Abstract
BACKGROUND
Isoflavones from soy and red clover exert modest hormonal effects in women, but the relevance to risk of breast cancer is unclear. The aim of this meta-analysis was to assess the effects of isoflavone-rich foods or supplements on a biomarker of breast cancer risk, women's mammographic density.
METHODS
Electronic searches were performed on The Cochrane Library, Medline and EMBASE (to June 2009), and reference lists and trial investigators were consulted to identify further studies. Randomized controlled trials (RCTs) of isoflavone-rich foods or supplements versus placebo with a duration of at least 6 months were included in our analysis. Inclusion/exclusion, data extraction and validity assessment were carried out independently in duplicate, and meta-analysis used to pool study results. Subgrouping, sensitivity analysis, assessment of heterogeneity and funnel plots were used to interpret the results.
RESULTS
Eight RCTs (1287 women) compared isoflavones with placebo for between 6 months and 3 years. Meta-analysis suggested no overall effect of dietary isoflavones on breast density in all women combined [mean difference (MD) 0.69%, 95% confidence interval (CI) −0.78 to 2.17] or post-menopausal women (MD −1.10%, 95% CI −3.22 to 1.03). However, there was a modest increase in mammographic density in premenopausal women (MD 1.83%, 95% CI 0.25–3.40) without heterogeneity but this effect was lost in one of three sensitivity analyses.
CONCLUSIONS
Isoflavone intake does not alter breast density in post-menopausal women, but may cause a small increase in breast density in premenopausal women. Larger, long-term trials are required to determine if these small effects are clinically relevant.
Keywords: isoflavone, mammographic density, breast cancer risk, meta-analysis, menopause
Introduction
The structural similarity of plant-derived isoflavones to human 17β estradiol has stimulated significant interest in their importance to women's health (Balk et al., 2005; Messina et al., 2006), with some evidence that dietary isoflavones can influence hormonal levels in pre- and post-menopausal women (Hooper et al., 2009). However, the safety and efficacy of soy-derived isoflavones from different sources has not been fully evaluated, particularly in relation to breast cancer risk.
Increased circulating estradiol concentrations are associated with increased risk of breast cancer in post-menopausal women (Endogenous Hormones Breast Cancer Collaborative Group, 2002), whereas higher soy isoflavone intakes are associated with lower breast cancer risk in epidemiological studies. Cross-cultural studies suggest 3-fold lower rates of breast cancer in Asian populations, where soy consumption is high (Messina et al., 2006; Trock et al., 2006). A recent meta-analysis of cohort and case–control data on soy intake and breast cancer showed soy to be associated with a small reduction in risk (Trock et al., 2006) whereas another found a significant trend of reduced breast cancer risk with increasing soy food intake (Wu et al., 2008). In a large recent analysis of breast cancer patients from the Shanghai Breast Cancer Survival Study, soy food consumption (with average intakes of 47 mg/day of isoflavones) was inversely associated with risk of death [hazard ratio (HR) 0.71, 95% confidence interval (CI) 0.54–0.92 for the highest compared with the lowest quartiles of soy intake] and breast cancer recurrence (HR 0.68, 95% CI 0.54–0.87; Shu et al., 2009). Despite these epidemiological data, results from the available intervention trials have been equivocal. No large, long-term randomized controlled trials (RCTs), powered to assess breast cancer as an outcome, have been published. It remains unclear whether isoflavones from different sources increase or decrease breast cancer risk in humans. A recent meta-analysis of side effects of phytoestrogens observed only 16 cases of breast cancer diagnoses, insufficient to assess effects on this outcome (Tempfer et al., 2009).
The biological effects induced by isoflavones may result in long-term genomic actions mediated by intracellular estrogen-receptor (ER) induced changes in gene expression or as rapid non-genomic actions that modify a wide array of intracellular signal transduction cascades (Setchell and Cassidy, 1999; Losel et al., 2003; Messina, 2007). Available evidence suggest that their ER binding potential may be more important in vivo (Losel et al., 2003; Penttinen-Damdimopoulou et al., 2009) since the isoflavone concentrations required to stimulate certain non-genomic activities, such as inhibition of cellular protein tyrosine kinases (>10 µM) and topoisomerase-II typically exceed the plasma levels that can be attained via a habitual dietary intake of soy-rich foods (∼2−5 µM; Messina et al., 2009).
There are conflicting experimental data on the effects of isoflavones on breast cancer cells in vitro and estrogen-sensitive induced mammary tumours in vivo (Hsieh et al., 1998; Allred et al., 2001; Kanno et al., 2003; Messina et al., 2006, 2009; Cline and Wood, 2009; Power et al., 2006a, b, 2007; Saarinen et al., 2009). Isoflavones are not estrogenic in cynomolgus monkeys, even following high isoflavone intake; these reduced estrogen-induced proliferation responses occur via effects on estrogen metabolism, or are mediated through ER interactions (Cline and Wood, 2009). Paradoxically, in rodents and in vitro cell models isoflavones induce estrogen-like effects in breast cancer cells lines and cause uterine enlargement in rodents (Wang et al., 1996; Hsieh et al., 1998; Allred et al., 2001; Kanno et al., 2003; Power et al., 2006a, b, 2007; Seo et al., 2006; Saarinen et al., 2009). This mixed estrogen agonist/antagonist activity of isoflavones was recently reported in an estrogen reporter mouse model, where ER signalling was modulated following ingestion of isoflavones (Penttinen-Damdimopoulou et al., 2009); soy isoflavones exerted antiestrogenic effects in the presence of estradiol and estrogenic effects in the absence of estradiol. Rodent studies and epidemiological data suggest that these differential biological effects may be explained by timing of exposure or dose of isoflavones in relation to endogenous estrogens (Hsieh et al., 1998; Lamartiniere et al., 1998, 2002; Lamartiniere, 2000; Wu et al., 2002; Korde et al., 2009). These observed estrogenic effects in animal models have heightened concerns over the potential estrogenic effects of isoflavone consumption in humans.
Although there are currently no reliable biomarkers of breast cancer risk, mammographic density has consistently been one of the best independent biomarkers; moderate to high-density confers a 1.8 to 5-fold risk for developing breast cancer compared with low-density in both pre- and post-menopausal women (McCormack and dos Santos Silva, 2006; Vachon et al., 2007; Cummings et al., 2009). Despite the evidence that it is an established risk factor for breast cancer development, there remains some uncertainty about the use of mammographic density as an intermediate marker of risk for the disease (Becker and Kaaks, 2009). Available data suggest that several hormonal and dietary factors can modify breast density; density increases with estrogen and progestin therapy and decreases following exposure to tamoxifen or ovarian suppression (Boyd et al., 1997, 2001; Greendale et al., 2003).
Given the current controversies and complex relationship between isoflavone intake and breast cancer risk and the lack of RCTs on the effects of isoflavones on breast cancer incidence, we conducted a systematic review and meta-analysis of the available randomized controlled studies of isoflavones using mammographic breast density as a surrogate marker. The primary objective was to assess the effect of isoflavone-rich foods and isoflavone extracts from soy and red clover on breast density in women. Secondary objectives included understanding effects in pre- and post-menopausal women, by baseline breast cancer risk, by duration of exposure and by source and dose of isoflavone. No protocol for this review has been published.
Methods
Search and inclusion criteria
An electronic search was performed on The Cochrane Library, Medline and EMBASE (from inception of each database until June 2009). The search was in the format: [isoflavones or soy or red clover] and [RCT filter] and [breast density or breast cancer]. The search was not limited to English language publications but no relevant non-English publications were located. In addition to the electronic searches the reference lists of included studies and relevant related systematic reviews were checked for further trials. Attempts were made to contact authors of all included studies for information about ongoing trials, as well as further details on their published studies.
Included studies were randomized controlled parallel arm trials of at least 6 months duration which compared increased intake of traditional soy foods, isoflavone-rich soy products or isolated isoflavones from soy or red clover compared with usual or control/placebo diet. Included participants were women of any age, at any baseline risk of breast cancer, with or without a history of breast cancer and outcomes included breast density.
All potential titles and abstracts were assessed for their relevance by two independent reviewers. Potentially relevant papers were collected in full text for further assessment of inclusion. One reviewer initially excluded studies that were clearly not relevant (such as in vitro or case–control studies) then the remainder were assessed independently for inclusion by two reviewers using an inclusion/exclusion form designed for the review. Reviewers met to discuss differences in data extraction, all differences were decided by discussion.
Data collection and assessment of validity
Data extraction and validity assessment were carried out together onto a data extraction form developed for the review. Extracted data included bibliographic details, participants' characteristics (menopausal status, mean age, baseline cancer risk, country), type of intervention (source, isoflavone dose, type of placebo, compliance), duration of intervention, numbers of participants randomized to and completing each study arm, method used to assess breast density, side effects and breast cancer diagnoses. In addition, details of the number of participants, mean breast density change (absolute change or as change per year) and variance of that change (or end breast density and its variance where change data were not available) were collected for each arm of each included study at the latest time point available. Baseline risk of breast cancer was defined as follows: high risk included participants with family history of breast cancer, presence of genetic risk markers or high risk according to the Gail model, the standard tool for assessing a woman's future risk for breast cancer (Gail et al., 1989), high mammographic density or Wolfe parenchymal pattern [based on patterns of ducts, nodularity and densities seen on mammography (Wolfe, 1976)]; moderate risk was participants with a history of any type of cancer or with first degree relatives with breast cancer; low risk was all other participants (Vachon et al., 2007). Where more than one method was used to asses breast density, as in the study by Atkinson (Atkinson et al., 2004; Kataoka et al., 2008), data for the main analysis were used from the semi-automated assessment, rather than the entirely visual or entirely automated assessments, as semi-automated assessment appeared to be most commonly used through the studies, although the visual and fully automated data were also used in subgroup analyses. Data for the main analysis were taken from the latest follow-up in each trial on the basis that any effect of isoflavones are likely to be cumulative and so any differences are more likely to be observed after a longer duration. Where studies did not provide data on the change in breast density from baseline to study end, but only data on mean breast density at study end, the end data were used within the meta-analysis (as is considered appropriate in the Higgins and Green, 2008a), to make best use of the available data. Authors were contacted for further data or clarification where questions arose.
Validity assessment of included studies was based on allocation concealment, masking of participants, masking of outcome assessors, industry funding or involvement, whether compliance with the intervention was measured and reported, whether isoflavone doses in intervention and control arms were reported, and whether dropouts were clearly reported. These characteristics [based on the method used by the Higgins and Green (2008a)] were:
Allocation concealment (concealment of the ability of those recruiting participants to assess which arm participants will be randomized into before recruitment is complete, coded as adequate, unclear or inadequate);
participant blinding (concealment of the participants to whether they are part of the intervention or control condition, coded as yes, unclear or no);
outcome assessor blinding (concealment of the outcome assessor, here the reader of the mammogram, to whether participants are part of the intervention or control condition, coded as yes, unclear or no);
industry funding or involvement [level of financial involvement of industries that may have a financial interest in the study results, coded as yes (study mainly funded by industry, or at least one author is employed by industry), partly (other impartial funding, but includes some industry funding that may include free provision of supplements) or no];
compliance assessed and reported [measurement of the degree to which participants complied with taking the intervention and control foods or supplements, and reporting of these data, coded as yes (both reported), partly (one or the other) or no]; and
reporting of withdrawals (numbers of withdrawals in each group clear and reasons reported coded as done, partial and not done).
Statistical analysis
Characteristics of included studies and study validity were tabulated (Tables I and II). Differences in percentage mammographic density between the isoflavone-rich intervention and control periods were combined across studies using mean differences (MD) using a random effects model in Review Manager 5.0 software (2008).
Table I.
Characteristics of included studies.
| Study | Participants | Interventions | Outcomes |
|---|---|---|---|
| Atkinson 2004, UK (Atkinson et al., 2004; Kataoka et al., 2008) | Participants: pre-, post- and peri- menopausal women without breast cancer but with Wolfe P2 or DY mammographic patterns | Form: tablet | Duration: 52 weeks |
| Randomized: int 102, cont 103 | Intervention: 1 tablet/day, Promensil (isolated isoflavones from red clover) versus 1 tablet/day placebo | Method of assessment: three methods used: visual assessment by two independent radiologists, computer-assisted using Cumulus and fully automated using SMF—data on Cumulus used in our analysis (% density) | |
| Analysed: int 76, cont 84 | Total isoflavone dose: int ∼44 mg/day, including 26 mg/day biochanin A, 16 mg/day formonetin, 1 mg/day genistein, 0.5 mg/day daidzein, cont unclear (nil?) | ||
| Mean age (sd): int 55.1 (4.7), cont 55.2 (4.9) | |||
| Baseline risk: high | |||
| Marini 2008, Italy (Marini et al., 2007, 2008; D'Anna et al., 2009; Atteritano et al., 2009) | Participants: post-menopausal osteopenic women aged 49–67 | Form: tablets | Duration: 156 weeks |
| Randomized: int 198, cont 191 | Intervention: 2 tablets/day purified genistein (isolated aglycone genistein) versus 2 tablets/day placebo | Method of assessment: computer-assisted assessment (IMI) | |
| Analyzed: at 2 years int 150, cont 154; At 3 years int 71, cont 67 | Total isoflavone dose: int 54 mg/day genistein (aglycone units), cont nil | ||
| Mean age (sd): int 53.8 (2.9), cont 53.5 (2.0) | |||
| Baseline risk: low | |||
| Maskarinec 2002, USA (Maskarinec et al., 2002, 2003) | Participants: premenopausal women aged 35–46 with recent normal mammogram | Form: tablet | Duration: 52 weeks |
| Randomized: int 17, cont 17 | Intervention: 2 × 50 mg tablets/day soy extract (isolated isoflavones) versus placebo (maltodextrin) | Method of assessment: computer-assisted assessment (% density) | |
| Analyzed: unclear, int 15, cont 15?? | Total isoflavone dose: int 76 mg/day (aglycone equivalents, 100 mg isoflavones) of total isoflavones, including 38.8 mg/day daidzein, 33.4 mg/day genistein, 3.8 mg/day glyceitin, cont unclear (nil?) | ||
| Mean age: int 41.1 (3.1), cont 43.3 (1.7) | |||
| Baseline risk: low | |||
| Maskarinec 2004a,b, USA (Maskarinec et al., 2004a, b) | Participants: premenopausal women with clear mammograms | Form: soy foods | Duration: 104 weeks |
| Randomized: int 109, cont 111 | Intervention: 2 servings/day soy foods versus usual diet | Method of assessment: computer-assisted assessment (% density) | |
| Analyzed: int 98, cont 103 | Total isoflavone dose: int ∼50 mg/day soy isoflavones (aglycone equivalents), cont unclear (usual diet) | ||
| Mean age (sd): int 43.2 (3.1), cont 42.8 (2.9) | |||
| Baseline risk: low | |||
| Maskarinec 2009 (OPUS), USA (Maskarinec et al., 2009) | Participants: early post-menopausal women aged 40–60 | Form: tablets | Duration: 104 weeks |
| Randomized: int A 136, int B 135, cont 135 | Intervention: 80 or 120 mg/day isoflavones from soy germ (isolated isoflavones) versus placebo tablets | Method of assessment: computer-assisted assessment by one assessor (% density) | |
| Analyzed (2 yrs): int A 109, int B 100, cont 116 | Total isoflavone dose: int A 120 mg/day isoflavones (aglycone equivalents), including 1.2 mg/day genistein, 15.6 mg/day genistin, 2.4 mg/day daidzein, 50.4 mg/day daidzein, 46.8 mg/day glyceitin, 3.6 mg/day glyceitein, int B 80 mg/day isoflavones, 2/3 content int A for subfractions, cont unclear | ||
| Mean age (sd): int A 54.7 (3.8), int B 55.2 (4.0), cont 54.8 (3.6) | |||
| Baseline risk: low | |||
| Powles 2008, UK (Powles et al., 2008) | Participants: pre-, post-, and peri-menopausal women aged 35–69 with a first degree relative with breast cancer | Form: tablet | Duration: 156 weeks |
| Randomized: int 199, cont 202 | Intervention: 1/day red clover isolate tablet, Promensil (isolated isoflavones) versus placebo tablet | Method of assessment: visual assessment (% density) | |
| Analyzed: int 119 (111 pre-, 8 post-menopausal), cont 112 (111 pre- and 11 post-menopausal) | Total isoflavone dose: 40 mg/day total isoflavones | ||
| Mean age: mean unclear, medians: int 45, cont 45 | |||
| Baseline risk: moderate | |||
| Tice 2009 (PREVENT), USA (Tice et al., 2005; Tice, 2006) | Participants: otherwise healthy premenopausal women with breast density ≥50% | Form: protein powder (taken in smoothies or added to other foods and drinks) | Duration: 26 weeks |
| Randomized: int 24, cont 23 | Intervention: 25 g/day soy protein (ISP) versus 25 g/day milk protein | Method of assessment: computer-assisted threshold method, using craniocaudal view (% density) | |
| Analyzed: int 20, cont 20 | Total isoflavone dose: int 50 mg/day isoflavones (aglycone equivalents), cont 0 mg/day isoflavones | ||
| Mean age (sd): int 44.8 (sd unclear), cont 44.6 (sd unclear) | |||
| Baseline risk: moderate | |||
| Verheus 2008 (Finesse) Netherlands (Kok et al., 2004, 2005a, b; Kreijkamp-Kaspers et al., 2005, 2009; Verheus et al., 2009) | Participants: healthy post-menopausal women aged 60–75 with a recent normal mammogram | Form: powder to be mixed with food or drink | Duration: 52 weeks |
| Randomized: int 100, cont 102 | Intervention: 25.6 g/day Solae soy protein (ISP) versus 25.6 g/day milk protein | Method of assessment: computer-assisted assessment (% density) | |
| Analyzed: int 70, cont 56 | Total isoflavone dose: int 99 mg/day isoflavones (aglycone units), including 52 mg/day genistein, 41 mg/day daidzein, 6 mg/day glyceitin, cont nil | ||
| Mean age (sd): int 66.3 (4.3), cont 65.3 (4.0) | |||
| Baseline risk: low |
ISP, isolated soy protein; Cont, control group; Int, intervention group; Isoflav, isoflavone/s; FP, food provided; DO, dropouts; sd, standard deviation; ISP, isolated soy protein; % density, percent density; SMF, Standard Mammogram Form, which is a fully automated volumetric computer method.
Table II.
Validity of included studies.*
| Study | Allocation concealment | Masking of participants | Masking of outcome assessors | Industry funding or involvement | Compliance assessed and reported | Reporting of withdrawals |
|---|---|---|---|---|---|---|
| Atkinson 2004 | Yes | Yes | Yes | Partly (some funding from non-industry sources, some from Novogen Ltd) | Done, urinary isoflavones | Partial |
| Marini 2008 | Unclear | Yes | Yes | Partly (some funding from non-industry sources, some from Primus Pharmaceuticals, two authors work for Primus Pharmaceuticals) | Done, serum genistein | Done |
| Maskarinec 2002 | Unclear | Yes | Yes | Yes, funding from Pharmavite Corporation | Done, urinary isoflavones and tablet count | Done |
| Maskarinec 2004 | Unclear | No | Yes | Partly (funding from non-industry source, food donations from Aloha Tofu, Dr Soy, Solae Company) | Done, urinary isoflavones, intake logs, 24 h recalls | Partial |
| Maskarinec 2009 OPUS | Unclear | Yes | Yes | Unclear (funded by NIH but unclear if study paid for the tablets or not) | Partly done, blood isoflavones and pill counts, results not reported | Done |
| Powles 2008 | Unclear | Yes | Unclear | Partly (funding from non-industry source, supplement supplied by Novogen Ltd) | Not done | Partial |
| Tice 2009 (PREVENT) | Yes | Yes | Yes | None (US Army) | Done, packet count at 6 mo. | Done |
| Verheus 2008 (Finesse) | Yes | Yes | Yes | Partly (some funding from non-industry sources, some from DuPont Protein Technologies) | Done, serum genistein, counts of powder bags, diary | Done |
*Trial quality characteristics assessed included: (i) allocation concealment (concealment of the ability of those recruiting participants to assess which arm participants will be randomized into before recruitment is complete, coded as adequate, unclear or inadequate); (ii) participant masking (concealment of the participants to whether they are part of the intervention or control condition, coded as ‘yes’ where there was a clear and realistic attempt to mask, ‘no’ where not, or ‘unclear’); (iii) outcome assessor blinding (concealment of the outcome assessor, here the reader of the mammogram, to whether participants are part of the intervention or control condition, coded as ‘yes’ where there was a clear and realistic attempt to mask, ‘no’ where not, or ‘unclear’); (iv) industry funding or involvement [level of financial involvement of industries that may have a financial interest in the study results, coded as yes (study mainly funded by industry, or at least one author is employed by industry), partly (other impartial funding, but includes some industry funding that may include free provision of supplements) or no]; (iv) compliance assessed and reported (measurement of the degree to which participants complied with taking the intervention and control foods or supplements, and reporting of these data, coded as ‘done’ when compliance was both assessed and reported, ‘partly done’ when it was assessed but not reported or reported without any indication of the method used, and ‘not done’ when neither was addressed adequately); and (v) reporting of withdrawals [numbers of withdrawals in each group clear and reasons reported coded as done, reported as ‘done’ when numbers randomized, completed and analysed all clear, plus reasons for dropouts given (by intervention arm), ‘partially done’ when some of the above, ‘not done’ when not].
Where a study provided data in several different ways the data were dealt with as follows for the main analysis: the data used were from all available participants (so that if there were two intervention groups and one control group the data from the two intervention groups were combined using the methods recommended in the Cochrane Handbook); from the longest duration available; and using the semi-automated system of assessment of breast density. In subgroup analyses, we used all available data; the Powles study (Powles et al., 2008) which provided data at 1, 2 and 3 years provided data into each of the three subgroups; the Atkinson study (Atkinson et al., 2004; Kataoka et al., 2008) which used three methods of assessment of breast density provided data into all three assessment type subgroups; and the OPUS study (Maskarinec et al., 2009) which provided two doses of isoflavones provided data to two dose subgroups, with the full control group used twice, so subgroups were not pooled.
The main analysis included all included trials with relevant outcome data, but data on no participants were included twice. Subgrouping was used to explore the effects of the following factors on breast density:
source of isoflavones (soy-based foods, soy protein, soy germ, isoflavones isolated from soy, isoflavones isolated from red clover, pure genistein, pure daidzein);
participants baseline risk of breast cancer (high, moderate or low);
isoflavone dose (in aglycone equivalents, <50, 50 to <100, 100+ mg/day)
duration of intervention (6 to <18, 18 to <30 and 30+ months); and
method of assessment of breast density (visual assessment, semi-automated and fully automated assessment).
It was intended that we would also subgroup by participants who were equol producers compared with those who were non-equol producers, however, there were insufficient data on breast density by equol producer status for this to be feasible. The equol producer phenotype is thought to be important as levels of this gut metabolite of the soy isoflavone daidzein have been inversely associated with breast cancer risk. In humans only 30–50% of the population harbour the bacteria capable of converting daidzein to equol, and this metabolite has biological activities that differ from its parent compound, e.g. relative binding affinity to estrogen receptors (Cassidy et al., 1994; Duncan et al., 1999; Lampe, 2009).
Sensitivity analysis was used to assess robustness of results. Sensitivity analyses were carried out to:
include all breast density measures using standardized mean difference analysis [which allowed the Marini data (Marini et al., 2008), not measured using percentage breast density but using image mean index (IMI) which cannot be translated into percent breast density, to be included],
exclude studies wholly funded by industry (Lexchin et al., 2003), and
use the P-values provided for the Powles study ((Powles et al., 2008), as the 95% CIs did not appear to correlate with the P-values provided in the same table. As we were not able to initiate discussion with the authors, both the CIs and the P-values were used to compute standard deviations used in the analyses (2008a), the P-value data were used as a sensitivity analysis).
Type and frequency of side effects, diagnosis of breast cancer, deaths and study withdrawals were tabulated and compared between different studies. Heterogeneity was assessed using Cochran's test and the I2 test (and assumed to be present when I2 > 50%; Higgins et al., 2003). Funnel plots were used to assess for evidence of publication bias (Egger et al., 1997). This manuscript follows the criteria suggested for systematic reviews in the PRISMA guidelines (Moher et al., 2009).
Results
Description of included studies
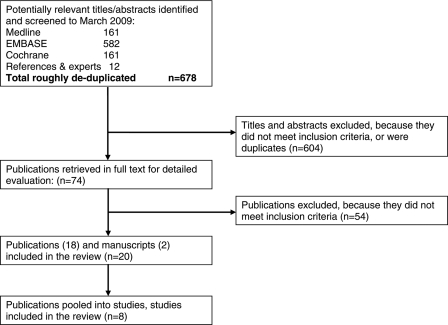
A total of 678 titles and abstracts were screened following the electronic and bibliographic searches. Of these 74 appeared potentially relevant and were ordered as full text papers to be assessed for inclusion independently in duplicate. Eight of these RCTs (published in 18 full text papers, one unpublished manuscript and an abstract) were included in the review (Fig. 1).
Figure 1.
Flowchart illustrating movement of papers from search to inclusion.
The eight included studies randomized 1904 women between them, and analysed data on 1287 after study durations of between 6 months and 3 years (Table I). Five of the studies included post-menopausal women, and five included premenopausal women (two studies included both groups). The baseline risk of breast cancer was low in most studies, moderate in one (including pre-, post- and peri-menopausal women with a first degree relative with breast cancer (Powles et al., 2008), and high in two studies (including premenopausal women with a Gail risk of at least 1.67 and >50% breast density at initial mammogram (Tice, 2006), and pre-, post- and peri-menopausal women with Wolfe P2 or DY mammographic patterns but without breast cancer (Atkinson et al., 2004). Four studies were conducted in Europe, four in the USA.
Two studies provided red clover-based isoflavone supplements, three soy-based isoflavone supplements (one pure genistein, one soy germ based isoflavones and one mixed soy isoflavones), one provided additional soy foods and two provided soy protein powder compared with milk protein powder. Isoflavone doses ranged from 40 to 120 mg/d. Control groups received other ‘inert’ or ill-defined substances low in isoflavones, advice to eat their usual diet or milk protein in place of soy protein.
All studies calculated mammographic percentage density except one (Marini et al., 2008) which calculated IMI, in arbitrary units. This outcome was assessed visually in one study, using a computer-assisted method in six and assessed using three methods (a visual method, a computer-assisted method and a fully computerized method) in one study.
All included studies were fully published in at least one journal article, except for the study by Tice which was informally published (Tice et al., 2005; Tice, 2006). The unpublished study by Tice was a double blinded, randomized trial of 6 months of daily soy protein containing 50 mg of isoflavones with a primary outcome of change in percentage mammographic breast density timed to the menopause cycle. For this trial data on the study were extracted from the study protocol and final report to the funders (Tice, 2006). As with other included studies the author was asked for further information.
Risk of bias
Allocation concealment was unclear in all studies except those by Atkinson, Verheus and Tice (Atkinson et al., 2004; Tice, 2006; Verheus et al., 2009), where it was clearly concealed. Attempts were made to mask participants in all studies except one (Maskarinec et al., 2004a, b), which was a food-based intervention. Masking of outcome assessors was attempted in all of the studies, except that by Powles et al. (2008) where masking of outcome assessors was unclear. Most studies were funded jointly by industry and non-industry sources, except for one of the Maskarinec trials (Maskarinec et al., 2003) which was funded solely by industry, the Tice study (Tice, 2006) which was exclusively funded by non-industry sources and OPUS (Maskarinec et al., 2009) which was unclear (though appeared to be mainly funded by non-industry sources). Most studies assessed compliance with the intervention and placebo, and reported results of this assessment, except that OPUS (Maskarinec et al., 2009) reported the method of assessment but not any results, and the Powles study (Powles et al., 2008) did not report either a methodology for assessing compliance or any results. Reporting of withdrawals was done for five studies and partially done for three (Atkinson et al., 2004; Maskarinec et al., 2004a, b; Powles et al., 2008).
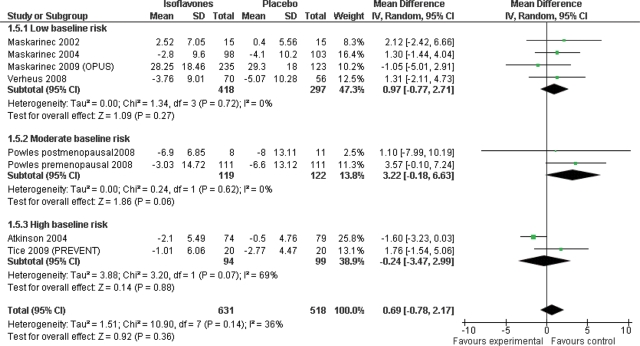
Effect of isoflavones on breast density
There was no evidence of an overall effect of isoflavones on percentage breast density from seven studies including 1149 participants for at least 6 months each (mean difference 0.69%, 95% CI −0.78 to 2.17) and no evidence of heterogeneity [Fig. 2; this analysis excluded the eighth study, that did not use percentage breast density as a measure (Marini et al., 2008)]. Sensitivity analyses including the Marini study [the study assessing breast density using a different measure (Marini et al., 2008)], or excluding studies wholly funded by industry, or using Powles P-value data [(Powles et al., 2008) rather than the CIs presented] similarly did not suggest any overall effect (Table III).
Figure 2.
Main analysis, subgrouping by baseline risk of breast cancer, using only percentage breast density data (mean difference analysis).
Table III.
Subgrouping and sensitivity analyses.
| Factor | Subgroup | Number of studies | Number of participants | Mean difference (95% CI) | P-value for heterogeneity, I2 |
|---|---|---|---|---|---|
| Main analysis | Overall analysis (no subgroups) | 7 | 1149 | 0.69 (−0.78 to 2.17) | 0.14, 36% |
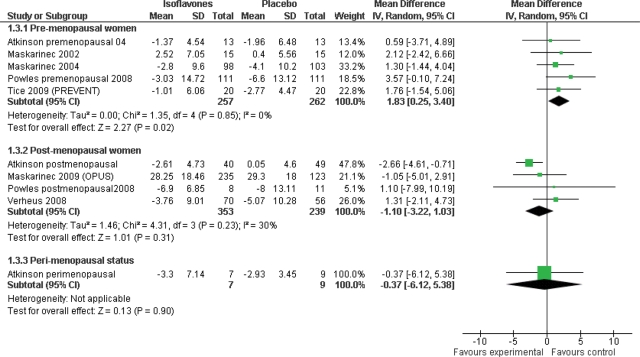
| Menopausal status* | Premenopausal | 5 | 519 | 1.83 (0.25 to 3.40) | 0.85, 0% |
| Post-menopausal | 4 | 592 | −1.10 (−3.22 to 1.03) | 0.23, 30% | |
| Peri-menopausal | 1 | 16 | −0.37 (−6.12 to 5.38) | NR | |
| Baseline risk of breast cancer | Low risk | 4 | 715 | 0.97 (−0.77 to 2.71) | 0.72, 0% |
| Moderate risk | 2 | 241 | 3.22 (−0.18 to 6.63) | 0.62, 0% | |
| High risk | 2 | 194 | −0.24 (−3.47 to 2.99) | 0.07, 69% | |
| Isoflavone source | Isolated red clover isoflavones | 3 | 394 | 0.70 (−3.37 to 4.76) | 0.04, 69% |
| Isolated soy isoflavones | 1 | 30 | 2.12 (−2.42 to 6.66) | NR | |
| Soy foods | 2 | 559 | 0.54 (−1.71 to 2.79) | 0.34, 0% | |
| Soy protein | 2 | 166 | 1.54 (−0.83 to 3.92) | 0.85, 0% | |
| Any soy intervention | 5 | 755 | 1.14 (−0.40 to 2.68) | 0.82, 0% | |
| Dose, mg/day total isoflavones** | <50 | 3 | 394 | 0.70 (−3.37 to 4.76) | 0.04, 69% |
| 50 to <100 | 5 | 635 | 0.90 (−0.74 to 2.54) | 0.36, 8% | |
| 100+ | 1 | 243 | 1.30 (−3.38 to 5.98) | NR | |
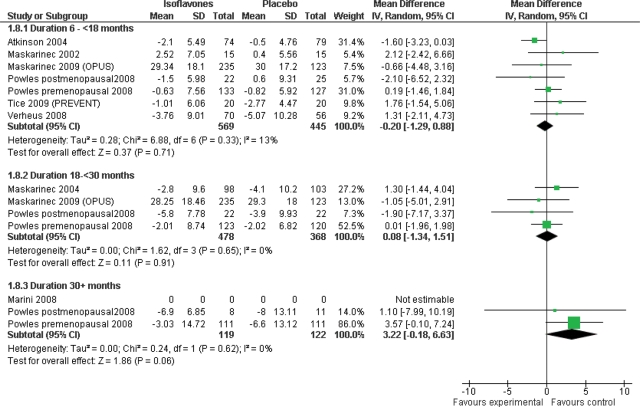
| Duration** | 6 to <18 months | 7 | 1014 | −0.20 (−1.29 to 0.88) | 0.33, 13% |
| 18 to <30 months | 4 | 846 | 0.08 (−1.34 to 1.51) | 0.65, 0% | |
| 30+ months | 2 | 241 | 3.22 (−0.18 to 6.63) | 0.62, 0% | |
| Type of density assessment** | Visual assessment | 1 | 160 | 0.70 (−2.95 to 4.35) | NR |
| Semi-automated | 7 | 1023 | 0.67 (−1.01 to 2.36) | 0.11, 43% | |
| Fully automated | 2 | 277 | −0.22 (−1.57 to 1.13) | 0.34, 0% | |
| Sensitivity analyses | |||||
| SMD analysis including all breast density measures | Overall analysis | 8 | 1287 | 0.06 (−0.09 to 0.21) | 0.09, 39% |
| Premenopausal | 4 | 479 | 0.21 (0.04 to 0.38) | 0.95, 0% | |
| Post-menopausal | 5 | 730 | −0.07 (−0.33 to 0.18) | 0.05, 57% | |
| Peri-menopausal | 1 | 16 | −0.07 (−1.05 to 0.92) | NR | |
| Excluding studies fully funded by industry | Overall analysis | 7 | 1119 | 0.60 (−1.00 to 2.19) | 0.12, 41% |
| Premenopausal | 4 | 489 | 1.79 (0.11 to 3.47) | 0.72, 0% | |
| Post-menopausal | 5 | 730 | −0.07 (−0.33 to 0.18) | 0.05, 57% | |
| Peri-menopausal | 1 | 16 | −0.07 (−1.05 to 0.92) | NR | |
| Using Powles P-value data | Overall analysis (no subgroups) | 7 | 1149 | 0.43 (−0.91 to 1.77) | 0.24, 23% |
| Premenopausal | 5 | 519 | 1.63 (−0.03 to 3.30) | 0.94, 0% | |
| Post-menopausal | 4 | 592 | −0.89 (−3.04 to 1.26) | 0.18, 38% | |
| Peri-menopausal | 1 | 16 | −0.37 (−6.12 to 5.38) | NR | |
CI, confidence intervals; NR, Not relevant (assessment of heterogeneity is not relevant when only one study is included).
*As the menopausal status of some of the Atkinson study participants was not known the total numbers of participants are smaller when subgrouped by menopausal status than in the overall analysis.
**In some studies data were measured at more than one time point or using more than one technique, so numbers do not add up to total numbers of study participants. One study used two different dose levels and as these arms fell in separate dose subgroupings the full control group was used once in each subgroup, increasing the apparent number of participants.
Analysis subgrouping by menopausal status resulted in an analysis that included slightly fewer women than were included in the overall analysis as some women from the Atkinson study had not been clearly allocated to a particular menopausal status. In premenopausal women isoflavone intake resulted in a modest increase in mammographic percent density compared with controls (mean difference 1.83%, 95% CI 0.25–3.40, n = 519, 5 trials) with no evidence of heterogeneity (P = 0.85, I2 = 0%; Fig. 3). In contrast, in post-menopausal women, isoflavone intervention had no effect on breast density (mean difference −1.10%, 95% CI −3.22 to 1.03, n = 592, 4 trials) and there was no evidence of heterogeneity (P = 0.23, I2 = 30%). There was no significant effect in the few peri-menopausal women (mean difference −0.37%, 95% CI −6.12 to 5.38, n = 16, 1 trial).
Figure 3.
Subgrouping by menopausal status, using only percentage breast density data (mean difference analysis).
Sensitivity analyses, using the P-value data from the Powles study (Powles et al., 2008), rather than the CI data (as these did not correspond, see the statistical analysis section of the Methodology above), resulted in marginal loss of statistical significance in this subgroup (P = 0.05). Including, or not, the Marini study data [(Marini et al., 2008), not expressed as breast density] did not alter these findings (the effects in premenopausal women were still statistically significant, see Table III). Removal of studies with some industry funding would remove almost all studies, but removal of the one study which was solely industry funded (Maskarinec et al., 2002) did not result in loss of the statistically significant effect of isoflavones on breast density in premenopausal women.
Subgrouping by duration suggested that there may possibly be an increase in breast density compared with controls in the long-term trials (3 years) although the significance was only marginal (mean difference 3.22%, 95% CI −0.18 to 6.63, P = 0.06, 2 trials including 241 participants, with no evidence of heterogeneity; Fig. 4).
Figure 4.
Subgrouping by study duration, only percentage breast density data (mean difference analysis).
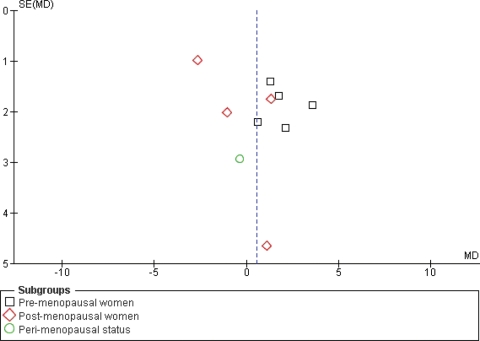
From the available evidence, there was little suggestion of differential effects of isoflavone source, isoflavone dose, baseline risk of breast cancer or type of assessment technique for breast density (Table III). The funnel plot was ineffective in assessing whether there was a risk of publication bias as most studies were of a similar size, and so the plot is difficult to interpret (Fig. 5).
Figure 5.
Funnel plot assessing risk of publication bias (plotting mean difference in breast density vs. the standard error of the mean difference).
Effect of isoflavones on breast cancer, dropouts and adverse events
As anticipated there were too few cases of breast cancer or deaths reported to draw conclusions on the effects of isoflavones on these outcomes (Table IV).
Table IV.
Breast cancer diagnoses, mortality and side effects in included studies.
| Study | Diagnoses of breast cancer during study |
Deaths from any cause during the study |
Reported side effects |
|||
|---|---|---|---|---|---|---|
| Isoflavone arm(s) | Placebo arm | Isoflavone arm(s) | Placebo arm | Isoflavone arm(s) | Placebo arm | |
| Atkinson 2004 | 1/102 | 0/103 | NR | NR | NR | NR |
| Marini 2008 | 0 | 0 | 0 | 0 | 37/150 GI complaint | 15/154 GI complaint |
| Maskarinec 2002 | NR | NR | NR | NR | NR | NR |
| Maskarinec 2004 | NR | NR | NR | NR | 0/108 adverse effects | 0/110 adverse effects |
| Maskarinec 2009 OPUS | NR | NR | NR | NR | Several dropped out due to GI complaints in both arms, but numbers unclear | |
| Powles 2008 | 3/199 | 5/202 | NR | NR | 9/111 GI complaint, 7/111 weight gain | 6/111 GI complaint, 13/111 weight gain |
| Tice 2009 PREVENT | 0/24 | 0/23 | 0/24 | 0/23 | 3/20 upset stomach, 3/20 constipation, 2/20 heartburn, 0/20 hot flashes, 1/20 diarrhoea | 4/20 upset stomach, 3/20 constipation, 1/20 heartburn, 2/20 hot flashes, 1/20 diarrhoea, |
| Verheus 2008 (Finesse) | 0 | 0 | 0 | 0 | 7/70 GI complaint | 8/56 GI complaint |
GI, gastrointestinal; NR, not reported.
Gastrointestinal complaints were commonly reported side effects, and meta-analysis of studies reporting dropouts due to gastrointestinal problems did not suggest a statistically significant difference between intervention and control arms (RR 1.49, 95% CI 0.69–3.24, 4 studies, 870 participants). However, there was strong heterogeneity between studies (P 0.07, I2 63%), which will have been accommodated through use of random effects meta-analysis. Individually, one study suggested significantly greater numbers of dropouts due to gastrointestinal problems in the intervention group (Marini et al., 2008), although the others suggested no significant differences (Maskarinec et al., 2004a, b; Powles et al., 2008; Verheus et al., 2009).
Other side effects were reported by Powles et al. (2008) (they noted breast abnormalities, weight gain, skin problems and lethargy, of which only weight gain appeared to be different between intervention and control arms—those on the intervention were less likely to report weight gain than those in the control group). Weight gain caused one participant to drop out of the control group of the Tice study (Tice, 2006), but did not affect other participants.
There was no significant difference in dropouts due to any cause between intervention and control arms (RR 1.14, 95% CI 0.96–1.35, 7 studies, 1870 participants, no significant heterogeneity).
Discussion
We included eight RCTs of isoflavones versus placebo or control diet with a duration of at least 6 months that assessed effects on breast density in women. These analyses included 1287 participants included in dietary intervention trials for between 6 months and 3 years. Although the available studies individually suggest that there is no effect overall on breast density following dietary isoflavone intervention, data from this meta-analysis provide evidence of a modest increase in breast density in premenopausal women taking isoflavones compared with control, and the effect may be greater in longer studies. These effects were small (a 1.83% increase in breast density compared with controls for premenopausal women) and although the clinical relevance of this potential relationship merits further investigation there are no immediate implications for practice.
In contrast, no significant effect was observed in post-menopausal women and to date only one trial has conducted analysis on a small number of peri-menopausal women (Atkinson et al., 2004; Kataoka et al., 2008). There were insufficient data to directly assess effects of isoflavones on breast cancer or mortality.
The doses of isoflavone fed in the trials ranged from 40 to 120 mg/day (aglycone equivalents). However, across this wide range in intake, there was little suggestion of differential effects by dose. The source of isoflavone did not significantly alter the observed effect.
The method used to assess breast density varied across the trials and this may affect the relative readings of breast densities in different groups. Kataoka assessed breast density using three separate techniques and found that the methods were not equivalent, confirming previous studies (Kataoka et al., 2008; Yaffe, 2008). We used the semi-automated assessment data from Atkinson and Kataoka within the main analyses (as this was the most common type of assessment across the different studies—this was chosen by a researcher blinded to the outcome measures), however, these suggest a larger effect of isoflavones on breast density than either of the other methods. Development of more robust methodology would be helpful in allowing assessment of effects in future studies (Yaffe, 2008).
Other dietary components and hormonal factors have previously been shown to modify breast density (Boyd et al., 1997, 2001; Greendale et al., 2003) and our previous systematic review of isoflavone and hormonal status suggested modest effects of isoflavones on gonadotrophins and estradiol in women (Hooper et al., 2009). However, to our knowledge this is the first systematic review of the available RCTs examining the effects of isoflavones on mammographic density.
In epidemiological settings, studies in high soy-consuming Asian populations suggest a reduction in breast cancer risk with increased habitual intake of soy; risk was lowest in those consuming ≥20 mg/day although the data were largely based on case–control studies and an inverse association was observed in both pre- and post-menopausal women (Wu et al., 2008). Recent data from Chinese breast cancer patients, where the range in soy intake is large enough to provide heterogeneity in isoflavone intake, showed that soy food consumption was significantly associated with decreased risk of death and recurrence and a linear dose–response effect was observed up to an isoflavone intake of 40 mg/day (Shu et al., 2009). However, these protective effects stem from consumption of traditional soy food and there is currently no epidemiological evidence for beneficial effects of dietary supplements derived from soy or red clover, such as were tested in many of our included studies.
The beneficial effects seen in observational studies may relate to lifetime or early life exposure to soy isoflavones, and the strongest and most consistent effect is related to childhood soy intake (Wu et al., 2002; Korde et al., 2009). Isoflavones may exert their potential protective effects early in life by stimulating breast cell differentiation (Lamartiniere, 2000; Lamartiniere et al., 2002) but these positive epidemiological data are in contrast to the conflicting experimental data from in vitro models and studies in animal models including the ovarectomized athymic nude mouse model implanted with MCF-7 cells (an estrogen-sensitive breast cancer cell line; Wang et al., 1996; Hsieh et al., 1998; Allred et al., 2001, 2004a, b; Ju et al., 2001; Kanno et al., 2003; Power et al., 2006a, b, 2007; Seo et al., 2006; Cline and Wood, 2009; Messina et al., 2009; Messina and Wu, 2009; Saarinen et al., 2009). Our findings from the available RCTs support the epidemiological data observed for post-menopausal women but not for premenopausal women as we observed a small increase in breast density following isoflavone consumption in the younger women.
Relationships exist between physical activity, body weight or BMI and breast density (Masala et al., 2009; Reeves et al., 2009) which might confound the relationship between isoflavones and breast density in epidemiological studies. However, there was no evidence of weight or BMI being consistently different in intervention and control groups in a way that could confound the relationship between isoflavone supplementation and breast density in our analyses of these dietary intervention trials. There was little information provided on physical activity, and although it is possible that individual relatively small studies may have been unbalanced with regards to physical activity, this is unlikely across the whole set of studies unless there was a specific bias in place. Despite the limitations of the included studies, their randomized controlled design will have controlled for any confounding by socioeconomic status, BMI, differential weight change, other health behaviours such as physical activity or interest in healthy eating for example, that may be manifest in epidemiological studies.
A further factor that may affect breast density readings is the timing of a mammogram within the menstrual cycle in premenopausal women. A review (Martin and Boyd, 2008) found little evidence of effects of steroid sex hormones on premenopausal mammographic density, whereas two more recent studies have found relationships between levels of endogenous hormones and breast density in premenopausal women (Walker et al., 2009; Yong et al., 2009). The effect of the menstrual cycle on breast density has also been studied directly. Several studies showed that variations in density during the cycle are small and not clinically important (Buist et al., 2006; Hovhannisyan et al., 2009), whereas Ursin et al. (2001) found that while changes were generally small, in some women they were more significant, suggesting that mammograms should be collected in the follicular phase of the cycle. This study echoed the findings of an earlier study (White et al., 1998) which was specific to women in their 40s. In our systematic review, of the six studies in premenopausal women menstrual cycle phase was mentioned only in one (Tice, 2006), in which the final report stated that mammograms were collected during Days 7–13 of the cycle. In three studies it was unlikely that phase had been taken into account as mammograms were taken as part of normal screening activities (Atkinson et al., 2004; Maskarinec et al., 2002, 2004a, b), and no details of timing were provided in the others. If phase of the menstrual cycle is important in breast density then lack of timing of mammograms is likely to make it more difficult to see any changes in breast density that may occur differentially over time.
Overall, the studies included were of moderate risk of bias. Whereas masking, assessment of compliance and reporting of withdrawals were generally carried out appropriately and well reported, allocation concealment was unclear in all but two trials, and industry at least partly funded almost all of the studies. Allocation concealment has been associated with bias in a pooled analysis of methodological studies, suggesting that inadequate or unclear reporting of allocation concealment treatments were 18% more beneficial than in studies with adequate allocation concealment (Pildal et al., 2007). However, this bias appears to be manifest in studies with subjective outcomes, and not objective ones, suggesting that the masking of outcome assessors to allocation, and method of assessment of breast density may be important in study outcomes (Wood et al., 2008). The predominance of industry funding for the studies may be expected to potentially lead to exaggerated suggestions of effectiveness, minimized suggestions of risk or publication bias (Lesser et al., 2007). Data from the Powles study were difficult to interpret as the 95% CIs presented did not correspond with the P-values presented for the same effect, and we were unable to elicit any discussion on this with the authors (Powles et al., 2008). As a result we ran the analysis first using the 95% CIs (as these were the most accessible), then ran a sensitivity analysis using the data from the P-values. The observed effect in premenopausal women was attenuated when the data from P-values, rather than 95% CIs, were used in analysis, but the pooled effect was still apparent (Table III, the effect in premenopausal women became a mean difference of 1.63% (95% CI −0.03 to 3.30, P = 0.05)).
Conclusions
Implications for practice
Isoflavones from different sources had no effect on breast density in post-menopausal women and a small effect in premenopausal women. The average 5 year absolute risk for breast cancer for a 50-year-old woman in the USA is 1.3%. The relative risk of breast cancer for a women with a breast density of 5–24% (∼15%), compared with a woman with a breast density of <5% (∼3%), is 1.79 (95% CI 1.48–2.16; McCormack and dos Santos Silva, 2006). As there appears to be an approximately linear relationship between RR and breast density moving from 3% density to 15% density is associated with a RR of 1.79, and increasing breast density by 1.83% would equate with a RR of 1.12. The 5 year risk for the average woman would therefore move from 1.3 to 1.46%. This does not seem to be of sufficient magnitude to justify any recommendation regarding isoflavone foods or supplements. Although the clinical relevance of this potential relationship merits further investigation there are no immediate implications for practice.
Implications for research
In the absence of sufficient data on the effects of isoflavones on breast cancer diagnoses it would be helpful to assess effects of isoflavones on breast density in premenopausal women in a large and high quality RCT of at least 4 years duration and which assesses breast density at a consistent point in the menstrual cycle.
Authors' roles
The protocol was drafted by G.M., L.H. and A.C., and all authors contributed to, and agreed, the final protocol. G.M. and L.H. ran the searches, assessed titles and abstracts for collection, collected relevant full text papers, assessed these for inclusion, merged papers into studies, extracted the data, assessed validity, wrote to authors for further information, collated data into tables and ran the analyses in RevMan. L.H. and A.C. prepared the first draft of the paper, and all authors contributed significantly to data interpretation, and preparation of the second draft of the manuscript. All authors agreed the final draft. L.H. is the guarantor of the study.
Funding
Funding to pay the Open Access publication charges for this article was provided by the University of East Anglia.
Acknowledgements
The following authors of included studies provided additional clarification of data and quality issues: Charlotte Atkinson, Masako Kataoka, Gertraud Maskarinec, Yvonne van der Schouw, Francesco Squadrito. Thanks also to the referees who commented and offered their expertise.
References
- Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Clausen LM, Doerge DR, Schantz SL, Korol DL, Wallig MA, Helferich WG. Dietary genistein results in larger MNU-induced, estrogen-dependent mammary tumors following ovariectomy of Sprague-Dawley rats. Carcinogenesis. 2004a;25:211–218. doi: 10.1093/carcin/bgg198. [DOI] [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Goeppinger TS, Doerge DR, Helferich WG. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004b;25:1649–1657. doi: 10.1093/carcin/bgh178. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, Healey CS, Runswick S, Day NE, Bingham SA. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial. Breast Cancer Res. 2004;6:R170–R179. doi: 10.1186/bcr773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atteritano M, Mazzaferro S, Frisina A, Cannata ML, Bitto A, D'Anna R, Squadrito F, Macri I, Frisina N, Buemi M. Genistein effects on quantitative ultrasound parameters and bone mineral density in osteopenic postmenopausal women. Osteoporos Int. 2009;20:1947–1954. doi: 10.1007/s00198-009-0883-4. [DOI] [PubMed] [Google Scholar]
- Balk E, Chung M, Chew P, Ip S, Raman G, Kupelnick B, Tatsioni A, Sun Y, Wolk B, DeVine D., et al. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Effects of Soy on Health Outcomes. Evidence Report/Technology Assessment No. 126. (Prepared by Tufts-New England Medical Center Evidence-based Practice Center under Contract No. 290-02-0022.) AHRQ Publication No. 05-E024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Kaaks R. Exogenous and endogenous hormones, mammographic density and breast cancer risk: can mammographic density be considered an intermediate marker of risk? Recent Results Cancer Res. 2009;181:135–157. doi: 10.1007/978-3-540-69297-3_14. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Greenberg C, Lockwood GA, Little L, Martin L, Byng J, Yaffe M, Tritchler D. Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. J Natl Cancer Inst. 1997;89:488–496. doi: 10.1093/jnci/89.7.488. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–321. doi: 10.1007/s11912-001-0083-7. [DOI] [PubMed] [Google Scholar]
- Buist DS, Aiello EJ, Miglioretti DL, White E. Mammographic breast density, dense area, and breast area differences by phase in the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2006;15:2303–2306. doi: 10.1158/1055-9965.EPI-06-0475. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Setchell KD, Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–340. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- Cline JM, Wood CE. Estrogen/isoflavone interactions in cynomolgus macaques (Macaca fascicularis) Am J Primatol. 2009;71:722–731. doi: 10.1002/ajp.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst. 2009;101:384–398. doi: 10.1093/jnci/djp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna R, Cannata ML, Marini H, Atteritano M, Cancielleri F, Corrado F, Triolo O, Rizzo P, Russo S, Gaudio A, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 2-year randomized, double-blind, placebo-controlled study. Menopause. 2009;16:301–306. doi: 10.1097/gme.0b013e318186d7e2. [DOI] [PubMed] [Google Scholar]
- Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999;84:192–197. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endogenous Hormones Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer postmenopausal women: reanalysis of nine prospective studies. J Nat Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Stone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S Cochrane Handbook for Systematic Reviews of Interventions version 5.0.1 [updated September 2008] The Cochrane Collaboration. 2008a Available at www.cochrane-handbook.org . [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovhannisyan G, Chow L, Schlosser A, Yaffe MJ, Boyd NF, Martin LJ. Differences in measured mammographic density in the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2009;18:1993–1999. doi: 10.1158/1055-9965.EPI-09-0074. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Santell RC, Haslem SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- Kanno J, Onyon L, Peddada S, Ashby J. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dose–response studies. Environ Health Perspect. 2003;111:1530–1549. doi: 10.1289/ehp.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M, Atkinson C, Warren RM, Sala E, Day NE, Highnam R, Warsi I, Bingham SA. Mammographic density using two computer-based methods in an isoflavone trial. Maturitas. 2008;59:350–357. doi: 10.1016/j.maturitas.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Kok L, Kreijkamp-Kaspers S, Grobbee DE, van der Schouw YT. Design and baseline characteristics of a trial on health effects of soy protein with isoflavones in postmenopausal women. Maturitas. 2004;47:21–29. doi: 10.1016/s0378-5122(03)00243-3. [DOI] [PubMed] [Google Scholar]
- Kok L, Kreijkamp-Kaspers S, Grobbee DE, Lampe JW, van der Schouw YT. Soy isoflavones, body composition, and physical performance. Maturitas. 2005a;52:102–110. doi: 10.1016/j.maturitas.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kok L, Kreijkamp-Kaspers S, Grobbee DE, Lampe JW, van der Schouw YT. A randomized, placebo-controlled trial on the effects of soy protein containing isoflavones on quality of life in postmenopausal women. Menopause. 2005b;12:56–62. doi: 10.1097/00042192-200512010-00011. [DOI] [PubMed] [Google Scholar]
- Korde LA, Wu AH, Fears T, Nomura AM, West DW, Kolonel LN, Pike MC, Hoover RN, Ziegler RG. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–1059. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005;81:189–195. doi: 10.1093/ajcn/81.1.189. [DOI] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. J Am Med Assoc. 2009;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr. 2000;71:1705S–1707S. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Murrill WB, Manzolillo PA, Zhang JX, Barnes S, Zhang X, Wei H, Brown NM. Genistein alters the ontogeny of mammary gland development and protects against chemically-induced mammary cancer in rats. Proc Soc Exp Biol Med. 1998;217:358–364. doi: 10.3181/00379727-217-44245. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser LI, Ebbeling CB, Goozner M, Wypig D, Ludwig DS. Relationship between funding source and conclusion among nutrition-related scientific articles. PLoS Med. 2007;4:e5. doi: 10.1371/journal.pmed.0040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. Br Med J. 2003;326:1167–1170. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stephano V, Minutoli L, Atteritano M, Levy RM, D'Anna R, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787–4796. doi: 10.1210/jc.2008-1087. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Boyd NF. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masala G, Assedi M, Ambrogetti D, Sera F, Salvini S, Bendinelli B, Ermini I, Georgi D, Rosselli del Turco M, Palli D. Physical activity and mammographic breast density in a Mediterranean population: the EPIC Florence longitudinal study. Int J Cancer. 2009;124:1654–1661. doi: 10.1002/ijc.24099. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:195–201. [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165–169. doi: 10.1097/00008469-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004a;13:1736–1744. [PubMed] [Google Scholar]
- Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004b;134:3089–3094. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK, Lewis RD, Murray MJ, Young RL, Wong WW. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:1–6. doi: 10.3945/jn.108.102913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I. Breast Density and Parenchymal Patterns as Markers of Breast Cancer Risk: A Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- Messina M. The safety and benefits of soybean isoflavones. A natural alternative to conventional hormone therapy? Menopause. 2007;14:958. doi: 10.1097/gme.0b013e31812e5258. [DOI] [PubMed] [Google Scholar]
- Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr. 2009;89:1673S–1679S. doi: 10.3945/ajcn.2009.26736V. [DOI] [PubMed] [Google Scholar]
- Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- Messina M, Watanabe S, Setchell KD. Report on the 8th International Symposium on the Role of Soy in Health Promotion and Chronic Disease Prevention and Treatment. J Nutr. 2009;139:796S–802S. doi: 10.3945/jn.108.104182. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen-Damdimopoulou PE, Power KA, Hurmerinta TT, Nurmi T, van der Saag PT, Makela SI. Dietary sources of lignans and isoflavones modulate responses to estradiol in estrogen reporter mice. Mol Nutr Food Res. 2009;53:996–1006. doi: 10.1002/mnfr.200800487. [DOI] [PubMed] [Google Scholar]
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol. 2007;36:847–857. doi: 10.1093/ije/dym087. [DOI] [PubMed] [Google Scholar]
- Power KA, Saarinen NM, Chen JM, Thompson LU. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int J Cancer. 2006a;118:1316–1320. doi: 10.1002/ijc.21464. [DOI] [PubMed] [Google Scholar]
- Power KA, Ward WE, Chen JM, Saarinen NM, Thompson LU. Genistein alone and in combination with the mammalian lignans enterolactone and enterodiol induce estrogenic effects on bone and uterus in a postmenopausal breast cancer mouse model. Bone. 2006b;39:117–124. doi: 10.1016/j.bone.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Power KA, Ward WE, Chen JM, Saarinen NM, Thompson LU. Flaxseed and soy protein isolate, alone and in combination, differ in their effect on bone mass, biomechanical strength, and uterus in ovariectomized nude micewith MCF-7 human breast tumor xenografts. J Toxicol Environ Health A. 2007;70:1888–1896. doi: 10.1080/15287390701549179. [DOI] [PubMed] [Google Scholar]
- Powles TJ, Howell A, Evans DG, McCloskey EV, Ashley S, Greenhalgh R, Affen J, Flook LA, Tidy A. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int. 2008;14:6–12. doi: 10.1258/mi.2007.007033. [DOI] [PubMed] [Google Scholar]
- Reeves KW, Stone RA, Modugno F, Ness RB, Vogel VG, Weissfield JL, Habel LA, Sternfeld B, Cauley JA. Longitudinal association of anthropometry with mammographic breast density in the Study of Women's Health Across the Nation. Int J Cancer. 2009;124:1169–1177. doi: 10.1002/ijc.23996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer program] Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration; 2008. Version 5.0. [Google Scholar]
- Saarinen NM, Power KA, Chen JM, Thompson LU. Flaxseed attenuates the tumor growth stimulating effect of soy protein in ovariectomized athymic mice with MCF-7 human breast cancer xenografts. Int J Cancer. 2009;119:925–931. doi: 10.1002/ijc.21898. [DOI] [PubMed] [Google Scholar]
- Seo HS, DeNardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99:121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Cassidy A. Dietary isoflavones: Biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy Food Intake and Breast Cancer Survival. J Am Med Assoc. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempfer CB, Froese G, Heinze G, Bentz E-K, Hefler LA, Huber JC. Side Effects of Phytoestrogens: A Meta-analysis of Randomized Trials. Am J Med. 2009;122:939–946. doi: 10.1016/j.amjmed.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Tice JA. Soy for breast cancer prevention in high risk pre-menopausal women: Final report for the US Army Medical Research and Material Command. 2006. Storming Media, at www.stormingmediaus/59/5920/A592064html .
- Tice JA, Guthrie N, Shepherd J, Kerlikowske K, Esserman L. Soy for the Prevention of Breast Cancer—a randomized trial; 8–11 June 2005; Era of Hope meeting Baltimore, MD. [Google Scholar]
- Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- Ursin G, Parisky YR, Pike MC, Spicer DV. Mammographic density changes during the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2001;10:141–142. [PubMed] [Google Scholar]
- Vachon CM, Van Gils CH, Sellers TA, Ghosh K, Pruthi S, Brandt KR, Pankratz VS. Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res. 2007;9:217. doi: 10.1186/bcr1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheus M, Van Gils CH, Kreijkamp-Kaspers S, Kok L, Peeters PHM, Grobbee DE, van der Schouw YT. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;17:2632–2638. doi: 10.1158/1055-9965.EPI-08-0344. [DOI] [PubMed] [Google Scholar]
- Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, Gibson L, Hillier SG, Holly JM, Moss S, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69:6490–6499. doi: 10.1158/0008-5472.CAN-09-0280. [DOI] [PubMed] [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- White E, Velentgas P, Mandelson MT, Lehman CD, Elmore JG, Porter P, Yasui Y, Taplin SH. Variation in mammographic breast density by time in menstrual cycle among women aged 40–49 years. J Natl Cancer Inst. 1998;90:906–910. doi: 10.1093/jnci/90.12.906. [DOI] [PubMed] [Google Scholar]
- Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Wood L, Egger M, Gluud LL, Schultz K, Juni P, Altman DG, Gluud C, Martin RM, Wood AJG, Sterne JAC. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. Br Med J. 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe MJ. Mammographic density. Measurement of mammographic density. Breast Cancer Res. 2008;10:209. doi: 10.1186/bcr2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong M, Atkinson C, Newton KM, iello Bowles EJ, Stanczyk FZ, Westerlind KC, Holt VL, Schwartz SM, Leisenring WM, Lampe JW. Associations between endogenous sex hormone levels and mammographic and bone densities in premenopausal women. Cancer Causes Control. 2009;20:1039–1053. doi: 10.1007/s10552-009-9321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]