Abstract
The current study examined the concentration of mothers’ peripherally produced oxytocin after close physical interactions with their biological and non-biological children. Each of 35 mothers and children participated in a computer game that promoted physical contact. In one interaction context, mothers interacted with their own children, and in the other context, mothers interacted with unfamiliar children. After the activity, urine samples were collected from the mothers and were assayed for oxytocin. Data from 26 mothers were available for oxytocin analyses. Oxytocin levels were higher among mothers following interactions with unfamiliar children than following interactions with their own children. Possible explanations for the differences in oxytocin levels across contexts are discussed.
Keywords: Mother-child interaction, Oxytocin
The role of oxytocin in relation to social behavior has been studied extensively among non-human mammals, but less is known about whether these processes function similarly among humans (Cho, DeVries, Williams, & Carter, 1999; Insel, Wang, & Ferris, 1994; Kendrick, Keverne, & Baldwin, 1987; Kendrick, Keverne, Baldwin, & Sharman, 1986; Kendrick, Lévy, & Keverne, 1991; Keverne, Lévy, Poindron, & Lindsay, 1983; Lim and Young, 2006; Williams, Insel, Harbaugh, & Carter, 1994; Winslow, Hastings, Carter, & Harbaugh, 1993; Young, Lim, Gingrich, & Insel, 2001). Oxytocin has been found to relate to maternal behavior in female adult and adolescent non-human mammals (Kendrick et al., 1987, 1991; Lim & Young, 2006; Olazábal & Young, 2006; Panksepp, Nelson, & Bekkedal, 1997; Pedersen, Ascher, Monroe, & Prange, 1982; Pedersen, Caldwell, Walker, Ayers, & Mason, 1994). Among adult humans, oxytocin has been associated with aspects of maternal care including attachment related thoughts and feelings during toward infants at pre-natal and post-partum time points (Feldman, Weller, Zagoory-Sharon, & Levine, 2007). As an attempt to further understanding the association between oxytocin production and maternal behavior toward biological and non-biological young, the current study explores differences in oxytocin levels after mothers participate in close, physical interactions with their biological children versus unknown, unfamiliar children.
In studies of non-human mammals, increased levels of oxytocin in the brain have been associated with social motivation and approach behavior, including maternal nurturance (Insel & Winslow, 1991; Panksepp, et al., 1997; Pedersen et al., 1994). For example, in non-pregnant sheep that had previously given birth, both vagino-cervical stimulation and centrally injected oxytocin levels induced maternal behaviors toward a non-biological lamb (Kendrick et al., 1987, 1991). Similar connections between oxytocin and maternal behavior have been found in adult rats. Prior to giving birth, adult female rats are repelled by rat pups, but begin to show maternal behavior immediately after the birth process. Oxytocin is thought to play a role in the onset of maternal behavior in this species, as estrogen-primed virgin adult rats that received injections of oxytocin to their central nervous system showed motivation to care for rat pups, instead of the typical aversion to pups in the absence of such injections (Pedersen et al., 1982).
The prairie vole provides an especially good model for studying associations between bonding and oxytocin production. Prarie voles are a socially monogamous species, with both males and females caring for young (Lim & Young, 2006). Further, alloparenting is seen among prairie voles, with juvenile voles displaying maternal behavior toward non-biological young (Solomon, 1991; Wang & Novak, 1994). Oxytocin has been found to be an important regulator of adult and juvenile prairie voles’ expression of parental behavior toward biological and non-biological young (Olazábal & Young, 2006). In juvenile prairie voles, correlations between the number of oxytocin receptors in the brain and the quality of alloparental care have been found. Furthermore, like sheep, injections of an oxytocin antagonist into the brain of prairie voles impede the expression of maternal behavior (Olazábal & Young, 2006).
Whereas centrally produced oxytocin is frequently measured in studies with many non-human mammals, this is typically not practical in primates or humans. As discussed by Seltzer and Ziegler (2007), the process of measuring oxytocin through cerebrospinal fluid is not only costly, but it is highly stressful. Given that oxytocin release is associated with the stress response system, the elevated stress levels that occur when measuring cerebrospinal fluid may alter the concentration of oxytocin (Amico, Mantella, Vollmer, & Li, 2004; Carter & Altemus, 1997; Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Kalin, Gibbs, Barksdale, Shelton, & Carnes, 1985; Seltzer & Ziegler, 2007). Therefore, research on humans and primates has often relied on less-invasive, peripheral measures of oxytocin in blood plasma and urine.
One example of a research study that relied on peripheral oxytocin measurements is Feldman, Weller, Zagoory-Sharon, & Levine’s (2007) investigation of oxytocin production during mother-infant bonding. In a group of 62 pregnant women, plasma levels of oxytocin were assessed during the first and third trimester of pregnancy and after infants were born. Blood plasma oxytocin levels remained stable through the prenatal phases until after birth. Oxytocin levels were related to a host of maternal behaviors including the mothers’ preoccupation with the infant, attachment-related thoughts, and bonding behaviors (Feldman et al., 2007). In another study examining peripheral levels of neuropeptide production, Wismer-Fries, Ziegler, Kurian, Jacoris, & Pollak (2005) compared the urine concentrations of oxytocin among 18 children who had lived in institutional care during the first years of their lives with those of 21 children who had lived continuously with their biological parents. For both groups of children, oxytocin levels were examined after children experienced close, physical contact with their biological or adoptive mothers in one condition and with unfamiliar adult females in another condition. Institutionally-raised children showed marginally lower levels of oxytocin after activities involving physical contact with their mothers when compared with biological family-reared children. Together, these two studies provide evidence that peripherally produced oxytocin is associated with aspects of social attachment, bonding, and affiliation among humans.
The current study extends the methodology used by Wismer-Fries et al. (2005) to examine associations between peripherally produced oxytocin in a sample of biological mothers and children. We were especially interested in differential levels of oxytocin in the contexts of close physical interactions between in biological versus non-biological mother-child interactions. Therefore, maternal oxytocin levels were examined after mothers interacted with their biological children in one condition, and with unfamiliar children in a second condition. It was plausible that oxytocin production would be greater when mothers interacted with their own children than with unfamiliar children, due to an association between oxytocin production and established, biological bonds between mothers and children. However, given that oxytocin production has not only been associated with alloparental behavior but has been found to promote prosocial behavior by inhibiting fear to novel conspecifics, it was also plausible that oxytocin levels in the unfamiliar condition would be greater than oxytocin levels in the biological child condition (Olazabal & Young, 2006).
Methods
Participants
Thirty-five biological mothers and children participated in this study. Fifteen percent of the dyads were African American, 63% were White/non-Hispanic, 7% were Asian American, and 15% were bi-racial. Twenty two percent of the mothers had completed graduate school, 28% had completed college, 27% had completed an associate’s degree, 12% had completed high school, and 11% had not completed high school. At the time of the first visit, children’s ages ranged from 29 to 54 months, with an average of 40.7 months of age. Mothers and children who participated in this research study had no medical or developmental disabilities. Data for 26 mothers were included in the analysis of oxytocin. Data were not included in the analyses for the following reasons: there was an insufficient amount of urine to detect oyxtocin levels (4 mothers) and oxytocin values were outliers (5 mothers).
Procedure
Upon approval by the (Deleted for blind review) Institutional Review Board, mothers were invited to participate in the research study over the phone. If interested, a consent visit was scheduled in the home to allow mothers to hear more about the study and decide whether they would like to participate. During the home visit, the purpose and description of the research, participant activities, risks and benefits, confidentiality, and other matters pertaining to the study were explained in detail. Participants had the option to decline or agree to participate in the study at this visit and to withdraw from participation at any point in the study without penalty. Questions or concerns were addressed in the consent visit, and throughout the study.
Upon consent, the mothers and children were asked to come to a university laboratory pre-school two times, approximately one week apart (range = 6 days to 14 days). Each child and mother dyad was scheduled to participate in the lab visit with at least one other mother and child dyad who also agreed to participate that day. Mothers were asked to avoid caffeine, nicotine, and alcohol before appointments, as these substances can interfere with oxytocin production. To control for diurnal patterns of oxytocin production, all lab visits were scheduled to begin at 9:00 a.m., so that urine samples would be collected at approximately the same time each day. The range in time that the visits began was from 9:05 to 9:30 a.m.
At the beginning of each lab visit, biological mothers and children were separated from each other for 45 minutes so that post-interaction samples of oxytocin levels were not affected by physical contact apart from the experimental manipulation. During this separation, children were allowed to play with various toys and with the other children in the lab and were offered snacks and juice. Mothers were asked to sit in a separate room with each other where there was an assortment of beverages and snacks. After the first 30 minutes of this 45 minute separation, the mothers were asked to void in private bathrooms. The purpose of doing so was to rid their systems of oxytocin that may have accumulated before mothers were separated from their child. In research on non-human animals, peak levels of oxytocin are found approximately 15 minutes after physical contact (Juszczak & Stempniak, 1997). Research on humans has demonstrated that increased levels of oxytocin occur as little as five minutes after the onset of physical contact (Turner, Altemus, Enos, Cooper, & McGuinness, 1999). Therefore, requesting that mothers and children void 30 minutes after being separated from one another was thought to sufficiently reduce the effect of pre-separation oxytocin production.
After the 45 minute separation, mothers and children engaged in a video-taped, interactive computer game. The onset of the game ranged from 9:50 a.m. to 10:15 a.m. During one of the two lab visits, biological mothers and children participated in the interactive computer game with each other. During the other visit, mothers participated in the game with unfamiliar children, and children participated with unfamiliar women (i.e., the mothers of the other children). The order of interaction type was counterbalanced in that half of the mothers first interacted with their own children whereas the other half of the mothers first interacted with the unfamiliar children. To encourage continuous physical contact, children were asked to sit on the mother’s lap for the duration of the game. The interactive computer game consisted of timed, physical contact that lasted 25 minutes. These procedures carefully followed the protocol used by Wismer-Fries et al. (2005). Examples of physical contact activities included playing tickling games, whispering to each other, counting while holding each others’ hands, and sitting together while listening to short stories. Prior to the game, mothers were informed that the interactions would be stopped if their children became distressed when interacting with unfamiliar mothers. (Although this occurred on one occasion, the mother of the upset child was able to complete the research activities without interruption).
After the completion of this task, mothers were asked to provide urine samples as soon as they were ready. The time in which final urine samples were collected ranged from 10:25 a.m. to 12:05 p.m. During sessions involving unfamiliar interactions, biological mothers and children were kept in separate rooms until the mothers provided urine samples. Mothers were encouraged to drink liquids before and during the visits to facilitate the process of voiding on two occasions (before and after the interaction). Mothers were compensated 25 dollars for their participation in each visit. Children received a toy at the end of each visit for their participation.
Oxytocin Assays
After biological and unfamiliar interactions, mothers provided urine samples in sterile specimen cups. The samples were then transferred to sterile cryogenic vials. Samples were placed on dry ice immediately after collection and transferred to a −80 °C freezer until they were thawed for assay in a laboratory in the University of Wisconsin. Samples were subsequently sent to the Wisconsin National Primate Research Center, which assayed data for the Wismer-Fries et al. (2005) study. Appropriate procedures were followed regarding the transfer of biological materials.
The use of an enzyme-immunoassay has been validated for the measurement of oxytocin in human urine (EIA, Assay Designs, MI). Through the use of HPLC (High Performance Liquid Chromatography) separation, the antibody used for this assay has been shown to be specific for human urinary oxytocin. For validation, human urine was purified before HPLC by solid phase extraction (SPE) (Waters, SepPak, C18, 100 mg/1CC). Solid Phase Extraction columns were prepared with 1 ml of methanol and 1 ml of purified water. After the addition of samples in 1 ml of urine, the column was washed with 10% acentonitrile in water with 1% trifluroacetic acid (TFA). Samples were eluted with 80% acentonitrile, dried, and reconstituted in 30 µl of 50% acentonitrile for injection through the HPLC system as described in Wismer-Fries et al. (2005). A urine sample was collected at 1 minute fractions for 10 minutes and the EIA antibody was used to detect cross-reactivity in each fraction. The antibody was specific for the fraction where oxytocin elutes. Pooled human urine was parallel to the oxytocin standards (t = 1.36, p > .05) accuracy was 99.476 + 1.60. Sensitivity of the assay was 0.78 pg. Recovery of added oxytocin to the assay procedure was 94.82%. Coefficient variation of the assay was: intra = 2.50, inter = 19.47. Oxytocin samples were adjusted for creatinine levels to control for fluid variability as described in Ziegler et al. (1995). For sample analyses, 1 ml of each sample was purified by SPE, dried in a water bath with air, and reconstituted in the assay buffer. The EIA for oxytocin was run according to the company’s recommendations.
Behavioral ratings
In both biological and unfamiliar interaction contexts, blind coders rated the level of “stress or unease” of the mother and the overall “positivity and closeness” of the adult-child interaction on a scale of 1 (not at all) to 5 (consistently). Because close physical touch has been associated with oxytocin production (Holst, Uvnas-Moberg, & Peterson, 2002), the percentage of time in which the mother and child spent touching each other of the entire interaction was also calculated for each interaction.
Maternal Sensitivity
Maternal sensitivity was assessed using the abbreviated version of the Maternal Behavior Q-sort (Pederson & Moran, 1995). The abbreviated maternal behavior Q-sort is a 25 item assessment of maternal behavior between mothers and infants. Q-sort items are first sorted into five categories ranging from least descriptive (1) to most descriptive (5) of the mothers. The resulting Q-sort is then correlated with a criterion Q-sort of a sensitive mother. This measure has been shown to have adequate psychometric properties including test-retest reliability and predictive validity (Pederson, Gleason, Moran, & Bento, 1998; Pederson & Moran, 1995; Tarabulsy, Avgoustis, Phillips, Pederson, & Moran, 1997). Coders achieved acceptable levels of inter-rater reliability (80% or higher) prior to coding interactions in this data set.
Results
Preliminary Analyses
First, differences in the participants who were included versus excluded in analyses were examined as one-way analyses of variance. No significant differences emerged with regard to children’s ethnicity (F (3, 38) = .745, p =.53), mothers’ ethnicity (F (3, 38) = 1.382, p =.26), children’s gender (F (1, 40) = .027, p =.87), or mothers’ educational levels (F (4, 37) = .527, p =.72).
Next, oxytocin values were assessed for the presence of outliers. Oxytocin values that were more than 3 standard deviations from the mean for each context (biological and unfamiliar interactions) were considered outliers and were not included in the analyses. This excluded only mothers with high oxytocin values because the lowest possible value (0) was within 3 standard deviations of the mean for each context. Data for 5 mothers were excluded because of high oxytocin values. Next, the normality of the distributions of oxytocin was assessed. Examination of data revealed a positive skew in the distribution of oxytocin and values. A Log 10 transformation was employed to normalize the distributions for each context and time point, as is conventional in the handling of skewed biological data (e.g., Dettling, Parker, Lane, Sebanc, & Gunnar, 2000).
Next, we explored whether the lag time between the end of the interaction and the post interaction sample differed significantly across interaction contexts. Mothers’ lag time between the time at which they started the interaction sequence to the time at which they provided the post interaction sample ranged from 25 to 50 minutes (M = 35, SD = 6). Paired samples t-tests revealed no significant differences between lag time of biological or unfamiliar interactions for mothers or in the length of time it took for mothers to void after biological or unfamiliar interactions (all p values >.20). Further, there were no significant associations between lag time and oxytocin concentrations for mothers in either interaction context, p’s > .10. Therefore, lag time between the end of the interaction and time of void was not included as a covariate in analyses.
Next, we examined whether the association between context and oxytocin concentration was a function of variability in the quality of maternal behavior in biological and non-biological interactions. Differences in maternal behavior across contexts were examined using within-subjects analyses of variance. Context (biological v. non-biological context) was included as the within-subjects variable and maternal behavior was included as the dependent variable. Maternal sensitivity, positivity and closeness, and stress and unease were not significantly different in biological versus unfamiliar dyads, (F (1, 24) = .58, p = .453), (F (1, 24) = 3.80, p = .064), and (F (1, 24) = 1.29, p = .268), respectively. Finally, percent of time spent engaged in physical contact was compared across interaction context. Analyses revealed no significant differences in the amount of time mothers and children engaged in close physical contact in biological versus unfamiliar interactions (F (1, 24) = .37, p = .549). These analyses did not indicate the need for including behavioral measures as covariates in analyses of primary interest.
Whether child age and gender should be included as covariates in primary analyses was also explored. Analyses revealed that child age was not associated significantly with oxytocin production for biological mothers or for unfamiliar adult females, p values > .05. Child gender was not associated with differential production of oxytocin for adults, p values > .20. Therefore, the primary analyses did not include age and gender as covariates.
Primary Analyses
Differences in oxytocin production were analyzed as within-subjects analyses of variance with context (biological vs. non-biological context) as the within-subjects variable and oxytocin level as the dependent variable. To ensure that results were not affected by the exclusion of outliers, analyses were conducted with and without the presence of outlying oxytocin values. A main effect for context emerged for mothers when post-interaction oxytocin was included as the dependent variable, F (1, 25) = 11.27, p = .003. Mothers showed higher levels of oxytocin following interactions with unfamiliar children than following interactions with their own children (see Figure 1). Similar results were found when outlying oxytocin values were not removed, F (1, 30) = 8.59, p = .006. Mean values for maternal oxytocin are presented in Table 2.
Figure 1.
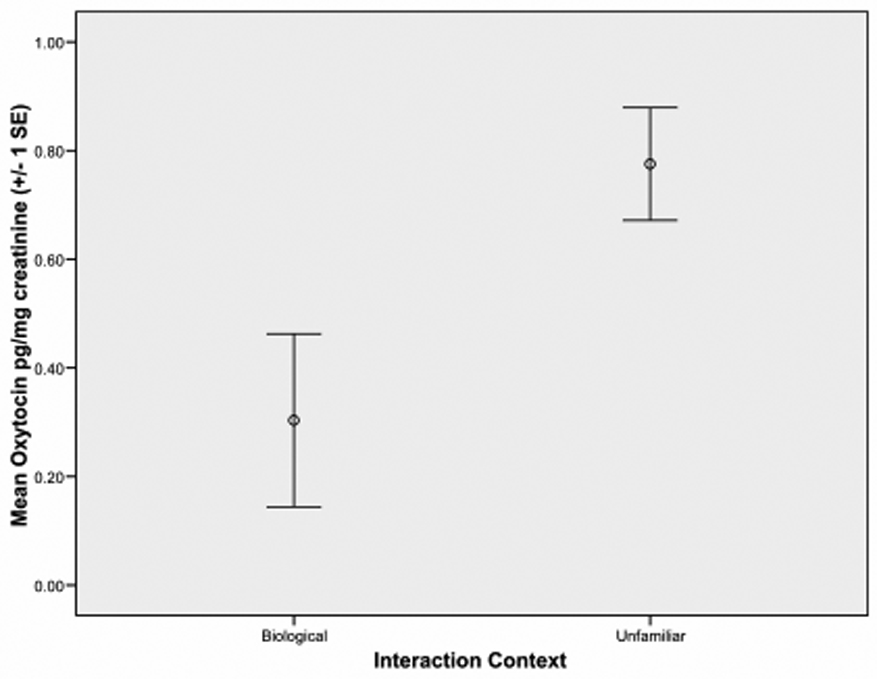
Maternal oxytocin urine concentrations after biological and unfamiliar interactions.
Table 2.
Descriptive Statistics of Dependent and Independent Variables for Mothers
| Mothers | M | SD |
|---|---|---|
| Oxytocin (pg/mg Creatinine) | ||
| Biological | .30 | .81 |
| Unfamiliar | .77 | .52 |
Discussion
This study extends previous research on the role of oxytocin after close physical interactions between mothers and children. Oxytocin levels were higher in mothers following interactions with unfamiliar children than following interactions with their own children. These results did not appear to have been driven by the differences in the quality of maternal behavior across conditions. Several possible explanations for the differential levels of oxytocin across interaction contexts exist.
Past research implicating oxytocin as important in the onset (as opposed to the maintenance) of maternal behavior and selective attachment to offspring may be useful when interpreting the results form the current study (Farbach, Morrell, & Pfaff, 1985; Pedersen, Caldwell, Fort, & Prange, 1985; Van Leengood, Kerker, & Swanson, 1987). For example, oxytocin antibodies, antagonists, and PVN lesions have been found to inhibit maternal attachment behavior during immediate post-partum phases (Insel, 2000; Kendrick, 2000). Remarkably, antagonists were not found to inhibit maternal attachment behavior among rats once post-partum maternal behaviors were established (Insel & Harbaugh, 1989). Therefore, oxytocin has been considered to play a role in initiating, but not maintaining, maternal behavior or attachment to offspring (Insel, 1997), by facilitating the transition from avoidance of pups to caring for them. With respect to the findings of the current study, it is possible that greater levels of oxytocin produced when mothers interacted with unfamiliar children are related to the onset of their maternal behavior in this context. However, given that humans do not exhibit the same transitions from avoidance of young to maternal behavior as is seen among adult rats, the generalizability of the findings from the research on rats to humans is limited.
In view of this, it is important to consider alternative explanations for the higher levels of oxytocin mothers produced after unfamiliar interactions. For example, the mere novelty of the unfamiliar interaction may have explained the differential levels of oxytocin production. Maternal stress levels at the time of the interaction may also be responsible for the differential levels of post-interaction oxtyocin concentrations. It has been widely established that oxytocin production serves as an anxiolytic function among humans and animals. For example, studies on rodents and humans have indicated that oxytocin administration reduces amygdala activation, increases parasympathetic activity, inhibits CRF neurons, decreases corticosteroid release, and results in lower levels of fearful behavior (Dreifuss et al., 1992; McCarthy et al., 1996; Uvnas-Moberg, 1997; Windle et al., 2004). Furthermore, when animals are confronted with stress of a social nature, the production of oxytocin has been found to buffer behavioral and biological stress responses by inhibiting animals’ defensive behavior and facilitating pro-social behavior (Heinrichs & Domes, 2008). Therefore, the greater levels of oxytocin that occurred when mothers interacted with unfamiliar children may have actually served an anxiolytic function in response to the potential stress of interacting with an unknown child. Alternatively, it is possible that mothers experienced stress on arrival to the laboratory. Their subsequent interaction with their biological children may have reduced this stress and the production of oxytocin that occurred in response to this stress. If so, the post interaction oxytocin levels may have reflected a reduction in maternal oxytocin levels in the biological condition, but not in the unfamiliar condition.
Like animal research, the production of oxytocin in the unfamiliar contexts may have served to inhibit mothers’ defensive behavior and promote maternal behavior toward the unknown children. Although differences in ratings of mothers’ stress and unease were not found across interaction contexts, obtaining subjective ratings of stress or unease in addition to obtaining a biological measurement of stress, such as cortisol, would be especially useful to address this issue. Collecting a baseline sample of both cortisol and oxtyocin prior to all interaction contexts might also be useful when interpreting results. However, a baseline sample will be of limited usefulness in the absence of control of previous interactions.
In addition to its production in response to stress, oxytocin has been found to play a role in the expression of pro-social behavior in non-stressful contexts. Specifically, intra-nasal infusions of oxytocin have been associated with social cognition, such as memory toward faces, as well as pro-social behavior, such as empathy and trust toward unknown individuals (Baumgartner et al, 2008; Domes et al., 2007; Heinrichs & Domes, 2008; Kosfeld et al, 2005; Rimmele, 2008). Similarly, oxytocin may be required for mothers in exhibit a specific type of pro-social behavior, maternal behavior, toward the unknown children in the current study.
Regardless of the mechanisms underlying these findings, it is important to consider research on the relationship between peripheral versus central levels of oxytocin production when interpreting the findings from this study. Although associations between peripheral levels of oxytocin and behavior have been found, causal mechanisms (such as association with areas in the brain or involvement in the HPA system and reward pathways) remain unknown. Nevertheless, evidence establishing the validity for using peripheral measures is growing. As reviewed by Seltzer and Ziegler (2007), several animal research studies provide evidence for an association between the central and peripheral systems (Polito, Goldstein, Sanchez, Cool, & Morris, 2006; Cushing & Carter, 2000; Cushing, Martin, Young, & Carter, 2001). In terms of human research, results of multiple studies have established links with peripheral oxytocin levels and social behavior in a normative group of children, adult mothers, and children with autism (Feldman et al., 2007; Modahl, 1998; Wismer-Fries et al., 2005).
In conclusion, the results regarding mother-child interactions and oxytocin concentrations contribute to the understanding of maternal oxytocin production with biological versus non-biological children. Ongoing research could explore whether similar patterns of oxytocin concentrations are found during actual alloparental interactions, such as when adults bond with foster or adoptive children. Are these effects maintained when adult individuals interact with unfamiliar adults in a similar manner, such in newly formed romantic relationships? Do these differential patterns of oxytocin production generalize to males? Although many questions remain unanswered, the results from the current study add to the growing body of literature on associations between oxytocin production and maternal behavior in biological and non-biological contexts.
Table 1.
Biological and Unfamiliar Oxytocin Levels Regressed on Child Age and Gender
| Child’s Age | |||||
| Oxytocin | B | SE | β | T | p value |
| Familiar | .006 | .013 | .071 | .427 | .67 |
| Unfamiliar | .007 | .011 | .107 | .627 | .53 |
| Child’s Gender | |||||
| Oxytocin | B | SE | β | T | p value |
| Familiar | −.316 | .199 | −.253 | −1.59 | .12 |
| Unfamiliar | −.008 | .180 | −.007 | −.042 | .97 |
B=unstandardized coefficient, SE=standard error, β=standardized coefficient, T=t ratio.
References
- Amico J, Mantella R, Vollmer R, Li X. Anxiety and stress responses in female OT deficient mice. Journal of Neuroendocrinology. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Carter C, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Annals of the New York Academy of Science. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Hormones and Behavior. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Hormones and Behavior. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. Quality of care and temperament determine changes in cortisol concentrations over the day for young children in childcare. Psychoneuroendocrinology. 2000;25:819–836. doi: 10.1016/s0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- Farbach SE, Morrell JL, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgarten T, Kirshbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: Effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Holst S, Uvnas-Moberg K, Peterson M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Autonomic Neuroscience. 2002;99:85–90. doi: 10.1016/s1566-0702(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. The American Journal of Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Insel TR. Toward a neurobiology of attachment Review of General Psychology. Special Issue: Adult Attachment. 2000;4(2):176–185. [Google Scholar]
- Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiology & Behavior. 1989;45(5):1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang Z, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Central administration of oxytocin modulates the infant rat’s response to social isolation. European Journal of Pharmacology. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Juszczak M, Stempniak B. The effect of melatonin on suckling-induced oxytocin and prolactin release in the rate. Brain Research Bulletin. 1997;44:253–258. doi: 10.1016/s0361-9230(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Kalin N, Gibbs D, Barksdale C, Shelton S, Carnes M. Behavioural stress decreases plasma oxytocin concentrations in primates. Life Science. 1985;36:1275–1280. doi: 10.1016/0024-3205(85)90272-3. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Oxytocin, motherhood and bonding. Experimental Physiology. 2000;85:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in sheep. Neuroendocrinology. 1987;46:56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA, Sharman DF. Cerebrospinal fluid levels of acetylcholinesterase, monoamines, and oxytocin during labour, parturition, vaginocervical stimulation, lamb separation, and suckling in sheep. Neuroendocrinology. 1986;44:149–156. doi: 10.1159/000124638. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Lévy F, Keverne EB. Importance of vaginocervical stimulation for the formation of maternal bonding in primiparous and multiparous parturient ewes. Physiological Behavior. 1991;50:595–600. doi: 10.1016/0031-9384(91)90551-x. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Levy F, Poindron P, Lindsay DR. Vaginal stimulation: An important determinant of maternal bonding in sheep. Science. 1983;219:81–83. doi: 10.1126/science.6849123. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein, et al. Plasma OT levels in autistic children. Biological Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Hormones and Behavior. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward: Evolutionary antecedents and neuropeptide intermediaries. Annals of the New York Academy of Science. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Caldwell J, Fort S, Prange A. Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides. 1985;6:175–182. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Caldwell J, Walker C, Ayers G, Mason G. Oxytocin activates the postpartum onset of rat behavior in the ventral tegmental and medial preoptic area. Behavioral Neuroscience. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pederson DR, Gleason K, Moran G, Bento S. Maternal attachment representations, maternal sensitivity, and the infant-mother attachment relationship. Developmental Psychology. 1998;34:925–933. doi: 10.1037//0012-1649.34.5.925. [DOI] [PubMed] [Google Scholar]
- Pederson DR, Moran G. A categorical description of attachment relationships in the home and its relation to Q-sort measures of infant-mother interaction. In: Vaughn B, Waters E, editors. Constructs, caregiving, and cultures: New growing points of attachment theory and research (pp. 111–132). Monographs of the Society for Research in Child Development. Vol. 60. 1995. (Serial No. 244) [Google Scholar]
- Polito A, Goldstein D, Sanchez L, Cool D, Morris M. Urinary oxytocin as a non-invasive biomarker for neurohypophyseal hormone secretion. Peptides. 2006;11:2877–2884. doi: 10.1016/j.peptides.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. Journal of Neuroscience. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer L, Ziegler T. Non-invasive measurement of small peptides in the common marmoset (callithrix jacchus): A radiolabeled clearance study and endogenous excretion under varying social conditions. Hormones and Behavior. 2007;51:436–442. doi: 10.1016/j.yhbeh.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Solomon NG. Current indirect fitness benefits associated with philopatry in juvenile prairie voles. Behavioral Ecology and Sociobiology. 1991;29:277–282. [Google Scholar]
- Tarabulsy GM, Avgoustis E, Phillips J, Pederson DR, Moran G. Similarities and differences in mother’s and observer’s descriptions of attachment behaviors. International Journal of Behavioral Development. 1997;21:599–619. [Google Scholar]
- Turner R, Altemus M, Enos T, Cooper B, McGuinness T. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Van Leengood KE, Swanson H. Inhibition of postpartum maternal behavior in the rat by injecting an oxytocin antagonist into the cerebral ventricles. Journal of Endocrinology. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- Wang Z, Novak MA. Alloparental care and the influence of father presence on juvenile prairie voles, microtus ochrogaster. Animal Behaviour. 1994;47:281–288. [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Neuroendrocrinology. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wismer-Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceeding National Academy Science USA. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and Behavior. Special Issue: ICHB & SBN Proceedings. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, saguinus oedipus. Hormones and Behavior. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]


