Abstract
An effective isolation protocol for outgrowth endothelial cells (OEC) resulting in higher cell numbers and a reduced expansion time would facilitate the therapeutical application. In this study a standard protocol based on the isolation of mononuclear cells from adult peripheral blood was modified by adding a passaging step 7 days after the isolation. OEC colonies gained by both protocols were evaluated after 28 days and resulted in different frequencies of OEC colonies depending on the donor and culture protocol. Accordingly, we defined two groups, namely, high colony-forming cultures (HCC) and low colony-forming cultures (LCC) for further analysis. LCC revealed no increase in OEC colonies by the modified protocol, whereas in HCC the frequency of OEC colonies was significantly improved by the passaging step. Quantitative real-time polymerase chain reaction, flow cytometry, and immunofluorescence for endothelial markers indicated an enrichment of OEC by protocol modification in HCC. In addition, HCC revealed higher expression of CD34 and CD133 compared to LCC and resulted in higher numbers of OEC gained per donor, which was further improved by the modified protocol. We conclude that the modified protocol supports the selection of OEC from adult peripheral blood with a high clonogenic potential and results in a better efficacy in OEC isolation.
Introduction
Endothelial progenitor cells (EPC) from adult peripheral blood or cord blood have raised significant interest as a potential cell source for proangiogenic cell therapies. Nevertheless, the definition of the therapeutically most promising cell population is still a matter of debate. One subpopulation occurring within EPC cultures isolated from the peripheral blood mononuclear fraction is characterized by a series of endothelial markers or functions and therefore often designated as outgrowth endothelial cells (OEC) or endothelial colony-forming cells.1–3 OEC appear as individual colonies with cobblestone-like morphology after 3 to 4 weeks in culture and can be expanded in vitro without loosing their endothelial phenotype. Several groups have shown that OEC are able to contribute to the neovascularization process in vivo by forming functional vessels anastomosed to the host's vasculature.4,5 Nevertheless, the therapeutical success critically depends on the experimental settings such as coimplantation strategies4–6 with stabilizing cells or carrier-based delivery approaches allowing a site-directed therapy.6,7
In laboratory practice the expansion procedure for OEC is time consuming and thus incompatible with acute cell therapy of ischemic tissues. Different isolation protocols aiming at the specific isolation of OEC from heterogeneous EPC cultures have been described using surface markers such as CD348 or CD146,9 although the origin and potentially the marker profile of cells appearing as colonies in heterogeneous EPC cultures might differ. Nevertheless, also in isolation procedures based on surface markers isolated cells have to be expanded in vitro to achieve adequate cell numbers for therapeutic applications. Despite the fact that the phenotype of OEC seems to be stable during the in vitro expansion phase, the angiogenic potential of OEC in vivo appears to decrease with passage number or to be reduced in cells from adult blood compared to cord-blood-derived OEC.5 In addition, long-term in vitro expansion of OEC might be associated with genetic instability and chromosomal aberrations.10 Thus, there is a need for alternative culture protocols ensuring both a reduction of in vitro expansion time and the optimization of OEC numbers gained from one individual donor. In this study we propose a simple modification of a standard protocol for the isolation of OEC from peripheral blood by including a passaging step in the early phase of EPC culture. For several donors we directly compared the effect of this modified protocol on the number of OEC colonies, the total number of OEC gained per donor, and the enrichment of OEC from heterogeneous endothelial progenitor cultures. Further, we compared potential effects on the cellular phenotype of OEC selected by the individual isolation protocols. Two groups of donors were observed that differed in the abundance of OEC colonies and permitted a classification of the cultures as high colony-forming cultures and low colony-forming cultures (HCC and LCC, respectively). Significantly, the protocol modification had a marked positive selection effect on the former (HCC), but not on the latter (LCC). These two groups of donors were compared in terms of EPC-relevant markers such as CD34 and CD133 by quantitative real-time polymerase chain reaction (PCR) and flow cytometry in dependence on the culture protocol.
Materials and Methods
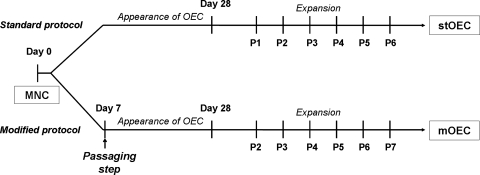
The use of the human cells was approved by the local ethics committees and included also the consent of the individual donors. In this study we compared two different protocols for the isolation and culture of OEC from mononuclear cells (MNC) isolated from human peripheral blood buffy coats. Human peripheral blood MNC were isolated by Ficoll (Sigma-Aldrich, Steinbach, Germany) density centrifugation from buffy coats as previously published.11 After this common step for both protocols, half of MNC from each individual donor was cultured according to a standard protocol or according to a modified protocol as described in the following sections. A schematic overview of the process is depicted in Figure 1.
FIG. 1.
Schematic diagram of the standard and modified protocol for the isolation of OEC from MNC. OEC, outgrowth endothelial cells; MNC, mononuclear cells.
Isolation and culture of MNC according to the standard protocol
MNC were seeded on 24-well culture plates coated with collagen type-I (BD Biosciences, Heidelberg, Germany) at a density of 5 × 106/well. Cells were cultivated in endothelial cell growth medium-2 BulletKit (CC-3162; Lonza, Verviers, Belgium) supplemented with growth factors provided by the manufacturer, with 5% fetal calf serum (Gibco Life Technologies, Karlsruhe, Germany) and 1% penicillin/streptomycin liquid (Gibco Life Technologies) in a humidified atmosphere (37°C, 5% CO2). Medium was changed three times a week. After 3 to 4 weeks single colonies of OEC with cobblestone-like morphology and endothelial characteristics appeared. These cells were designated as stOEC (OEC derived from standard protocol) in the following sections. After reaching confluency OEC were trypsinized and expanded on fibronectin-coated 24-well plates over several passages using a splitting ratio of 1:2.
Isolation and culture of MNC according to the modified protocol
In this protocol MNC from peripheral blood isolated according to the standard protocol as described above and seeded in a density of 5 × 106 cells/well were trypsinized after 7 days of culture and seeded on fibronectin-coated (Millipore, Schwalbach, Germany) 24-well culture plates in a seeding density of 0.5 × 106 cells/well. At the time point of cell passaging the cultures were characterized by a mixed population of cells as described in a previous publication.11 After the passaging step cells were maintained under the same culture conditions as described for the culture for the MNC in the standard protocol. In these cultures, colonies with cobblestone-like morphology similar to stOEC developed. These cells derived from this modified protocol were defined in the following sections as mOEC (OEC derived from the modified protocol).
OEC colony formation and comparison of total cell numbers
OEC colonies characterized by cobblestone-like morphology were identified by light microscopy. The number of colonies obtained from the standard protocol or from the modified protocol was compared for each individual donor 28 days after the isolation and standardized to a reference value for the number of seeded MNCs (mean value for the number of MNC/2) to exclude influences by different numbers of MNC gained per isolation. In addition, the total number of OEC per donor harvested from the individual protocols was monitored over the entire culture period.
Immunofluorescence of cell fraction isolated from peripheral blood
Twenty-eight days after the isolation of MNC, whether isolated according to the standard or the modified protocol, cells were investigated by immunofluorescence for the endothelial markers von Willebrand factor (vWF) and CD31. In brief, cells were fixed with 3.7% paraformaldehyde (Merck, Darmstadt, Germany) and permeabilized using 0.1% Triton® X-100 (Sigma-Aldrich). For staining the anti-human vWF (A0082; Dako, Hamburg, Germany) antibody was diluted 1:8000 and CD31 (M0823; Dako) was diluted 1:50 in 1% bovine serum albumin (Sigma-Aldrich) in phosphate-buffered saline (PBS). Cells from the different isolation protocols were incubated with both primary antibodies for 1 h at room temperature. After washing with PBS, cells were labeled with the corresponding secondary antibody, Alexa 488 anti-mouse (A-11029; MoBiTec, Göttingen, Germany) and Alexa 546 anti-rabbit (A-11010; MoBiTec) diluted 1:1000 in 1% bovine serum albumin in PBS for 1 h at room temperature. After incubation, cells were washed and cell nuclei were counterstained with Hoechst, diluted 1:1000 (Sigma-Aldrich). Samples were mounted with Gelmount (M01; Biomeda, Foster City, CA) and analyzed using confocal microscopy (Leica TCS-NT; Wetzlar, Germany) and fluorescence microscopy (Delta Vision and softWoRx; Applied Precision).
Flow cytometry analysis
The percentage of CD146- and CD34-positive cells resulting from MNC isolated and cultured according to the standard or the modified protocol was compared by flow cytometry 28 days after the isolation of MNC from the peripheral blood. Briefly, cells from same donor obtained by the individual protocols were incubated with anti CD146-Phycoerythrin (PE) (550315; BD Biosciences), CD34–fluorescein isothiocyanate (130-081-001; Miltenyi, Bergisch-Gladbach, Germany), or isotypic control anti immunoglobulin G1-PE (555749; BD Biosciences) or anti immunoglobulin G–fluorescein isothiocyanate (BD Biosciences) according to the manufacturer's protocol (20 min at 4°C). Analysis was performed for at least three individual donors using FACSCalibur (BD Biosciences) and CellQuestPro software (BD Biosciences). Results depicted in the histogram plots are representative for cells gained from several donors.
Gene expression analysis
Twenty-eight days after isolation cells from individual donors isolated and cultured according to the standard or the modified protocol were characterized by quantitative real-time PCR. Real-time PCR was performed for the endothelial markers vWF, CD146, Kinase insert domain–containing receptor (KDR), CD31, and caveolin-1; for the endothelial precursor markers CD34 and CD133; and for the hematopoietic marker CD45. For gene expression analysis total RNA was extracted from cells, isolated by the standard protocol or by the modified protocol using RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. RNA was transcribed into cDNA using Omniscript RT kit (Qiagen). Quantitative real-time PCR was performed using Applied Biosystems 7300 Real-Time PCR System (Applera Deutschland, Darmstadt, Germany). About 12.5 μL of QuantiTect™ SYBR® Green PCR Master Mix, 2.5 μL of QuantiTect SYBR Green primer assay, 6 μL of RNase-free water, all provided by Qiagen, and 4 μL of cDNA (1 ng/μL) were used for one reaction. Primer information is summarized in Table 1. Amplification was performed with the following thermocycler program—stage 1: 95°C, 15 min; stage 2: 94°C, 15 s; 55°C, 30 s; 72°C, 35 s (stage 2 was repeated for 40 cycles); stage 3: dissociation step. Glyceraldehyde 3-phosphate dehydrogenase was used as internal control and each gene was processed in triplicate. Relative quantification of gene expression was performed using Applied Biosystems Sequence Detection software v.1.2.2.
Table 1.
Primer Information for Primers Used in Real-Time Polymerase Chain Reaction
| Gene name | Primer assay name | Catalog number |
|---|---|---|
| vWF | Hs_VWF_1_SG QuantiTect Primer Assay (200) | QT00051975 |
| CD146 | Hs_MCAM_1_SG QuantiTect Primer Assay (200) | QT00079842 |
| KDR | Hs_KDR_1_SG QuantiTect Primer Assay (200) | QT00069818 |
| CD34 | Hs_CD34_1_SG QuantiTect Primer Assay (200) | QT00056497 |
| CD133 | Hs_PROM1_1_SG QuantiTect Primer Assay (200) | QT00075586 |
| CD45 | Hs_PTPRC_1_SG QuantiTect Primer Assay (200) | QT00028791 |
| GAPDH | Hs_GAPDH_1_SG QuantiTect Primer Assay (200) | QT00079247 |
| Gene name | Sequence | Annealing temperature |
|---|---|---|
| CD31 | Forward 5′- CCGGATCTATGACTCAGGGACCAT-3′ | 55°C |
| Reverse 3′-GGATGGCCTCTTTCTTGTCCAG-5′ |
vWF, von Willebrand factor; KDR, Kinase insert domain–containing receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
The gene expression levels in cells cultured under standard or modified conditions or from LCC or HCC was compared (reference value expression in LCC cultured under standard conditions set to 1) as indicated in the individual graphs, and the difference was considered to be significant if p-values obtained from the paired Student's t-test and analysis of variance were less than 0.05 or 0.03, respectively.
Proliferation curves of stOEC and mOEC
When cells of the same donors from the standard and the modified protocol reached confluence, cells were trypsinized and expanded over several passages using a splitting ratio of 1:2. Cell numbers of stOEC and mOEC were determined using a counting chamber and light microscopy. Growth curves of stOEC and mOEC were calculated.
Population doubling level (PDL) and population doubling time (PDT) were calculated using the following equations:
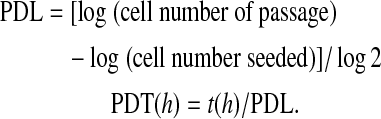 |
Statistical analysis
Significant differences were determined using the paired Student's t-test or analysis of variance. Statistical analysis was performed with Excel (Microsoft, München, Germany). Statistical significance was assessed when the p-value was less than 0.05 or 0.03, respectively.
Results
Evaluation of OEC colony formation by protocol modification
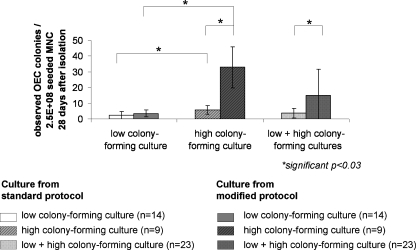
The isolation of OEC according to our previously published protocols usually results in OEC colony formation in 68% of the isolations of MNC from adult peripheral blood. In this study we modified the protocol as described above by including a passaging step after 7 days of culture of MNC. The effect on the number of OEC colonies formed in response to the protocol modification using an overall evaluation of 23 donors 28 days after the isolation of MNC from peripheral blood buffy coats is depicted in Figure 2. We observed two groups of cultures revealing different effects on the number of OEC colonies by the protocol modification. One group of cultures was characterized by an abundant appearance of OEC colonies, whereas in other cultures the number of OEC colonies was significantly lower. According to these characteristics the cultures were grouped for further analysis as described in detail in Table 2 into (1) LCC and (2) HCC. In the group of HCC the formation of OEC colonies (n = 9 donors) was significantly increased by the modified protocol (Fig. 2). Compared to this, there was no significant effect by the modified protocol in the group of LCC (n = 14). Nevertheless, the difference in the frequency of colonies in LCC and HCC was also observed when cells were isolated according to the standard protocol. HCC appear at a frequency of 39% in all tested donors from peripheral blood with the donor distribution according to age and sex as indicated in Table 2. Similar effects were observed using collagen as substrate, revealing the same beneficial effect of the protocol modification on the colony formation in HCC (data not shown).
FIG. 2.
Quantification of OEC colonies. Number of observed OEC colonies (28 days after isolation) per 2.5 × 108 seeded MNC cultured according to the standard protocol or the modified protocol. Donors were grouped as LCC and HCC. *Student's t-test was performed and considered significant if p ≤ 0.05. LCC, low colony-forming cultures; HCC, high colony-forming cultures.
Table 2.
Definition and Occurrence of High and Low Colony-Forming Cultures Depending on Donor's Sex and Age
| Low colony-forming culture | High colony-forming culture | |||
|---|---|---|---|---|
| Definition (n = 23) | <10 OEC colonies/2.5 × 108 seeded MNC in cultures from modified protocol | >10 OEC colonies/2.5 × 108 seeded MNC in cultures from modified protocol | ||
| Percentage of total (n = 23) | 61 | 39 | ||
| Sex | Male (n = 11) | Female (n = 3) | Male (n = 7) | Female (n = 2) |
| Percentage of male/female | 61 | 40 | 39 | 60 |
| Age of donor | 33.1 ± 9.9 | 39.5 ± 12.5 | 39.3 ± 10.1 | 44.7 ± 10.1 |
OEC, outgrowth endothelial cells; MNC, mononuclear cells.
Morphological appearance and immunofluorescent study of cultures after 28 days
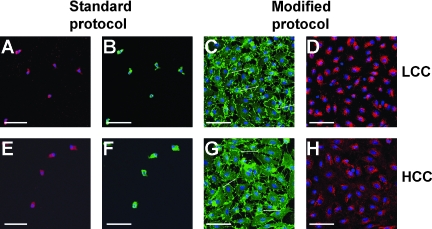
Using immunofluorescence for the endothelial markers CD31 and vWF (Fig. 3), we assessed the morphological appearance of cultures derived from the standard or the modified protocol or from LCC and HCC, respectively. In the cultures from the standard protocol (Fig. 3A, B, E, F) mainly single cells revealing no mature cobblestone-like morphology were observed. These cells revealed some fluorescent labeling for endothelial markers, although the immunofluorescent pattern typical of mature endothelial cells was not observed. In these heterogeneous cultures from the standard protocol only single-cell colonies with the morphological characteristics of mature endothelial cells typical of OEC were observed (data not shown), reflecting also the low frequency of OEC colonies as indicated in Figure 2. On the other hand, the protocol modification resulted in cultures that contained cells of a more mature endothelial phenotype indicated by larger patches of endothelial cells expressing the endothelial marker CD31 along the intercellular contacts (white arrows in Fig. 3C, G) and vWF in typical dotted pattern in the perinuclear region (Fig. 3D, H). The morphological appearance of the OEC colonies obtained by HCC (Fig. 3G, H) and LCC (Fig. 3C, D) or from the standard or modified protocol (data not shown) seemed to be uneffected.
FIG. 3.
Immunofluorescence staining for endothelial markers. Morphology and immunofluorescence pattern is depicted for cells obtained from the standard protocol (A, B, E, F) or from the modified protocol (C, D, G, H) 28 days after isolation. Representative images of low colony-forming cells (upper row) and high colony-forming cells (lower row) in dependence of the culture protocol are presented for CD31 (B, C, F, G) and vWF (A). (A, D, E, H) staining. Scale bars (A–H): 75 μm. vWF, von Willebrand factor. Endothelial contacts are highlighted by arrows.
Quantitative real-time PCR for the evaluation of gene expression in culture after 28 days
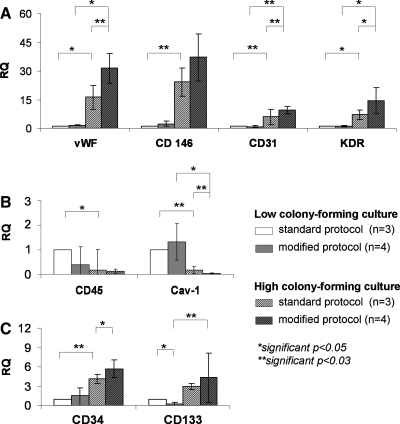
To obtain quantitative data about which cell types are potentially enriched by the protocol modification or how cells from HCC and LCC differ in their gene expression, cultures were analyzed by quantitative real-time PCR 28 days after isolation. For the analysis we used several markers for mature endothelial cells, such as CD31, vWF, CD146, KDR, and caveolin-1 also used in our previous studies for OEC,11 as well as markers associated with progenitor cell function, such as CD34,12–14 CD133,12,14 and CD4515–17 (Fig. 4). In HCC we observed significantly higher expression of markers for mature endothelial cells, such as vWF, CD31, CD146, and KDR, as well as upregulated expression of progenitor markers CD34 and CD133 compared to LCC. In HCC the relative expression rates of these markers could be further increased by protocol modification although these findings were only significant for vWF, CD31, KDR, and CD34. On the other hand, markers such as the hematopoietic marker CD45 and caveolin-1 were significantly reduced in HCC compared to LCC.
FIG. 4.
Gene expression analysis by quantitative real-time polymerase chain reaction. (A) Relative quantification of endothelial markers vWF, CD146, KDR, and CD31; (B) relative quantification of the hematopoietic marker CD45 and Cav-1; and (C) relative quantification of the endothelial precursor cell markers CD34 and CD133 depicted for LCC and HCC and their respective culture protocols. Gene expression was analyzed 28 days after the isolation and statistically evaluated by Student's t-test with *p ≤ 0.05 and **p ≤ 0.03.
Flow cytometry for single cell evaluation of cultures
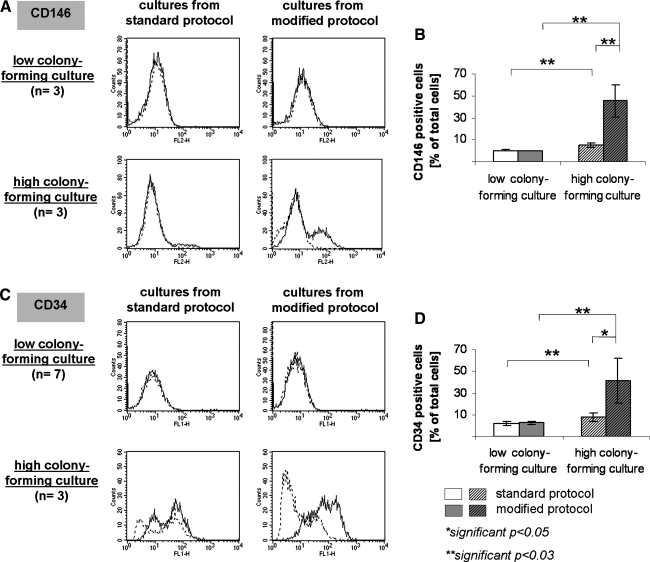
The frequency of cells carrying the mature endothelial marker CD146 or the endothelial progenitor marker CD34 in HCC and LCC derived from the standard or modified protocol was further investigated at the single cell level by flow cytometry after 28 days of culture. Histograms representative of several donors and quantitative data in percentage of total (mean value n = 3 donors) for individual markers are depicted in Figure 5. In HCC significantly more CD146-positive cells were observed, representing 5.3 ± 1.7% of total cells, compared to 0.5 ± 0.3% of total cells in LCC. Similar findings were observed for CD34, resulting in a significantly higher frequency of CD34-positive cells in HCC (7.5 ± 4.2%) compared to cultures derived from LCC (2.0 ± 2.1%). The protocol modification including the passaging step resulted in a significant enrichment of CD146-positive cells in HCC from 5.3 ± 1.7% to 45.3 ± 14.9% (Fig. 5B). In addition, the number of CD34-positive cells was also increased in cultures from the modified protocol from 7.5 ± 4.2% to 41.3 ± 20.6% (Fig. 5D). Flow cytometry was also used to assess the purity of the HCC and LCC after longer periods of expansion in vitro (64 days after the isolation). In LCC 99.4 ± 0.04% of the cells for the standard and 91.3 ± 5.3% for the modified protocol were positive for CD31. In HCC the purity with regard to CD31 was 99.0 ± 0.5% for the standard protocol and 98.8 ± 0.6% for the modified protocol.
FIG. 5.
Flow cytometry. Representative histogram plots for the endothelial markers CD146 (A) and CD34 (C) are depicted on the left side showing the specific staining (continuous line) and the isotypic controls (broken line). Histograms are depicted for HCC and LCC derived from the standard or modified protocol as indicated in the graph. Bar charts depict mean values of CD146-positive (B) and CD34-positive (D) cells for the individual groups determined as mean values of at least n = 3 donors. **p ≤ 0.03.
Cell proliferation and cell numbers obtained per donor
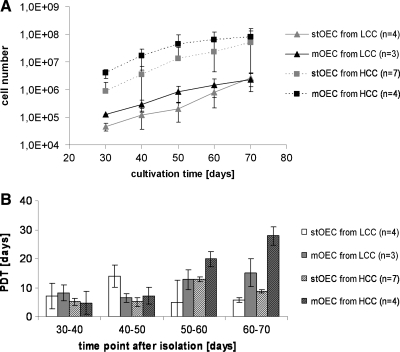
Cell proliferation of stOEC or mOEC or from HCC or LCC, respectively, was monitored over the culture period. Growth curves based on cell counting are depicted in Figure 6A as mean values for at least three donors per group. Growth curves depicted were limited to 70 days of culture because of the differences in the expansion time for the individual OEC donors (compare Table 3). After 70 days some of the donors entered a senescent state, whereas others proliferated over a much longer culture time. Therefore, the total cell numbers obtained per donor during the overall culture time are individually listed in Table 3. Isolations from HCC cultured according to the modified protocol resulted in the highest numbers of OEC. Comparing the total amount of cells received during in vitro expansion the protocol modification in HCC resulted in OEC numbers as twice as high as in the standard protocol (Table 3). The differences regarding the OEC cell numbers in HCC and LCC were already observed in the earlier phases of the cultures (Fig. 6A). This was also reflected by the effect of the modified protocol on the number of colonies. On the other hand, the number of OEC received from LCC was significantly lower and was not affected by the modified protocol. According to the growth curves (Fig. 6A) the proliferation of OEC slowed down with progressing culture time with a slightly higher effect on the cells derived from the modified protocol. This was also demonstrated by comparing the PDTs depicted in Figure 6B. PDTs seemed to increase with progressing culture time, with a slightly higher effect in cells derived from the modified protocols, irrespective of whether they derived from HCC or LCC. In addition, we observed no difference in the life span of cells derived from HCC or LCC (Table 3).
FIG. 6.
Cell proliferation. Growth curves of xOEC from HCC and LCC obtained by the standard or the modified protocol are depicted in (A) as indicated in the graph using a logarithmic scale. Panel (B) depicts the PDT for LCC and HCC depending on culture protocol and culture time. PDT, population doubling time.
Table 3.
Evaluation of Total Cell Numbers Gained Per Individual Donors Depending on Culture Time
| |
|
Standard protocol |
Modified protocol |
||||
|---|---|---|---|---|---|---|---|
| Donor | Number of OEC colonies 28 days after isolation | Total cell number | Day | Number of OEC colonies 28 days after isolation | Total cell number | Day | |
| Low colony-forming culture | 1 | 3 | 2.0 × 105 | 64 | 4 | 3.2 × 106 | 64 |
| 2 | 2 | 2.6 × 106 | 85 | 2 | 2.6 × 106 | 85 | |
| 3 | 2 | 2.4 × 107 | 78 | 3 | 6.3 × 106 | 78 | |
| Mean value | 8.9 × 106 | 4.0 × 106 | |||||
| High colony-forming culture | 1 | 2 | 6.8 × 105 | 69 | 28 | 4.8 × 107 | 69 |
| 2 | 4 | 1.5 × 108 | 104 | 23 | 5.2 × 108 | 104 | |
| 3 | 9 | 4.7 × 106 | 43 | 56 | 2.6 × 107 | 36 | |
| 4 | 7 | 2.2 × 108 | 71 | 24 | 2.7 × 108 | 65 | |
| Mean value | 9.4 × 107 | 2.2 × 108 | |||||
Discussion
The concept of cellular therapies to improve vascularization of tissue-engineered constructs or in tissues undergoing a regeneration process has been critically discussed over the last years. Despite several studies showing that this concept can lead to actively perfused vessels after implantation, the selection of therapeutically relevant EPC, their expansion in vitro, and the protocols for their application still need to be further improved. A reduction of the expansion time, as well as a higher number of OEC obtained per individual donor will be essential steps toward a clinical application. In this study we propose a modified protocol for the isolation of OEC, resulting in higher colony frequencies and cell numbers in HCC. In addition, HCC seem to be enriched in cells with characteristics reported for EPC, as indicated by data from real-time PCR and flow cytometry.
According to the current knowledge EPC are contained in very low numbers in peripheral blood and represent only 0.01% to 0.0001% of MNC.18 This low frequency, together with the lack of commonly accepted markers, complicates their accurate detection or directed isolation. Many of the cells termed EPC might support the neovascularization process more by paracrine or indirect and synergistic effects than by differentiation into mature and functional endothelial cells.19,20 In contrast to this, OEC are a distinct population with a promising angiogenic potential.21–23 Among the heterogeneous cell types obtained from peripheral blood MNC, OEC appear as endothelial colonies in the later stages of the culture. In this study 68% of cultures from different donors were able to generate OEC colonies. Twenty-eight days after standard isolation about two to six OEC colonies per 2.5 × 108 seeded MNC appeared, representing 0.0008% to 0.0025% of MNC. Because of considerable differences in the number of OEC colonies depending on the donor, cultures were classified into LCC (61% of total donors) and HCC (39% of total donors). The number of OEC colonies in HCC was significantly higher than in LCC independent of the culture protocol, although the protocol modification further improved the colony formation. Many factors associated with individual donors such as trauma, cardiovascular disease, or tumorigenesis might influence the number of EPC or endothelial colony-forming cells as reviewed in Ref.,24 which was not the scope of the present study. In our study the blood was obtained from healthy donors undergoing blood donations according to the standard control mechanisms. The present study was performed without additional information regarding the health conditions for individual donors. Sixty percentage of isolated cultures from female donors and only 39% of male donors could be assigned to HCC, whereas the age of the donor was irrelevant. These results are consistent with those of Hoetzer et al., who demonstrate that EPC isolated from middle-aged women have a higher colony-forming capacity than those from men of similar age.25 Further details on the potential reasons for the difference in HCC and LCC and the consequences for the protocol modification will have to be determined in future studies.
According to our study the modified protocol, which includes a passaging step 7 days after the isolation of MNC, leads to the enrichment of OEC in HCC from peripheral blood cultures. This was indicated by increased levels of markers for mature endothelial cells and endothelial cell colony-forming cells1,2 in real-time PCR (vWF, CD146, CD31) and by flow cytometry (CD146). On the other hand, the protocol modification showed no effect on LCC. Besides this difference for mature endothelial markers in HCC and LCC, we also observed differences in gene expression of markers associated with an EPC type, such as CD133 and CD34.12,14,26 This was accompanied by lower expression rates of caveolin-1 and CD45 expression in the real-time PCR and in higher rates of CD34-positive cells in the flow cytometry study in HCC. Although we have not directly shown that OEC colonies derive from CD34-positive cells, our study suggests a positive correlation of CD34 expression in HCC with the number of OEC colonies. This is in accordance with reports from the literature, providing increasing evidence that OEC derive from CD34+/CD45− cells in the blood.8,27 Nevertheless, CD34 is also expressed on hematopoietic cells as well as on mature endothelial cells but often lost during in vitro cultivation. Further, we also observed increased expression of CD133 in real-time PCR in HCC, which is also used by several groups as a marker for EPC. Nevertheless, Timmermans et al.8 showed that OEC do not derive from CD133+/CD45− cells, this being in accordance with a study by Case et al.,27 who showed that endothelial colony-forming cells occur in CD34+ CD45− cultures, but are absent in cell cultures selected for CD34+ CD133+ VEGFR-3+ cells.
Another marker differentially expressed in HCC and LCC is caveolin-1, which is highly expressed in mature endothelial cells,28 including OEC, but absent in cells often defined as early EPC.1,11 In addition, the upregulation of caveolin-1 correlates in many types with cellular senescence.29,30 In HCC cultures we observed significantly lower expression of caveolin-1 compared to LCC. According to these differences in the marker expression with regard to EPC markers and caveolin-1, one might suppose that HCC are potentially less differentiated than LCC or might derive from more stem-cell-like precursors. Nevertheless, whether these differences in marker expression in our study might indicate different origins of OEC is currently unclear and needs further investigation.
Differences in endothelial colony-forming cells from the peripheral blood or cord blood were reported by several groups mainly in terms of their proliferative capacity, which was associated with their origin from the vessel wall or from bone-marrow-derived stem cells. Studies by Lin et al.3 with sex-mismatched bone-marrow-transplanted patients showed that endothelial colony-forming cells or OEC originate from two different sources such as circulating angioblasts derived from the bone marrow donor or from circulating cells from the recipient. The proliferation capacity of OEC derived from angioblasts was markedly higher than the proliferation capacity derived from circulating cells including also mature cells shed from the vessel wall. Nevertheless, clonogenic analysis of colony-forming endothelial cells in other studies also suggests that endothelial cell colony-forming cells exhibit a hierarchy with differences in proliferation capacity and clonogenic potential.31 Moreover, in endothelial cells from the vessel wall proliferation capacity and clonogenic potential can range from cells with a limited replicating capacity to cells with a high proliferation capacity.32 Nevertheless, differences in the marker profile between progenitor-derived and vessel-wall-derived cells, or high and low proliferating cells, respectively, were not reported in these studies.
In our study the proliferation curves of OEC from LCC and HCC monitored over the culture time were similar and independent of the culture protocol, but nevertheless differed in the numbers of OEC contained in the early stages of cultures investigated after 28 days. The effect of the protocol modification on HCC including a passaging step might be associated with the higher clonogenic potential of HCC compared to LCC, as previously reported.31,32 Thus, passaging of cells at an earlier stage might support the selection of cells with a higher clonogenic potential, also resulting in higher numbers of OEC obtained per individual donor.
For a therapeutical application the identification of OEC in earlier phases of the culture, as well as a detailed analysis of their origin from specific cell types, would be desirable. Nevertheless, problems of identification are partly due to technical difficulties in detecting OEC or their progenitors appearing in very low frequencies in heterogeneous cell populations from the peripheral blood. We combined real-time PCR and flow cytometry to characterize OEC from HCC and LCC. Steurer et al. recently compared these two methods to detect circulating endothelial cells and EPC.33 In the so-called spiking experiments, using defined numbers of circulating endothelial cells added to heterogeneous blood MNCs, detection limits were found to be in the range of 0.001% for quantitative real-time PCR or 0.01% for flow cytometry. Although the real-time PCR was 10 times more sensitive than flow cytometry, the specifity of the real-time PCR was lower due to the methological problems associated with this technology. This includes the lack of markers exclusively expressed on the relevant cell types, such as expression of CD34 on hematopoietic cells. In addition, real-time PCR lacks the ability to identify specific cellular subpopulations by simultaneous analysis of multiple markers at the single-cell level. Using both methods was therefore essential to investigate the effect of the protocol modification in the present study.
In conclusion, an enrichment of relevant cell types by protocol modification as suggested in the present study might be valuable in further improving the detection of endothelial cell colony-forming cells or their progenitors. This method might serve as an alternative or as an additional step for currently used protocols for EPC isolations. Moreover, the protocol modification resulted in higher numbers of OEC gained per donor in HCC and is potentially associated with their higher clonogenic potential. Last but not least, initial findings concerning the differences in gene expression in HCC and LCC further support the current understanding of endothelial cell hierarchy in adult blood-derived endothelial cell colony-forming cells.
Acknowledgments
The authors would like to thank BMBF (German–Chinese Young Investigator Group), MAIFOR (funded by the University Medical Center, Johannes Gutenberg University), and Expertissues (funded by the European Commission) for financial support and Barbara Pavic for her excellent technical assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gulati R. Jevremovic D. Peterson T.E. Chatterjee S. Shah V. Vile R.G. Simari R.D. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 2.Hur J. Yoon C.H. Kim H.S. Choi J.H. Kang H.J. Hwang K.K. Oh B.H. Lee M.M. Park Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y. Weisdorf D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 5.Au P. Daheron L.M. Duda D.G. Cohen K.S. Tyrrell J.A. Lanning R.M. Fukumura D. Scadden D.T. Jain R.K. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs S. Ghanaati S. Orth C. Barbeck M. Kolbe M. Hofmann A. Eldenkamp M. Gomes M. Reis R.L. Kirkpatrick C.J. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30:526. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Silva E.A. Kim E.-S. Kong H.J. Mooney D.J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci. 2008;105:14347. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmermans F. Van Hauwermeiren F. De Smedt M. Raedt R. Plasschaert F. De Buyzere M.L. Gillebert T.C. Plum J. Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45 + hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 9.Delorme B. Basire A. Gentile C. Sabatier F. Monsonis F. Desouches C. Blot-Chabaud M. Uzan G. Sampol J. Dignat-George F. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- 10.Corselli M. Parodi A. Mogni M. Sessarego N. Kunkl A. Dagna-Bricarelli F. Ibatici A. Pozzi S. Bacigalupo A. Clinical scale ex vivo expansion of cord blood-derived outgrowth endothelial progenitor cells is associated with high incidence of karyotype aberrations. Exp Hematol. 2008;36:340. doi: 10.1016/j.exphem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs S. Hermanns M. Kirkpatrick C. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006;326:78. doi: 10.1007/s00441-006-0222-4. [DOI] [PubMed] [Google Scholar]
- 12.Peichev M. Naiyer A.J. Pereira D. Zhu Z. Lane W.J. Williams M. Oz M.C. Hicklin D.J. Witte L. Moore M.A. Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952. [PubMed] [Google Scholar]
- 13.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Salven P. Mustjoki S. Alitalo R. Alitalo K. Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 15.Dahlke M.H. Larsen S.R. Rasko J.E. Schlitt H.J. The biology of CD45 and its use as a therapeutic target. Leuk Lymphoma. 2004;45:229. doi: 10.1080/1042819031000151932. [DOI] [PubMed] [Google Scholar]
- 16.Pelosi E. Valtieri M. Coppola S. Botta R. Gabbianelli M. Lulli V. Marziala G. Masella B. Muller R. Sgadari C. Testa U. Bonanno G. Peschle C. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- 17.Shaw J.P. Basch R. Shamamian P. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells Mol Dis. 2004;32:168. doi: 10.1016/j.bcmd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Khan S.S. Solomon M.A. McCoy J.P., Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005;64:1. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 19.Yoon C.H. Hur J. Park K.W. Kim J.H. Lee C.S. Oh I.Y. Kim T.Y. Cho H.J. Kang H.J. Chae I.H. Yang H.K. Oh B.H. Park Y.B. Kim H.S. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 20.Rehman J. Li J. Orschell C.M. March K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs S. Hofmann A. Kirkpatrick C.J. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 22.Sieveking D.P. Buckle A. Celermajer D.S. Ng M.K.C. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 23.Finkenzeller G. Torio-Padron N. Momeni A. Mehlhorn A.T. Stark G.B. In vitro angiogenesis properties of endothelial progenitor cells: a promising tool for vascularization of ex vivo engineered tissues. Tissue Eng. 2007;13:1413. doi: 10.1089/ten.2006.0369. [DOI] [PubMed] [Google Scholar]
- 24.Aaron L. Barry F. O'Brien T. Endothelial progenitor cells: diagnostic and therapeutic considerations. Bioessays. 2006;28:261. doi: 10.1002/bies.20372. [DOI] [PubMed] [Google Scholar]
- 25.Hoetzer G.L. MacEneaney O.J. Irmiger H.M. Keith R. Van Guilder G.P. Stauffer B.L. DeSouza C.A. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99:46. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gehling U.M. Ergun S. Schumacher U. Wagener C. Pantel K. Otte M. Schuch G. Schafhausen P. Mende T. Kilic N. Kluge K. Schafer B. Hossfeld D.K. Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106. [PubMed] [Google Scholar]
- 27.Case J. Mead L.E. Bessler W.K. Prater D. White H.A. Saadatzadeh M.R. Bhavasar J.R. Yoder M.C. Ingram D.A. Human CD34 + AC133 + VEGFR-2 + cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Frank P.G. Woodman S.E. Park D.S. Lisanti M.P. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 29.Park W.Y. Park J.S. Cho K.A. Kim D.I. Ko Y.G. Seo J.S. Park S.C. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- 30.Volonte D. Zhang K. Lisanti M.P. Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13:2502. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K. Pollok K. Ferowicz M.J. Gilley D. Yoder M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 32.Ingram D.A. Mead L.E. Moore D.B. Woodard W. Fenoglio A. Yoder M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 33.Steurer M. Kern J. Zitt M. Amberger A. Bauer M. Gastl G. Untergasser G. Gunsilius E. Quantification of circulating endothelial and progenitor cells: comparison of quantitative PCR and four-channel flow cytometry. BMC Res Notes. 2008;1:71. doi: 10.1186/1756-0500-1-71. [DOI] [PMC free article] [PubMed] [Google Scholar]