk factor or a change in status of a categorical risk factor. To determine if the association between age and the prevalence of detectable BAC was modified by risk factors, the significance of age-by-risk factor terms was investigated.
Based on the results of the age-adjusted and age-by-risk factor interaction models, as well as risk factors that have been found to be significantly associated with BAC by others, a set of candidate multiple variable logistic regression models were considered to identify the most parsimonious model for the prevalence of BAC. We used the log-likelihood test to evaluate the significance of the variables in the models and the Akaike Information Criterion (AIC) to select the most parsimonious model.
Results
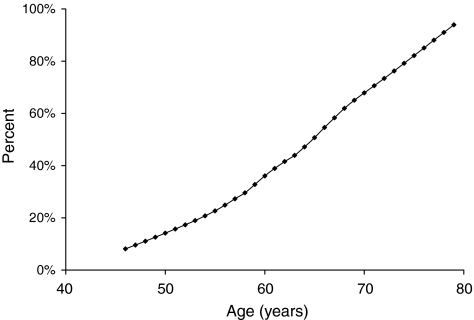
Table 1 presents descriptive characteristics of the women in the current study. Mean ± SD age was 62.1 ± 8.3 years (range 46.3–82.3 years), and mean ± SD absolute time between mammography and risk factor measurement was 4.8 ± 5.0 months (range 0.0–23.6 months). The mean ± SD number of live births was 3.9 ± 2.0 (range 1–12), and the mean ± SD age at first birth was 23.3 ± 3.8 years (range 16–36 years). Although one third (33.8%) of the women were hypertensive, the prevalence of diabetes was low (3.8%). Overall, the prevalence of BAC was 43.8%. Figure 1 shows the prevalence of BAC as a function of age. The prevalence of BAC increased significantly with increased age (p < 0.0001).
Table 1.
Descriptive Characteristics of 240 Asymptomatic, Postmenopausal Women in Epidemiology of Breast Arterial Calcification Study
| Characteristic | Mean or (%) | Standard deviation | Range |
|---|---|---|---|
| Age, years | 62.1 | 8.3 | 46.3–82.3 |
| Time between mammography and risk factor collection, months | 4.8 | 5.0 | 0.0–23.6 |
| Cholesterol, mg/dL | 209.3 | 32.3 | 121.5–290.5 |
| HDL-C, mg/dL | 60.8 | 16.4 | 29.4–127.2 |
| LDL-C, mg/dL | 122.5 | 31.2 | 14.1–223.0 |
| Glucose, mg/dL | 93.8 | 13.9 | 68.4–177.0 |
| Systolic blood pressure, mm Hg | 123.7 | 19.2 | 87.0–207.0 |
| Diastolic blood pressure, mm Hg | 69.7 | 10.2 | 43.0–98.0 |
| Weight, kg | 73.8 | 15.4 | 38.7–133.5 |
| Height, cm | 161.8 | 5.6 | 144.9–174.6 |
| Body mass index, kg/m2 | 28.2 | 5.8 | 16.1–50.1 |
| Waist, cm | 89.8 | 15.4 | 58.9–147.6 |
| Hip, cm | 106.8 | 11.6 | 75.6–144.4 |
| Pack-years of smoking | 7.7 | 15.2 | 0.0–78.0 |
| Number of live births | 3.9 | 2.0 | 1.0–12.0 |
| Age at first live birth, years | 23.3 | 3.8 | 16.0–36.0 |
| College education, % | (52.1) | ||
| Hypertension, % | (33.8) | ||
| Diabetes, % | (3.8) | ||
| Impaired fasting glucose or diabetes, % | (9.2) | ||
| Ever smoked, % | (40.8) | ||
| Current smoker, % | (7.9) | ||
| Breast arterial calcification, % | (43.8) |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
FIG. 1.
Age-specific prevalence of breast arterial calcification among 240 asymptomatic, postmenopausal women.
Associations with BAC
A 1-unit increase in the SD of age was associated with an OR (95% CI) of 3.1 (2.2–4.4) (p < 0.0001) for BAC (Table 2). After adjusting for age, DBP was the only CVD risk factor significantly associated with BAC. A 1-unit increase in the SD of DBP was associated with an OR of 1.4 (1.0–1.9) (p = 0.03). Having a college education was protective for BAC, with a 0.4 times lesser odds (0.2–0.7) (p = 0.003) of having BAC after adjusting for age. Having a first birth at an older age was also protective for BAC, and a 1-unit SD increase in the log-transformed age at first birth was associated with an 0.5 times lesser odds (0.4–0.7) (p < 0.0001) of having BAC. After adjusting for age, a 1-unit SD increase in the log-transformed number of live births resulted in a 1.8 times greater odds (1.3–2.6) (p = 0.0006) of having BAC. There was a significant age-by-waist interaction (p = 0.05), age-by-BMI interaction (p = 0.02), and age-by-weight interaction (p = 0.005) associated with having BAC (data not shown). There was a steeper increase in the probability of having BAC as a function of age for women with lower as compared with higher measures of body size.
Table 2.
Risk Factor Associations with Breast Arterial Calcification Among 240 Postmenopausal Women in Epidemiology of Breast Arterial Calcification Study
| Covariate | OR | 95% CI | pa |
|---|---|---|---|
| Age | 3.1 | 2.2-4.4 | <0.0001 |
| Cholesterol | 1.1 | 0.8-1.5 | 0.47 |
| HDL-C | 1.0 | 0.7-1.3 | 0.87 |
| LDL-C | 1.2 | 0.9-1.6 | 0.25 |
| Glucose | 0.8 | 0.6-1.1 | 0.20 |
| Systolic blood pressure | 1.3 | 0.9-1.8 | 0.12 |
| Diastolic blood pressure | 1.4 | 1.0-1.9 | 0.03 |
| Weight | 1.0 | 0.7-1.3 | 0.83 |
| Height | 1.1 | 0.8-1.6 | 0.40 |
| Body mass index | 0.9 | 0.7-1.2 | 0.56 |
| Waist | 0.8 | 0.6-1.1 | 0.15 |
| Hip | 0.9 | 0.7-1.2 | 0.53 |
| Pack-years of smoking | 0.8 | 0.6-1.1 | 0.14 |
| Number of live births | 1.8 | 1.3-2.6 | 0.0006 |
| Age at first live birth | 0.5 | 0.4-0.7 | <0.0001 |
| College education | 0.4 | 0.2-0.7 | 0.003 |
| Hypertension | 1.3 | 0.7-2.4 | 0.46 |
| Diabetes | 0.4 | 0.1-1.8 | 0.22 |
| Impaired fasting glucose or diabetes | 0.8 | 0.3-2.2 | 0.69 |
| Ever smoked | 0.8 | 0.4-1.4 | 0.43 |
| Current smoker | 0.5 | 0.1-1.4 | 0.17 |
Estimated odds ratio (OR) and 95% confidence interval (CI) associated with a 1-unit increase in the standard deviation of a quantitative variable or a change in status of a categorical variable.
All risk factor associations except age are adjusted for age.
Candidate models were evaluated to determine the most parsimonious model to predict the presence of BAC. Because of the strong association of age, log-transformed number of live births, and log-transformed age at first birth with BAC, the multiple variable logistic regression model that included these three risk factors as covariates was specified as the reduced model. This reduced model (−2*log-likelihood = 247.15 with 3 degrees of freedom) had an AIC of 255.15. Additional candidate models were adjusted for these three risk factors. Models that included each of the traditional CVD risk factors of SBP, DBP, hypertension status, having impaired fasting glucose or diabetes, being a current smoker, and log-pack-years of smoking were evaluated. Additionally, models that included having a college education, waist and age-by-waist interaction, BMI and age-by-BMI interaction, and weight and age-by-weight interaction were evaluated based on our earlier findings.
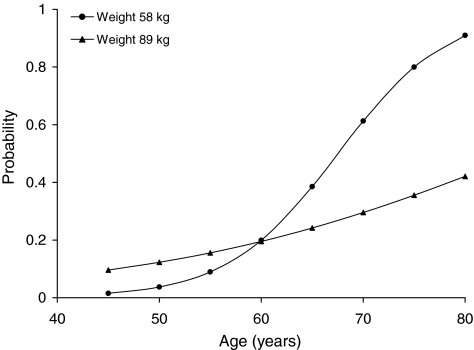
The most parsimonious model for the presence of BAC included age, log-transformed number of live births, log-transformed age at first birth, weight, and an age-by-weight interaction term. This most parsimonious model (−2*log-likelihood = 238.06 with 5 degrees of freedom) had an AIC of 250.07. A 1-unit SD increase in log-transformed number of live births resulted in a 1.6 times greater odds (1.1–2.3) (p = 0.01) of having detectable BAC, and a 1-unit SD increase in log-transformed age at first birth was associated with a 0.6 times lesser odds (0.4–0.8) (p = 0.002) of having detectable BAC. There was a significant (p = 0.004) interaction between age and weight on the probability of having detectable BAC. Figure 2 illustrates results of the most parsimonious model for women with a history of two live births and an age at first birth of 22 years. Figure 2 shows how the association between age and the probability of having detectable BAC was modified by weight. There was a steeper increase in the probability of having BAC as a function of age for women with a lower as compared with a higher weight.
FIG. 2.
Estimated probability of breast arterial calcification for women with history of 2 live births and first live birth at age 22 years based on most parsimonious logistic regression model results.
Discussion
In this study group of postmenopausal women from the community, almost 44% had BAC at mammography, and increased age was positively associated with an increased prevalence of BAC. In age-adjusted models, we found that increased parity and DBP were positively and significantly associated with BAC, whereas age at first birth and having a college education were inversely and significantly associated with BAC. To our knowledge, this is the first study to describe an association between age at first birth and BAC. Additionally, measures of body size modified the association between examination age and BAC. All these factors contribute to a woman's estrogen exposure across the life course. Thus, BAC at mammography may provide an assessment of a woman's lifetime estrogen exposure. The mechanisms by which these factors were associated with BAC may operate in pathways involving both biological and sociological factors.
Biological and sociological factors
The main growth spurt of the breast occurs at puberty, and the breast attains its maximum development during pregnancy, with differentiation of breast tissue completed by the end of the first full-term pregnancy.18 An early age at first birth is associated with an earlier differentiation or maturation of the breast tissue. The first full-term birth creates a specific genomic signature in the breast that is protective for breast cancer, especially when the first birth occurs at a young age.19 This genomic signature is still evident at menopause.20 Breast tissue also undergoes postlactational involution and an age-related involution after menopause.18 These changes in a woman's breast size and composition over a lifetime may contribute to injuries to the arteries of the breast. BAC is localized to the media, and medial arterial calcification is primarily localized to the elastin of media tunica.14 Elastin has been shown to have a strong affinity for calcium,21 and it has been postulated that disruption of the elastic fibers facilitates medial calcification.14,22
Measures of body size have been found to be positively associated with blood estrogen levels in postmenopausal women23 because estrogen levels may become elevated as a result of aromatization of androgens in adipose tissue. We found that older postmenopausal woman with a larger body size had a lower probability of having BAC than women of the same age with a smaller body size. Although we did not measure estrogen levels in the study participants, older women with a larger body size may have had higher estrogen levels than similarly aged women with a smaller body size. Interestingly, previous studies have found the use of hormonal therapy has been associated with a decreased prevalence of BAC.7,8
In a study of almost 13,000 women aged 40–79 years, BAC was found to be inversely associated with educational level.3 Our findings were similar: after adjusting for age, having a college education was significantly associated with a decreased probability of having BAC. Age at first birth was significantly higher among women with a college education compared with women without a college education after adjusting for age (p < 0.0001) (data not shown). In a logistic regression model including age, log-transformed number of live births, log-transformed age at first birth, and having a college education, log-transformed age at first birth was significantly associated with having BAC (OR = 0.61, (95% CI 0.42–0.88)) (p = 0.009), whereas having a college education was no longer significantly associated with BAC (OR = 0.76, 95% CI 0.38-1.51) (p = 0.44) (data not shown). The inverse association between having a college education and BAC may be explained by the association of age at first birth with educational attainment. Having a first child at a young age may delay or interfere with educational attainment, and women who attend college may delay parenthood.
In many studies,1–5 cigarette smoking was shown to have an inverse association with BAC. Smoking has been found to have effects on hormone secretion, reproduction, age at menopause,24,25 and fecundity. In a meta-analysis, women who smoked were at increased risk of infertility compared with women who did not smoke (OR = 1.60, 95% CI 1.34-1.91).26 Thus, an inverse association between smoking and BAC may be mediated, in part, by smoking's effects on hormone levels across the life course.
The factors found here (i.e., early age at first birth, increased parity, and low weight in older women) to be associated with the presence of BAC have all been associated with a lower risk of breast cancer in postmenopausal women.27 These associations with breast cancer noted in women of different ethnicities also have been documented in animal models.28 Although the parallels between factors associated with breast cancer risk and with BAC may be due to chance, they warrant further investigations to determine if common biological mechanisms are involved in both conditions.
Limitations
The current study was limited to non-Hispanic white women; therefore, our findings may not be generalized to other ethnic groups. The prevalence of BAC has been shown to vary by ethnicity,29 and fertility levels also have been shown to vary by ethnicity and education.30 We were not able to measure other biological or sociological factors during early life that may be important in the pathogenesis of BAC. For example, we did not have longitudinal information on measures of body size, nor did we have information on lactation history. Such information is important to understand the role of other biological and sociological factors within the context of the life course.
Conclusions
Calcification in the arteries of the female breast has been recognized as an incidental finding at routine mammography. Due, in part, to the strong association of calcified atherosclerotic plaques in the coronary arteries with future CVD events, there has been speculation that BAC at mammography also may be a marker of increased CVD risk. Among asymptomatic postmenopausal women in the current study, the presence of detectable BAC was associated with increased age, reproductive history, and body size factors. These findings suggest the etiology of BAC is associated with a woman's reproductive history and hormonal levels across the life course. The presence or absence of BAC at mammography may provide insight to identify women at risk for hormonally related conditions.
Acknowledgments
This research was supported by NIH grants R01 HL46292 and R21 HL77123 and by a General Clinic Research Center Grant from the NIH (M01-RR00585) awarded to Mayo Clinic Rochester.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kemmeren JM. van Noord PA. Beijerinck D. Fracheboud J. Banga JD. van der Graaf Y. Arterial calcification found on breast cancer screening mammograms and cardiovascular mortality in women: The DOM Project. Doorlopend Onderzoek Morbiditeit en Mortaliteit. Am J Epidemiol. 1998;147:333–341. doi: 10.1093/oxfordjournals.aje.a009455. [DOI] [PubMed] [Google Scholar]
- 2.Crystal P. Crystal E. Leor J. Friger M. Katzinovitch G. Strano S. Breast artery calcium on routine mammography as a potential marker for increased risk of cardiovascular disease. Am J Cardiol. 2000;86:216–217. doi: 10.1016/s0002-9149(00)00860-2. [DOI] [PubMed] [Google Scholar]
- 3.Iribarren C. Go AS. Tolstykh I. Sidney S. Johnston SC. Spring DB. Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health. 2004;13:381–389. doi: 10.1089/154099904323087060. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka M. Warren R. Luben R, et al. How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography? Am J Roentgenol. 2006;187:73–80. doi: 10.2214/AJR.05.0365. [DOI] [PubMed] [Google Scholar]
- 5.Maas AH. van der Schouw YT. Beijerinck D. Deurenberg JJ. Mali WP. van der Graaf Y. Arterial calcifications seen on mammograms: Cardiovascular risk factors, pregnancy, and lactation. Radiology. 2006;240:33–38. doi: 10.1148/radiol.2401050170. [DOI] [PubMed] [Google Scholar]
- 6.Leinster SJ. Whitehouse GH. Factors which influence the occurrence of vascular calcification in the breast. Br J Radiol. 1987;60:457–458. doi: 10.1259/0007-1285-60-713-457. [DOI] [PubMed] [Google Scholar]
- 7.Cox J. Simpson W. Walshaw D. An interesting byproduct of screening: Assessing the effect of HRT on arterial calcification in the female breast. J Med Screen. 2002;9:38–39. doi: 10.1136/jms.9.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Schnatz PF. Rotter MA. Hadley S. Currier AA. O'Sullivan DM. Hormonal therapy is associated with a lower prevalence of breast arterial calcification on mammography. Maturitas. 2007;57:154–160. doi: 10.1016/j.maturitas.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Schoenhagen P. Osteopontin, coronary calcification, and cardiovascular events: Future diagnostic and therapeutic targets for disease prevention? Eur Heart J. 2006;27:766–767. doi: 10.1093/eurheartj/ehi743. [DOI] [PubMed] [Google Scholar]
- 10.Goodman WG. London G. Amann K, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Demer LL. Tintut Y. Parhami F. Novel mechanisms in accelerated vascular calcification in renal disease patients. Curr Opin Nephrol Hypertens. 2002;11:437–443. doi: 10.1097/00041552-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Topal U. Kaderli A. Topal NB, et al. Relationship between the arterial calcification detected in mammography and coronary artery disease. Eur J Radiol. 2007;63:391–395. doi: 10.1016/j.ejrad.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Basalyga DM. Simionescu DT. Xiong W. Baxter BT. Starcher BC. Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: Role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dao HH. Essalihi R. Bouvet C. Moreau P. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–317. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Doherty TM. Fitzpatrick LA. Shaheen A. Rajavashisth TB. Detrano RC. Genetic determinants of arterial calcification associated with atherosclerosis. Mayo Clin Proc. 2004;79:197–210. doi: 10.4065/79.2.197. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.McClelland RL. Chung H. Detrano R. Post W. Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 18.Russo J. Russo IR. Development of the human breast. Maturitas. 2004;49:2–15. doi: 10.1016/j.maturitas.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Russo J. Balogh GA. Heulings R, et al. Molecular basis of pregnancy-induced breast cancer protection. Eur J Cancer Prev. 2006;15:306–342. doi: 10.1097/00008469-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Russo J. Balogh GA. Russo IH. Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol Biomarkers Prev. 2008;17:51–66. doi: 10.1158/1055-9965.EPI-07-0678. [DOI] [PubMed] [Google Scholar]
- 21.Bailey M. Pillarisetti S. Jones P. Xiao H. Simionescu D. Vyavahare N. Involvement of matrix metalloproteinases and tenascin-C in elastin calcification. Cardiovasc Pathol. 2004;13:146–155. doi: 10.1016/S1054-8807(04)00009-2. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson J. Age-related medial elastocalcinosis in arteries: Mechanisms, animal models, and physiological consequences. J Appl Physiol. 2008;105:1643–1651. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- 23.Lorincz AM. Sukumar S. Molecular links between obesity and breast cancer. Endocr Rel Cancer. 2006;13:279–292. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen TF. Graff-Iversen S. Sundby J. Bjertness E. Early menopause, association with tobacco smoking, coffee consumption and other lifestyle factors: A cross-sectional study. BMC Public Health. 2007;7:149. doi: 10.1186/1471-2458-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper GS. Sandler DP. Bohlig M. Active and passive smoking and the occurrence of natural menopause. Epidemiology. 1999;10:771–773. [PubMed] [Google Scholar]
- 26.Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2004;81:1181–1186. doi: 10.1016/j.fertnstert.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Russo J. Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Cruz CM. Moody SE. Master SR, et al. Persistent parity-induced changes in growth factors, TGF-beta3, and differentiation in the rodent mammary gland. Mol Endocrinol. 2002;16:2034–2051. doi: 10.1210/me.2002-0073. [DOI] [PubMed] [Google Scholar]
- 29.Reddy J. Son H. Smith SJ. Paultre F. Mosca L. Prevalence of breast arterial calcifications in an ethnically diverse population of women. Ann Epidemiol. 2005;15:344–350. doi: 10.1016/j.annepidem.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y. Morgan SP. How big are educational and racial fertility differentials in the U.S.? Soc Biol. 2003;50:167–187. doi: 10.1080/19485565.2003.9989070. [DOI] [PMC free article] [PubMed] [Google Scholar]