Lipid bilayer systems have been used extensively to study the structure and function of biomembranes including molecular recognition, permeation, adhesion, and fusion.1-5 Further interest arises from their potential use as biosensors.6,7 While classical black lipid bilayer membranes are highly suitable as such model systems, they suffer from their limited long-term stability.1 Additionally, to apply the broad range of surface sensitive experimental techniques developed in recent years for the characterization of ultrathin films, it is often necessary to immobilize lipid bilayers on solid supports. However, lateral fluidity of the lipid membrane is governed by the physical and chemical properties of the solid substrate. Thus, bilayer membranes prepared by the Langmuir-Blodgett (LB) technique or by vesicle fusion directly onto solid substrates are often polycrystalline or amorphous, and lateral lipid mobility within the membrane is restricted. While on hydrophilic oxidized silicon or glass a water layer (~1 nm thick) is formed which allows for free diffusion of phospholipid molecules (but not membrane-spanning proteins) within the bilayer,8-10 on most other metals and metal oxides, lipid mobility is strongly suppressed.11,12 To preserve the membrane's natural properties, one promising approach is to rest it on a water-swellable (hydrophilic) polymer or polyelectrolyte cushion which can act as a deformable and mobile substrate6,13,14 like the cytoskeletal support in living cells. Such softly supported lipid bilayers can, in principle, exist in an entirely fluid state, nearly undisturbed by the presence of the supporting polymer gel which provides an aqueous compartment between the solid substrate and the membrane. In our recent work, we have studied DMPC bilayers on a highly branched cationic polymer (polyethylenimine, PEI). Important structural information about these polymer-supported bilayer systems formed by various preparation methods was provided by neutron scattering experiments.15,16 Furthermore, measuring the intermembrane interaction forces using the surface forces apparatus (SFA) technique has shown the effect of fluidity on membrane functionality.17
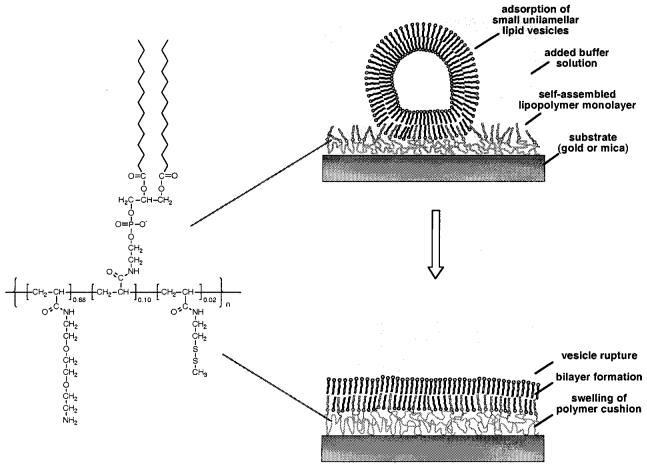
These physisorbed, polymer-supported bilayers are only the first step in the approach of creating artificial biomembranes that more closely resemble living cells. To provide more biologically relevant architectures, molecular assemblies of increased complexity and long-term stability have been tested, including a partial chemical fixation of the membrane's lipids to the underlying polymeric substrate.7,14,18,19 However, the construction of such “asymmetric” tethered supported membranes is not always straightforward, and each particular system needs to be optimized. The most versatile approach appears to be vesicle fusion onto polymer-supported lipid monolayers previously prepared by LB transfer or self-assembly (Figure 1).16 In general, this allows for both (a) a partial fixation of the inner (proximal) lipid layer to the underlying polymer gel and (b) the reconstitution of integral membrane proteins into the supported membrane.
Figure 1.
Formation of polymer-supported bilayers by adsorption of small unilamellar vesicles onto self-assembled lipopolymer monolayers. In the first step, vesicles adsorb and bind to the substrate (gold, specific adsorption via thiol groups; mica, electrostatic binding via amino functions). Subsequent vesicle rupture and fusion leads to bilayer formation.
Adopting a concept first introduced by Spinke et al.,14 we have recently synthesized and studied lipopolymers, which are comprised of an acrylamide backbone modified with lipid side chains (DMPE) and a disulfide moiety for the chemisorption to gold substrates (Figure 1).20-22 After adsorption onto gold or mica surfaces they form a hydrophilic, water-swellable polymer cushion underneath a layer of lipid side chains. Vesicles prepared from DPMC adsorb to these layers if at least 5 mol % of DMPE side chains are present and if some ionic groups are incorporated.21,22 On gold, the thickness increase after vesicle adsorption was slightly higher than expected for a polymer-supported lipid bilayer, as probed by surface plasmon resonance. Fluorescence measurements prove that the vesicles did not rupture under the conditions employed but formed an adsorbed vesicle layer.21 This finding is consistent with observations made during our earlier work on physisorbed DMPC bilayers on PEI.15,16 There, the successful preparation of uniform homogeneously supported lipid bilayers was found to depend on various factors. Most importantly, the polymer layer has to be kept quasi-dry before vesicle adsorption. A soft and fluctuating polymer substrates—although a desired property of the final systems—is unfavorable during the initial stages of the preparation, as it leads to vesicle trapping and aggregation on or within the swollen polymer layer.15,16
In addition, a more subtle effect became apparent: during that earlier study of physisorbed DMPC bilayers, a stock vesicle solution in pure water was generally prepared which subsequently was diluted in the particular buffer solution used in each experiment. This was initially done for convenience: during the subsequent SFA force measurements in different buffered electrolyte solutions, this procedure proved to give the most homogeneous and “clean” bilayer surfaces. However, in control experiments where solutions of small unilamellar vesicles were initially prepared in buffer solution, rather than in pure water, multilamellar or more complex vesicle aggregates (up to 150 nm thick) often appeared on the surfaces, as measured using the optical technique of the SFA. This observation was confirmed by neutron scattering studies.15,16 In this short Note we now present more concrete evidence by atomic force microscopy that hypotonic osmotic stress acting on lipid vesicles provides a driving force for the formation of homogeneous tethered or polymer-supported lipid bilayers from self-assembled lipopolymer monolayers.
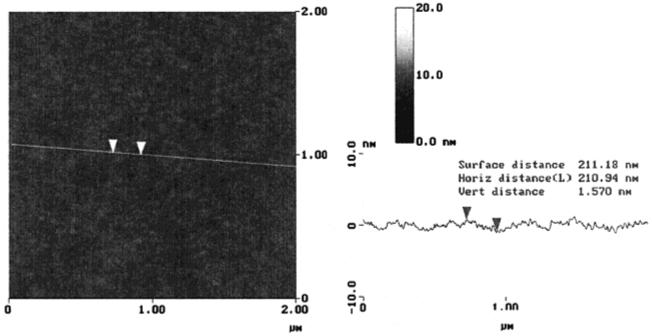
Freshly cleaved mica substrates were immersed into a solution of lipopolymer 1 (c ~ 1 mg/mL) in ethanol/chloroform (v/v ~ 10:1) overnight. The substrate was then thoroughly washed with solvent, as well as with pure ethanol, and dried. As on gold,21,22 the self-assembly process on mica leads to a complete surface coverage with a self-assembled monolayer which is very homogeneous, as shown by the atomic force microscope (AFM) image in Figure 2. From defects within the film, an average peakvalley distance of ~1.5 nm was determined.
Figure 2.
AFM image of a self-assembled lipopolymer monolayer adsorbed onto freshly cleaved mica from ethanol solution (c ~ 1 mg/mL, 3 h adsorption time followed by rinsing with ethanol and Millipore-filtered water). The image was taken in water at room temperature with a Nanoscope III Multimode AFM (Digital Instruments, Goleta, CA) in the tapping mode using a silicon nitride tip on a cantilever with a spring constant of 0.38 N/m.
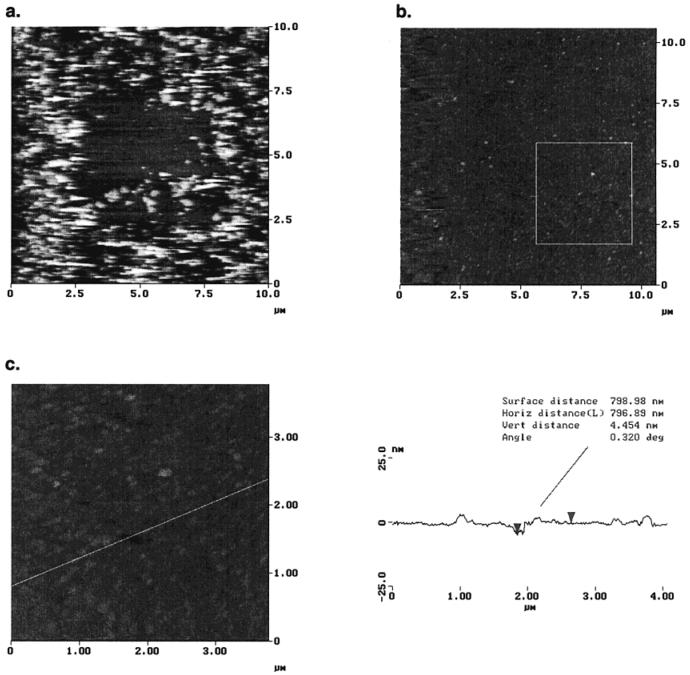
In first control experiments, two suspensions of “unstressed” DMPC vesicles in water and electrolytecontaining (0.5 mM KNO3) buffer were prepared by sonication and adsorbed onto the self-assembled lipopolymer monolayers. Inhomogeneous surface aggregates were observed in both cases, which confirms the findings of the earlier studies on these systems on gold.21 This is illustrated by the AFM image shown in Figure 3a, which was obtained after vesicle adsorption under purely aqueous conditions. Also, previous to the acquisition of Figure 3a, the 5 × 5 μm2 central area was scanned repeatedly under high applied load. While in the untreated regions of the sample, adhered vesicles remain at the surface (and do not rupture or fuse), the central squared area appears more homogeneous. Obviously, most adsorbed vesicles have been removed as a result of the tip-surface interaction. In addition, stimulated vesicle rupture and fusion may have occurred in the central region.
Figure 3.
AFM images obtained after adsorbing a solution of small unilamellar vesicles of DMPC (obtained by diluting 1 mL of an aqueous stock solution, c ~ 1 mg/mL with 30 mL of 0.5 mM KNO3 solution) onto a previously self-assembled lipopolymer monolayer (adsorption at 28 °C overnight). Imaging conditions: tapping mode, silicon nitride tip, T ~ 26 °C, i.e., above the phase transition temperature of DMPC bilayers, Tm) = 24 °C; z-range is 0 nm (black) to 50 nm (white). (a) Inhomogeneous surface structure of aggregated vesicles obtained after vesicle adsorption under purely aqueous conditions. The 5 × 5 μm2 central area was scanned repeatedly under high applied load in contact mode previous to imaging in tapping mode in which vesicles are mostly removed. (b) Tethered supported bilayer obtained from osmotically stressed vesicles, fifth scan of same region of sample. Note that edge effects are more pronounced on the left side of the images which may result from the fact that the AFM signal is only acquired in one direction. E.g., the second pass of the AFM tip over the sample is detected on the left side whereas the first pass is detected on the right edge (showing the mostly homogeneous “original” bilayer structure). (c) AFM image of a smaller area (4 × 4 μm2) obtained immediately after scan b (taken from the lower right region as indicated).
In a second experiment, an aqueous stock solution (~1 mg/mL) of vesicles was first prepared by sonication in water and then diluted with 0.5 mM KNO3 solution to a final concentration of ~50 μg/mL. With this procedure, the vesicles are directly subjected to an osmotic stress before the adsorption process. As a result, water should leave their interior; the vesicles will deflate. At the same time, the lipid bilayer's topology and curvature will become less defined. They will thus appear “rougher” and more deformable which may result in a steric undulation repulsion between individual vesicles. At the same time, high local curvatures may serve as nucleation centers for vesicle rupture and fusion. An almost complete surface coverage of lipid bilayers was obtained using this method as shown in Figure 3b. Most importantly, aggregated structures of adsorbed lipid vesicles are no longer observed. Since the screening of electrostatic repulsion in electrolyte buffers should favor vesicle aggregation, the successful formation of polymer-supported bilayers only from osmotically stressed vesicles indicates that a high salt concentration alone appears to play a minor role, at least for the particular system described here.
This AFM image of a polymer-supported lipid bilayer membrane can be obtained both reproducibly for different regions of the substrate and repeatedly for the same region (Figure 3b is the fifth scan over the same bilayer region).
Various smaller areas of the samples were also scanned (Figure 3c). Again, from defects within the film, i.e., natural imperfections and those obtained from the interaction between the bilayer and the AFM tip, an averaged thickness for the interfacial layer of ~6 nm was found. Although this method does not allow the most precise thickness determination,23 this value is in good agreement with the expected thickness of a lipid bilayer attached to a water-swollen polymer cushion.21 (Note that the few observed bilayer patches on top of the polymer-supported membrane consist of DMPC bilayers alone, which is reflected by their observed thickness of approximately 4 nm).
In the context of the imaging of soft bilayer surfaces by AFM, it should be mentioned that effects of tip-induced bilayer reconstitution have been discussed in the literature.24 It has been argued that if the appropriate amount of energy is provided, the AFM tip helps to convert unstable holes or disks into a lower energy lipid bilayer which is in accordance with our observations here. To minimize tip-surface interactions here, the AFM images were acquired in tapping mode with a low stress acting upon the system. Nevertheless, the edges in Figure 3b appear more rugged than the center of the image as a result of repeated scanning. At the same time, we have also observed some healing of defects and holes in the lipid bilayer system upon repeated scanning which could be indicative of reconstitution processes. Thus, the investigated membrane system still appears to provide sufficient fluidity for structural reorganization processes within the lipid bilayer. Such a preserved “dynamic behavior” is in good agreement with the recently observed “self-healing” of two polyethylenimine-supported DMPC bilayers after their hemifusion in the surface forces apparatus.17
In summary, this AFM study provides evidence of tethered lipid bilayer formation by vesicle adsorption onto lipopolymer monolayers. Polymer hydration has been found earlier to be an important factor governing this process. Here, it was found that hypotonic osmotic stress acting upon lipid vesicles stimulates their incorporation in homogeneous bilayers on polymeric substrates. Most importantly, the formation of multilamellar and more complex surface aggregates is prevented.
Finally, it is interesting to compare our observations with a strategy for tethered bilayer formation recently reported by Cornell et al.7 In their case, lipid was simply added from ethanolic solution onto a previously prepared tethered monolayer which was then flushed by 0.1 M NaCl solution. Obviously, the polymer-supported lipid membrane is indeed a thermodynamically stable structure which can self-organize spontaneously after lipid is supplied to a polymer-supported monolayer in hydrophilic and saline environment. However, a subtle balance of experimental parameters is needed for a successful realization of these highly interesting membrane structures.
Acknowledgment
This work was supported by NIH Grant 47334 and partially supported by the MRL Program of the National Science Foundation under Award No. DMR-9123048. M.S. was partially funded by a DFG postdoctoral fellowship (Se 855/1-1).
References
- (1).Ti Tien H. Bilayer Lipid Membranes (BLM): Theory and Practice. Marcel Dekker; New York: 1974. [Google Scholar]
- (2).(a) Lipowsky R, Sackmann E, editors. Handbook of Biological Physics. Elsevier; New York: 1995. [Google Scholar]; (b) Rosoff M. Vesicles. Marcel Dekker; New York: 1996. [Google Scholar]; (c) Ti Tien H, Ottova-Leitmannova A. Membrane Biophysics. Elsevier Science; New York: 2000. [Google Scholar]
- (3).(a) Tamm LK, McConnell HM. Biophys. J. 1985;47:105. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McConnell HM, Watts TH, Weis RM, Brian AA. Biochim. Biophys. Acta. 1986;864:95. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- (4).Helm CA, Israelachvili JN, McGuiggan PM. Science. 1989;246:919. doi: 10.1126/science.2814514. [DOI] [PubMed] [Google Scholar]
- (5).Helm CA, Knoll W, Israelachvili JN. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8169. doi: 10.1073/pnas.88.18.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sackmann E. Science. 1996;271:43. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- (7).(a) Cornell BA, Braach-Maksvytis VLB, King LB, Osman PDJ, Raguse B, Wieczorek L, Pace RJ. Nature. 1997;387:580. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]; (b) Raguse B, Braach-Maksvytis V, Cornell BA, King LG, Osman PDJ, Pace RJ, Wieczorek L. Langmuir. 1998;14:648. [Google Scholar]
- (8).Bayerl TM, Bloom M. Biophys. J. 1990;58:357. doi: 10.1016/S0006-3495(90)82382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Johnson SJ, Bayerl TM, McDermott DC, Adam GW, Rennie AR, Thomas RK, Sackmann E. Biophys. J. 1991;59:289. doi: 10.1016/S0006-3495(91)82222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Koenig BW, Krueger S, Orts WJ, Majkrzak CF, Berk NF, Silverton JV, Gawrisch K. Langmuir. 1996;12:1343. doi: 10.1007/978-1-4615-5847-7_19. [DOI] [PubMed] [Google Scholar]
- (11).Groves JT, Ulman N, Boxer SG. Science. 1997;275:651. doi: 10.1126/science.275.5300.651. [DOI] [PubMed] [Google Scholar]
- (12).Groves JT, Ulman N, Cremer PS, Boxer SG. Langmuir. 1998;14:3347. [Google Scholar]
- (13).Häussling L, Knoll W, Ringsdorf H, Schmitt FJ, Yang JL. Makromol. Chem., Makromol. Symp. 1991;46:145. [Google Scholar]
- (14).Spinke J, Yang J, Wolf H, Liley M, Ringsdorf H, Knoll W. Biophys. J. 1992;63:1667. doi: 10.1016/S0006-3495(92)81742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Majewski J, Wong JY, Park CK, Seitz M, Israelachvili JN, Smith GS. Biophys. J. 1998;75:2363. doi: 10.1016/S0006-3495(98)77680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wong JY, Majewski J, Seitz M, Park CK, Israelachvili JN, Smith GS. Biophys. J. 1999;77:1445. doi: 10.1016/S0006-3495(99)76992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wong JY, Park CK, Seitz M, Israelachvili JN. Biophys. J. 1999;77:1458. doi: 10.1016/S0006-3495(99)76993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Beyer D, Elender G, Knoll W, Kühner M, Maus S, Ringsdorf H, Sackmann E. Angew. Chem., Int. Ed. Engl. 1996;35:1682. [Google Scholar]
- (19).Seitz M, Wong JY, Park CK, Alcantar NA, Israelachvili JN. Thin Solid Films. 1998;327-329:767. [Google Scholar]
- (20).(a) Hausch M, Beyer D, Knoll W, Zentel R. Langmuir. 1998;14:7213. [Google Scholar]; (b) Hausch M, Zentel R, Knoll W. Macromol. Chem. Phys. 1999;200:174. [Google Scholar]
- (21).Hausch M. Ph.D. Thesis. University Mainz; Germany: 1999. [Google Scholar]
- (22).(a) Theato P, Zentel R. J. Macromol. Sci., Pure Appl. Chem. 1999;A36:1001. [Google Scholar]; (b) Theato P, Zentel R. Langmuir. 2000;16:1801. [Google Scholar]
- (23).We have also attempted a height determination by removing regions of the bilayer applying higher loads and determining step heights at the edges of the removed regions. However, this strategy gave much more ambiguous results. Particularly, as the proximal (surface-bound) monolayer is covalently attached to the surface-anchored polymer it could not be removed completely. However, a higher number of defects was observed. The measured step heights measured on those induced defects varied from 1 to 7.5 nm, i.e., were no larger than those measured from naturally occurring defects in a freshly prepared bilayer structure.
- (24).(a) Santos NC, Ter-Ovanesyan E, Zasadzinski JA, Castanho MARB. Biophys. J. 1998;75:2119. doi: 10.1016/S0006-3495(98)77654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Reviakine I, Brisson A. Langmuir. 2000;16:1806. [Google Scholar]