Abstract
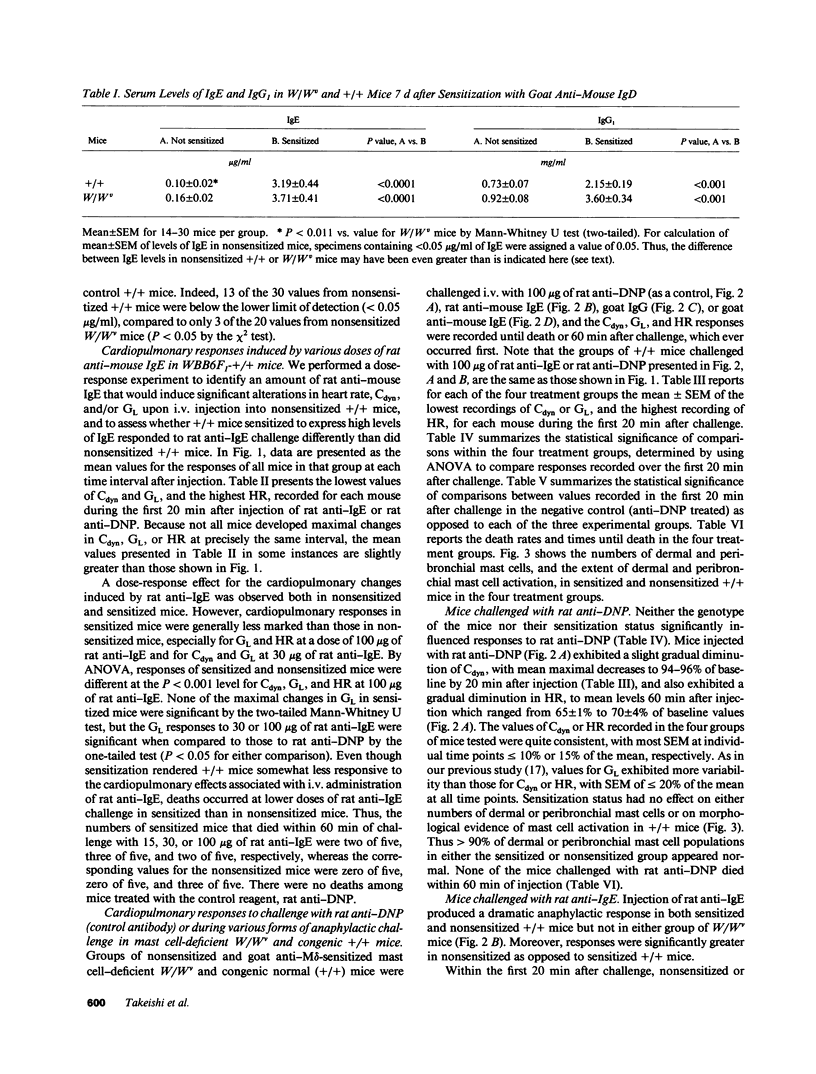
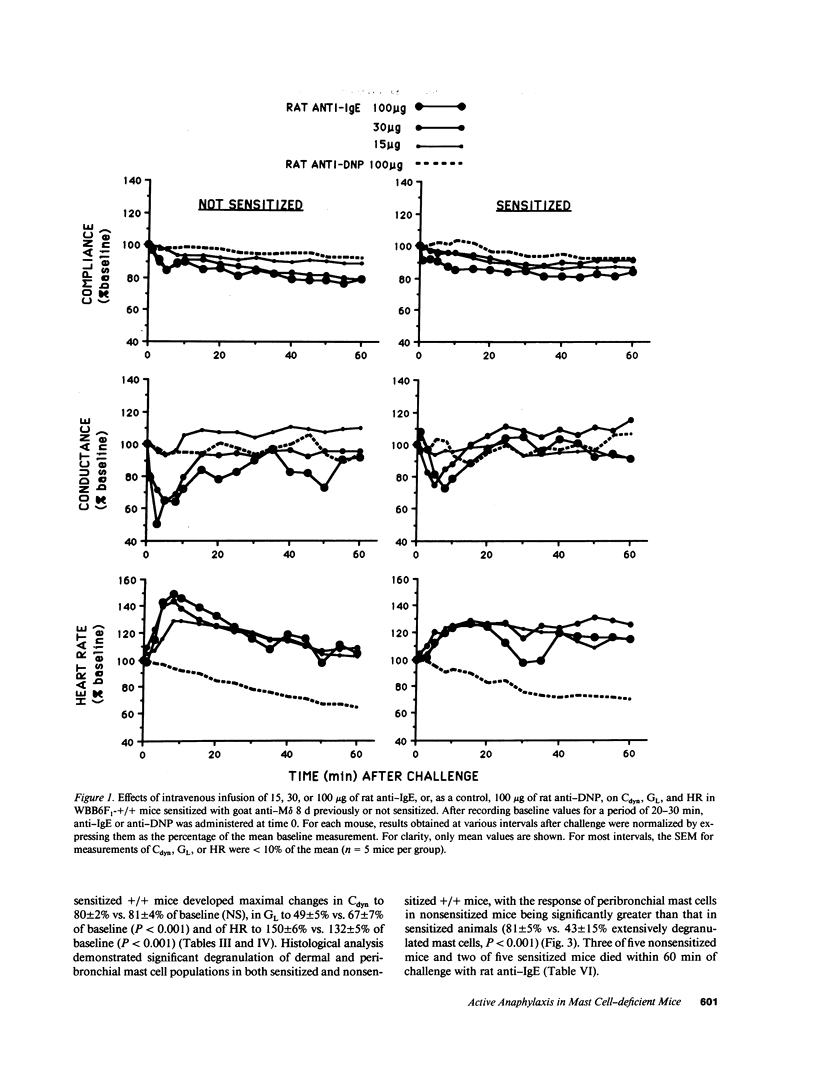
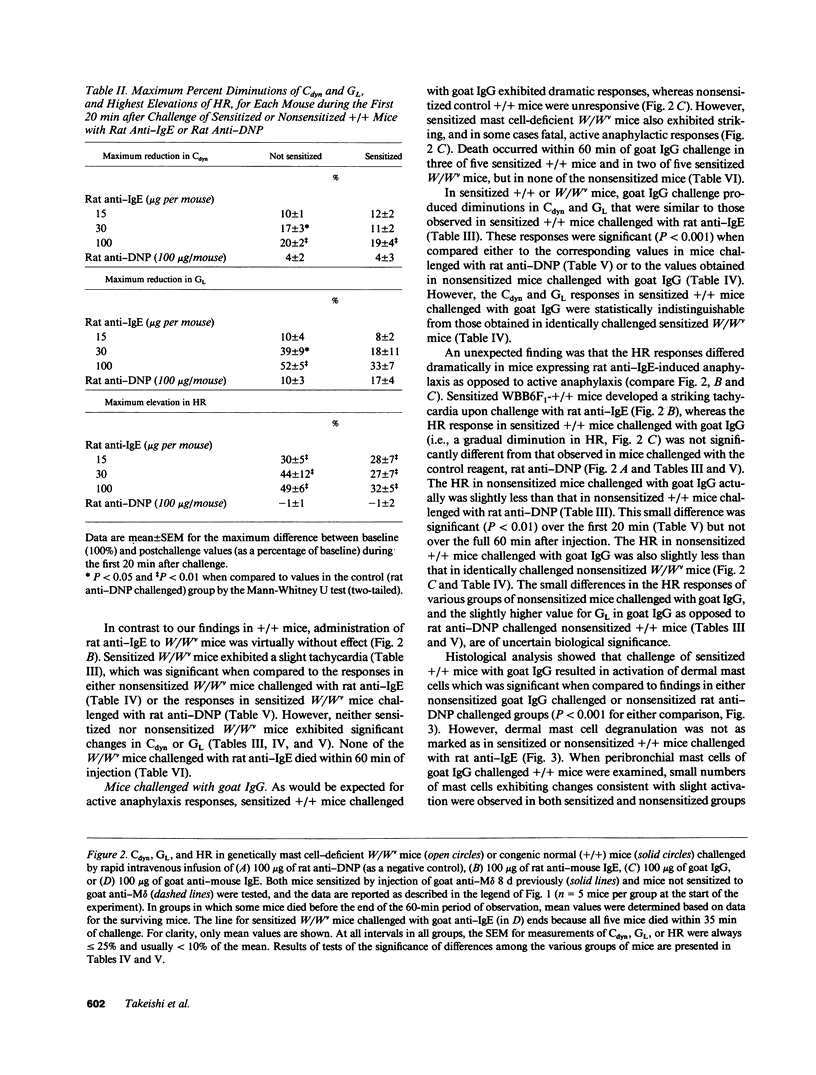
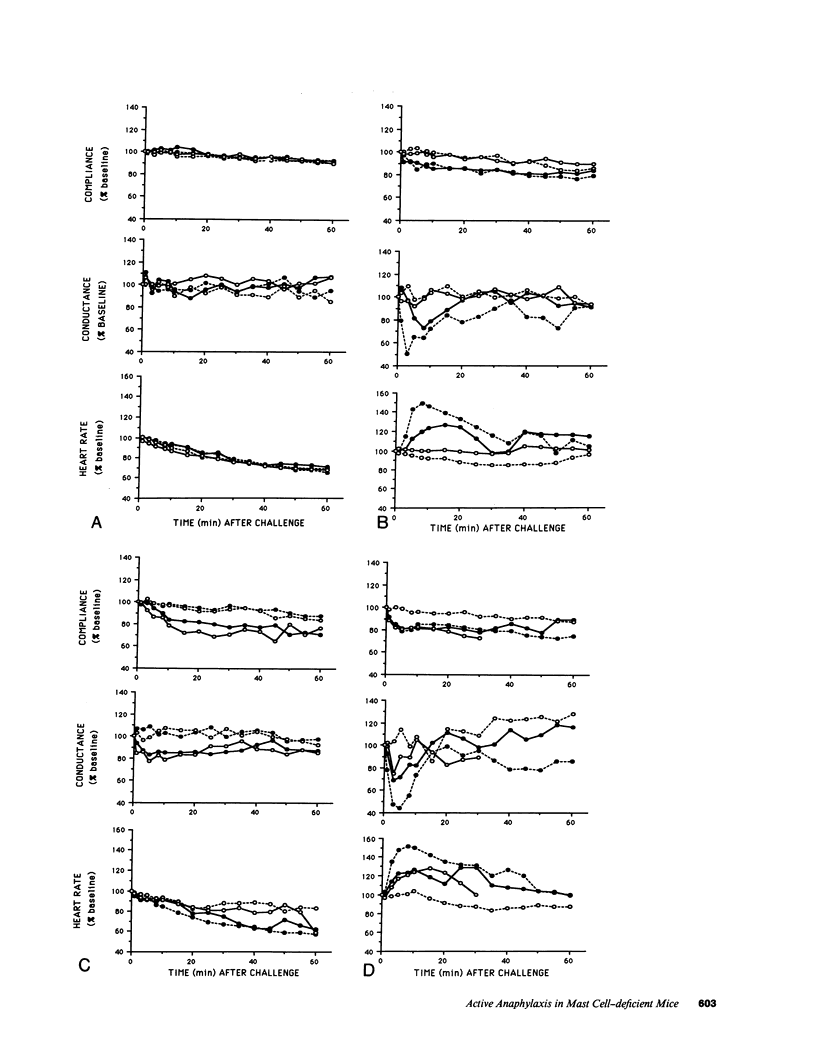
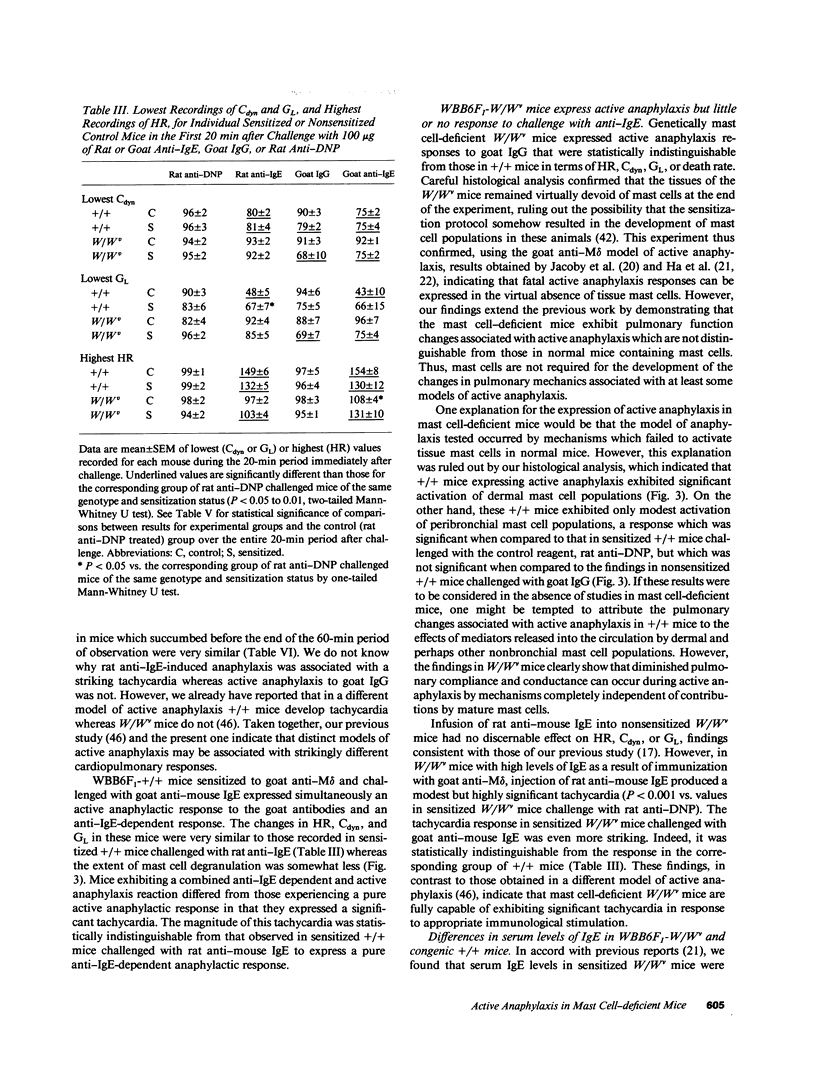
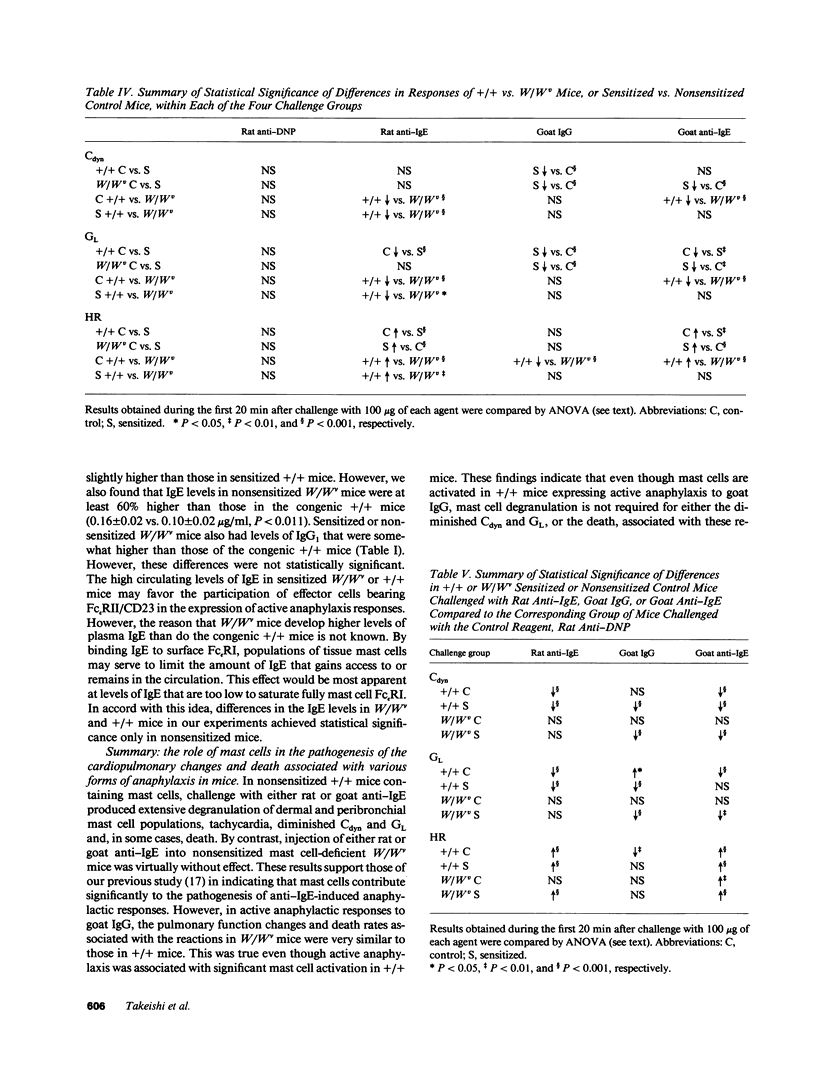
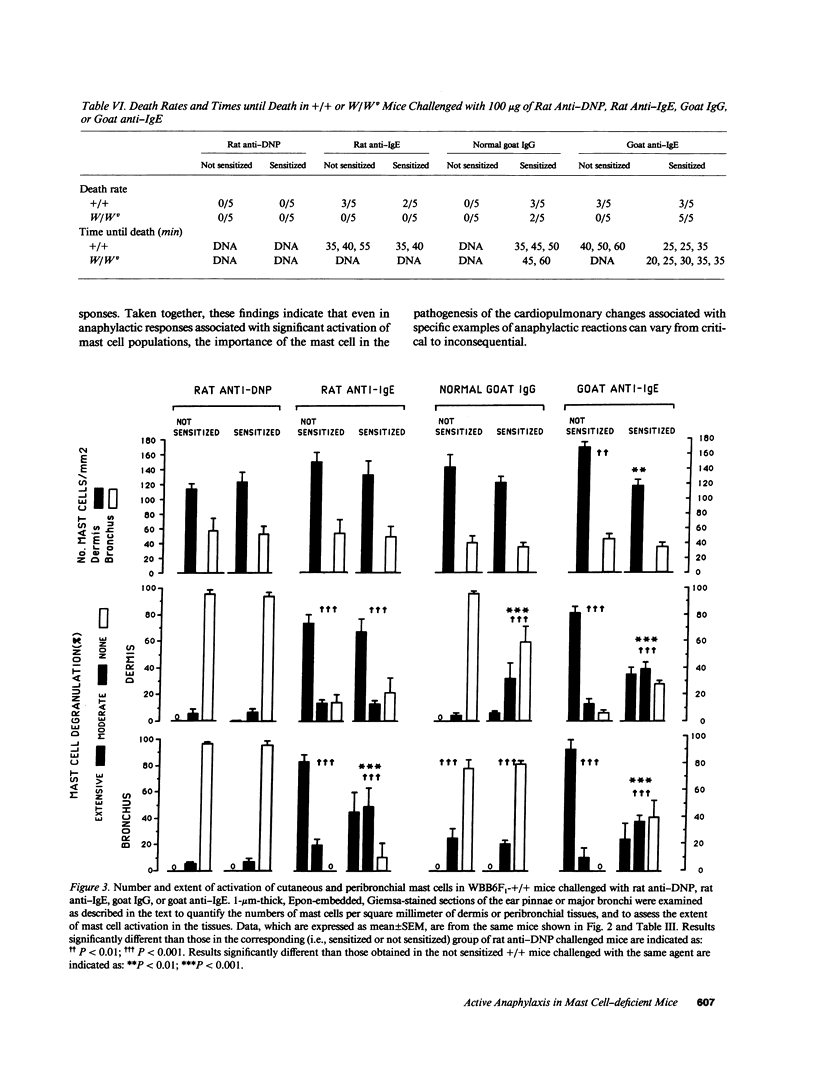
We compared the changes in heart rate (HR), pulmonary dynamic compliance (Cdyn), and pulmonary conductance (GL) associated with three different models of anaphylaxis in genetically mast cell-deficient WBB6F1-W/Wv and congenic normal (+/+) mice. Intravenous infusion of a monoclonal rat anti-mouse IgE produced a marked tachycardia, diminutions in Cdyn and GL, and death in +/+ but not W/Wv mice, and +/+ mice sensitized to develop high circulating levels of IgE exhibited HR, Cdyn, and GL responses to rat anti-IgE challenge which were significantly less intense than those in nonimmunized +/+ mice. By contrast, virtually identical cardiopulmonary responses were observed in either +/+ or W/Wv mice challenged to elicit pure active anaphylactic responses or simultaneous active and anti-IgE-dependent anaphylaxis. These findings show that anaphylactic responses associated with significant tachycardia, reductions in Cdyn and GL, and death can occur in the virtual absence of tissue mast cells. This is true even though, in normal mice, such responses are associated with extensive degranulation of tissue mast cells. By contrast, certain models of anaphylaxis, such as that induced in nonsensitized mice by anti-mouse IgE, can not be elicited in the absence of mast cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMDUR M. O., MEAD J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol. 1958 Feb;192(2):364–368. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- Baniyash M., Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur J Immunol. 1984 Sep;14(9):799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- Burrows B., Martinez F. D., Halonen M., Barbee R. A., Cline M. G. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989 Feb 2;320(5):271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- Capron A., Capron M., Grangette C., Dessaint J. P. IgE and inflammatory cells. Ciba Found Symp. 1989;147:153–170. doi: 10.1002/9780470513866.ch10. [DOI] [PubMed] [Google Scholar]
- Conrad D. H. Fc epsilon RII/CD23: the low affinity receptor for IgE. Annu Rev Immunol. 1990;8:623–645. doi: 10.1146/annurev.iy.08.040190.003203. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Donnell M. Pulmonary mechanics in normal rats. J Appl Physiol Respir Environ Exerc Physiol. 1977 Dec;43(6):942–948. doi: 10.1152/jappl.1977.43.6.942. [DOI] [PubMed] [Google Scholar]
- Drazen J. M., Austen K. F. Leukotrienes and airway responses. Am Rev Respir Dis. 1987 Oct;136(4):985–998. doi: 10.1164/ajrccm/136.4.985. [DOI] [PubMed] [Google Scholar]
- EINBINDER J. M., WALZER R. A., NELSON C. T. THE ROLE OF THE MAST CELL IN ANAPHYLAXIS IN THE MOUSE. J Immunol. 1964 Jul;93:165–175. [PubMed] [Google Scholar]
- Finkelman F. D., Kessler S. W., Mushinski J. F., Potter M. IgD-secreting murine plasmacytomas: identification and partial characterization of two IgD myeloma proteins. J Immunol. 1981 Feb;126(2):680–687. [PubMed] [Google Scholar]
- Finkelman F. D., Scher I., Mond J. J., Kessler S., Kung J. T., Metcalf E. S. Polyclonal activation of the murine immune system by an antibody to IgD. II. Generation of polyclonal antibody production and cells with surface IgG. J Immunol. 1982 Aug;129(2):638–646. [PubMed] [Google Scholar]
- Finkelman F. D., Smith J., Villacreses N., Metcalf E. S. Polyclonal activation of the murine immune system by an antibody to IgD. VI. Influence of doses of goat anti-mouse delta chain and normal goat IgG on B lymphocyte proliferation and differentiation. Eur J Immunol. 1985 Apr;15(4):315–320. doi: 10.1002/eji.1830150402. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Snapper C. M., Mountz J. D., Katona I. M. Polyclonal activation of the murine immune system by a goat antibody to mouse IgD. IX. Induction of a polyclonal IgE response. J Immunol. 1987 May 1;138(9):2826–2830. [PubMed] [Google Scholar]
- Froese A. Receptors for IgE on mast cells and basophils. Prog Allergy. 1984;34:142–187. [PubMed] [Google Scholar]
- Galli S. J., Arizono N., Murakami T., Dvorak A. M., Fox J. G. Development of large numbers of mast cells at sites of idiopathic chronic dermatitis in genetically mast cell-deficient WBB6F1-W/Wv mice. Blood. 1987 Jun;69(6):1661–1666. [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D., Crowle P. K. Immune response potential of mast cell-deficient W/Wv mice. Int Arch Allergy Appl Immunol. 1986;80(1):85–94. doi: 10.1159/000234031. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Reed N. D. Systemic anaphylaxis in mast-cell-deficient mice of W/Wv and Sl/Sld genotypes. Exp Cell Biol. 1987;55(2):63–68. doi: 10.1159/000163399. [DOI] [PubMed] [Google Scholar]
- Hanley S. P. Prostaglandins and the lung. Lung. 1986;164(2):65–77. doi: 10.1007/BF02713630. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Jacoby W., Cammarata P. V., Findlay S., Pincus S. H. Anaphylaxis in mast cell-deficient mice. J Invest Dermatol. 1984 Oct;83(4):302–304. doi: 10.1111/1523-1747.ep12340431. [DOI] [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Scher I., Kanellopoulos-Langevin C., Finkelman F. D. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J Immunol. 1983 Jan;130(1):350–356. [PubMed] [Google Scholar]
- Kikutani H., Yokota A., Uchibayashi N., Yukawa K., Tanaka T., Sugiyama K., Barsumian E. L., Suemura M., Kishimoto T. Structure and function of Fc epsilon receptor II (Fc epsilon RII/CD23): a point of contact between the effector phase of allergy and B cell differentiation. Ciba Found Symp. 1989;147:23–35. doi: 10.1002/9780470513866.ch3. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Martin T. R., Galli S. J., Katona I. M., Drazen J. M. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice. J Clin Invest. 1989 Apr;83(4):1375–1383. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Gerard N. P., Galli S. J., Drazen J. M. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol (1985) 1988 Jun;64(6):2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Kaliner M., Donlon M. A. The mast cell. Crit Rev Immunol. 1981 Sep;3(1):23–74. [PubMed] [Google Scholar]
- Metzger H., Kinet J. P., Blank U., Miller L., Ra C. The receptor with high affinity for IgE. Ciba Found Symp. 1989;147:93–113. [PubMed] [Google Scholar]
- Pepys J., Hargreave F. E., Chan M., McCarthy D. S. Inhibitory effects of disodium cromoglycate on allergen-inhalation tests. Lancet. 1968 Jul 20;2(7560):134–137. doi: 10.1016/s0140-6736(68)90419-4. [DOI] [PubMed] [Google Scholar]
- Piper P. J. Formation and actions of leukotrienes. Physiol Rev. 1984 Apr;64(2):744–761. doi: 10.1152/physrev.1984.64.2.744. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Dvorak A. M., Sharkis S. J., Kagey-Sobotka A., Niv Y., Finkelman F. D., Barbieri S. A., Galli S. J., Plaut M. Mouse splenic and bone marrow cell populations that express high-affinity Fc epsilon receptors and produce interleukin 4 are highly enriched in basophils. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois P., Borgeat P. From slow reacting substance of anaphylaxis (SRS-A) to leukotriene D4 (LTD4). Int J Immunopharmacol. 1980;2(4):281–293. doi: 10.1016/0192-0561(80)90028-4. [DOI] [PubMed] [Google Scholar]
- Wershil B. K., Murakami T., Galli S. J. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988 Apr 1;140(7):2356–2360. [PubMed] [Google Scholar]
- Wershil B. K., Wang Z. S., Gordon J. R., Galli S. J. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991 Feb;87(2):446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]



