Abstract
Excess of ferricytochrome c protects purified mitochondrial cytochrome c oxidase and bound cardiolipin from hydrogen peroxide-induced oxidative modification. All of the peroxide-induced changes within cytochrome c oxidase, such as oxidation of Trp19,IV and Trp48,VIIc, partial dissociation of subunits VIa and VIIa, and generation of cardiolipin hydroperoxide, no longer take place in the presence of ferricytochrome c. Furthermore, ferricytochrome c suppresses the yield of H2O2-induced free radical detectable by electron paramagnetic resonance spectroscopy within cytochrome c oxidase. These protective effects are based on two mechanisms. The first involves the peroxidase/catalase-like activity of ferricytochrome c, which results in the decomposition of H2O2, with the apparent bimolecular rate constant of 5.1 ± 1.0 M−1s−1. Although this value is lower than the rate constant of a specialized peroxidase, the activity is sufficient to eliminate H2O2-induced damage to cytochrome c oxidase in the presence of an excess of ferricytochrome c. The second mechanism involves ferricytochrome c-induced quenching of free radical(s) generated within cytochrome c oxidase. These results suggest that ferricytochrome c may have an important role in protection of cytochrome c oxidase and consequently the mitochondrion against oxidative damage.
Keywords: cytochrome c oxidase, cytochrome c, oxidative damage, peroxidase activity, free radicals, hydrogen peroxide
INTRODUCTION
The mitochondrion has a central role in the production of ATP as well as the generation of reactive oxygen species (ROS). Under physiological conditions, the mitochondrion converts ~1–2% of the consumed oxygen into superoxide anion [1]. Moreover, ROS concentrations increase significantly under certain pathological events such as ischemia-reperfusion [2], muscular dystrophy [3], atherosclerosis [4], Parkinson’s disease [5], or other age-related disorders. Mitochondrial superoxide radicals are generated primarily by two ubiquinone utilizing electron transport complexes, i.e., Complex I (NADH:ubiquinone oxidoreductase), and Complex III (ubiquinone-cytochrome c reductase) [6,7]. Normally, the resulting superoxide anion is converted to H2O2 by superoxide dismutase with subsequent conversion to H2O by catalase or glutathione peroxidase. However, in the presence of transition metals, H2O2 is converted into highly reactive hydroxyl radicals. Because the electron transfer chain components are a major source of superoxide, the local concentration of ROS near the inner membrane is potentially quite high, making the electron transfer chain itself a potential target for oxidative damage. If the resulting damage inhibits one or more of the complexes, ROS production by complexes I and III would be expected to increase in a manner analogous to that caused by electron transport inhibitors. As a result, ROS damage to the electron transfer chain could initiate a cascade of structural and functional alterations within inner mitochondrial membrane, the entire mitochondrion, and other cell components. In order to survive such a ROS attack, the mitochondrial electron transfer complexes must be equipped with efficient protective mechanisms.
Cytochrome c oxidase (CcO, EC 1.9.3.1.) is one of the mitochondrial electron transfer complexes known to be inactivated by ROS [8,9,10,11,12]. This enzyme catalyzes the transfer of electrons from ferrocytochrome c to oxygen, a reaction that proceeds through two oxy-intermediates, “peroxy-” and “ferryl-CcO”, both of which are potential free radical sources [13,14,15]. During normal electron flux, the concentration of these transient intermediates is quite low; however, during the lifetime of the mitochondrion, the potential exists for chronic free radical exposure. To avoid damage to redox-active sites, CcO is equipped with several defense mechanisms. For example, subunit III of CcO has been proposed to protect CcO from oxidative damage during enzymatic turnover [8]. An amino acid aromatic network within CcO, that facilitates radical transfer away from the redox-active, binuclear center, may also protect the enzyme from damage by radicals generated near the active site [16,17].
Cytochrome c is located in the mitochondrial inter-membrane space where its main function is to shuttle electrons from cytochrome bc1 to CcO during respiration. In addition to its function in mitochondrial respiration, cytochrome c has a significant role in activation of a programmed cell death cascade [18]. For example, dissociation of cytochrome c from the inner mitochondrial membrane is known to be a critical step in the initiation of apoptosis [19,20,21]. Cytochrome c can also function as a cardiolipin-specific oxygenase that chemically oxidizes cardiolipin to produce CL hydroperoxides [21]. The resulting oxidized CL then releases pro-apoptotic factors into the cytosol. In addition to these roles, cytochrome c is also known to alter both the generation and elimination of H2O2 [22,23,24], and to regenerate dioxygen from superoxide radical anion under conditions of oxidative stress [25].
In this study, we tested the hypothesis that CcO is protected from peroxide-induced oxidative damage by its natural mitochondrial partner, cytochrome c. This hypothesis is based upon the facts that: 1) cyt c3+ can destroy H2O2 [23,24]; and 2) cyt c3+ binding to CcO alters the conformation of the CcO catalytic binuclear center [26,27,28]. Therefore, we have examined the extent of H2O2-induced changes to purified, detergent-solubilized CcO in the presence and absence of excess ferricytochrome c.
EXPERIMENTAL PROCEDURES
Materials
Sodium cholate, hydrogen peroxide, horse heart cytochrome c (≥ 95% purity), ammonium iron sulfate hexahydrate, butylated hydroxytoluene, xylenol orange, and 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radical were purchased from Sigma-Aldrich Co. Dodecyl maltoside was from Anatrace Inc. Bovine cardiolipin was obtained from Avanti Polar Lipids. Triton X-100 was from Roche Diagnostics. Other chemicals were analytical grade.
Methods
Purification of Cytochrome c Oxidase from bovine heart
Two forms of purified CcO, designated as A and B, were used in the present work. Preparation A involves sodium cholate solubilization of sodium deoxycholate treated Keilin-Hartree heart muscle particles followed by purification of CcO by ammonium sulfate precipitation as previously described [29]. The resulting purified enzyme was dissolved at ~100 µM in pH 7.4 buffer containing 25 mM sodium cholate and stored at −60 °C. Before it was used, the enzyme was diluted to 5–10 µM with 2 mM dodecyl maltoside, pH 7.4 buffer followed by exhaustive dialysis at 4 °C against the same buffer to remove residual sodium cholate. Preparation B involves Triton X-100 solubilization of CcO from mitochondria at neutral pH followed by purification using ion-exchange chromatography in the presence of Triton X-100. Triton X-100 was replaced with dodecyl maltoside by the second ion exchange procedure [30,31]. The resulting enzyme was concentrated to ~200 µM in dodecyl maltoside containing buffer and stored at −60 °C. The first procedure produces a form of CcO that reacts slowly with heme a3 binding ligands; the second procedure produces a form of CcO that reacts quickly with these same heme a3 binding ligands [32]. The data presented in this work were obtained using five different preparations of CcO with essentially the same results. All assays were done at least in triplicate using at least two different enzyme preparations.
Reaction of Cytochrome c Oxidase with H2O2 in the Presence or Absence of Ferricytochrome c
Dodecyl maltoside-solubilized CcO, either preparation A or B, at 10 µM in 20 mM Tris-Cl containing 2 mM dodecyl maltoside was reacted with 500 µM H2O2 for 30 min at room temperature in the presence or absence 0–500 µM of cyt c3+. In experiments that included cyt c3+, H2O2 was added 5 min after cyt c3+ had been mixed with CcO. The reaction was stopped by removal of both H2O2 and cyt c3+ by HiTrap Q anion-exchange column chromatography [33]. This chromatographic procedure also removes almost all phospholipids bound to CcO, except for the 3–4 tightly bound cardiolipin [33]. The resulting CcO was subsequently analyzed for changes in activity, modification of subunits and peroxidation of bound cardiolipin. It made no difference in these experiments, whether oxidized cyt c was used directly as purchased, or fully oxidized by treatment with potassium ferricyanide followed by removal of potassium ferricyanide by dialysis for 15 hours at 4 °C. In either case, cyt c3+ concentration was determined using ε410nm = 106.1 mM−1 cm−1 [34], while the concentration of H2O2 was calculated using ε240nm = 40 M−1cm−1 [35]. The concentration of the “peroxy” and/or “ferryl” forms of CcO were determined from the difference spectra of H2O2-treated minus oxidized CcO using either ΔΔε434-412nm = 65 mM−1cm−1 [36] for the mixture of both forms, or ΔΔε607–630 = 11 mM−1cm−1 for P-form, and ΔΔε580–630 = 5.3 mM−1cm−1 for F-form [37]. Cyanide-inhibited cyt c was prepared by reacting of 500 µM cyt c3+ with 10 mM KCN for 1 hour at RT. The formation of the cyanide-cyt c3+ complex was confirmed by spectral changes at 695 nm. All visible spectra were collected using an SLM Aminco 3000 diode array spectrophotometer.
Determination of Cytochrome c Oxidase Activity
CcO activity was measured spectrophotometrically by following the pseudo-first order rate of oxidation of 25 µM ferrocytochrome c by 1.75 nM CcO at pH 7.0 in 25 mM phosphate buffer containing 2 mM dodecyl maltoside [33].
Subunit Analysis by High-Performance Liquid Chromatography
Quantitative RP-HPLC analysis of CcO subunit content was done using gradient elution from a Vydac C18 reverse-phase column (5 µm, 0.46 × 25 cm, 300-Ǻ pore size) and a Waters/Millipore liquid chromatography system [9,16,38]. The gradient was made from mixtures of solvent A (0.2% TFA in water) and solvent B (0.2% TFA in acetonitrile). The column was equilibrated with solvent A and subunits eluted at 1 mL/min with a linear gradient from 25 to 50 % solvent B in 50 min, followed by a linear gradient from 50 to 85 % solvent B in 17.5 min. Elution was monitored at 214 nm after loading 0.2 – 2.0 nmol of unmodified or H2O2-modified CcO. Percent yield of each subunit was based upon quantitative integration of peak areas as compared with unmodified, purified CcO [9,16,38]. Fractions (0.5 mL) were collected for analysis of unmodified and modified subunits by mass spectrometry.
Analysis of Cardiolipin Oxidation
The extent of conjugated diene generation within cardiolipin after exposure of CcO to H2O2 was determined spectrally after (Bligh and Dyer) extraction of all phospholipids from CcO using chloroform/methanol/water [39] and subsequent purification of cardiolipin by normal phase HPLC [40]. Conjugated diene formation was monitored by the increase in absorbance at 234 nm [41].
Mass Spectrometry
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectra were acquired on an Applied Biosystems Voyager-DE-STR operated in either the linear or reflectron mode. HPLC-electrospray ionization tandem mass spectra were obtained using a Finnigan LCQ in conjunction with a Michrom BioResources MAGIC 2002 micro HPLC connected to a home-built microspray interface. Mass spectrometry analysis of modified and unmodified CcO subunits was done as described previously [9,16,17].
EPR Spectroscopy
EPR spectra were recorded with a Varian E-6 spectrometer. Acquisition conditions of the spectra were: modulation amplitude 10 G, frequency 9.225 GHz, microwave power 200 µW and temperature 109 K. Two series of H2O2-treated samples were analyzed by EPR spectroscopy to determine the effect of cyt c3+ upon H2O2-induced radical generation within CcO. The first was a mixture of CcO and a variable amount of ferricytochrome c; the second was only cyt c3+. In CcO-containing samples, 50 µM CcO, solubilized in 1 mM dodecyl maltoside pH 7.4 buffer containing 20 mM Tris-Cl and 4 mM K2SO4, was incubated with ferricytochrome c at room temperature (RT) for 5 minutes, followed by the addition of 300 µM H2O2. After 10 sec, the samples were rapidly frozen by immersion in a methanol-dry ice bath and then transferred into liquid nitrogen. Time-dependence of the radical signal was obtained by warming the samples to room temperature. After incubation of certain time the samples were frozen and used for EPR measurement. The concentration of radical within CcO was determined by the double integration of the EPR signal and comparison of the resulting area with that of DPPH standard. DPPH was solubilized in dimethyl sulfoxide and concentration calculated from the absorbance spectrum using an extinction coefficient of ε525nm = 11.9 mM−1cm−1 [42].
Rate of H2O2 Decomposition by Ferricytochrome c and Cytochrome c Oxidase
The H2O2 concentration was measured at discrete time intervals after mixing with either cyt c3+, CcO or mixture of both proteins using the ferrous ion oxidation-xylenol orange assay (FOX2) [43]. The reaction conditions were 20 mM Tris-Cl buffer at pH 7.4, 2 mM dodecyl maltoside and 500 µM H2O2 mixed with either: 1) 500 µM cyt c3+; 2) 10 µM CcO; or 3) the mixture of 500 µM cyt c3+ and 10 µM CcO. Each reaction was initiated by the addition of H2O2, stopped at 5 min intervals by removal of H2O2 using a Microcon, Ultracel YM-3 membrane filter, with 3000 Da cutoff. Peroxide concentration in the filtrate was determined by absorbance at 560 nm 30 min after addition of the FOX2 reagent (1/9 v/v) using a standard calibration curve.
RESULTS
Ferricytochrome c Prevents H2O2-induced Oxidative Damage to CcO
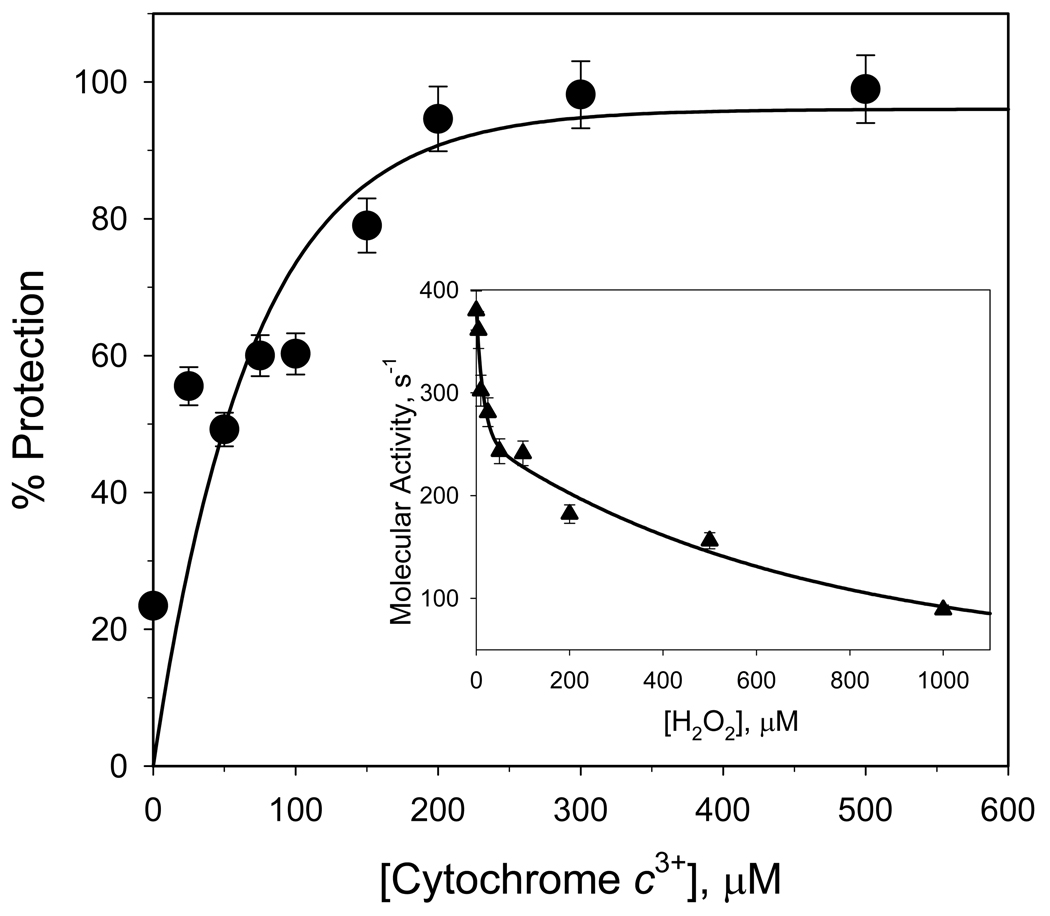
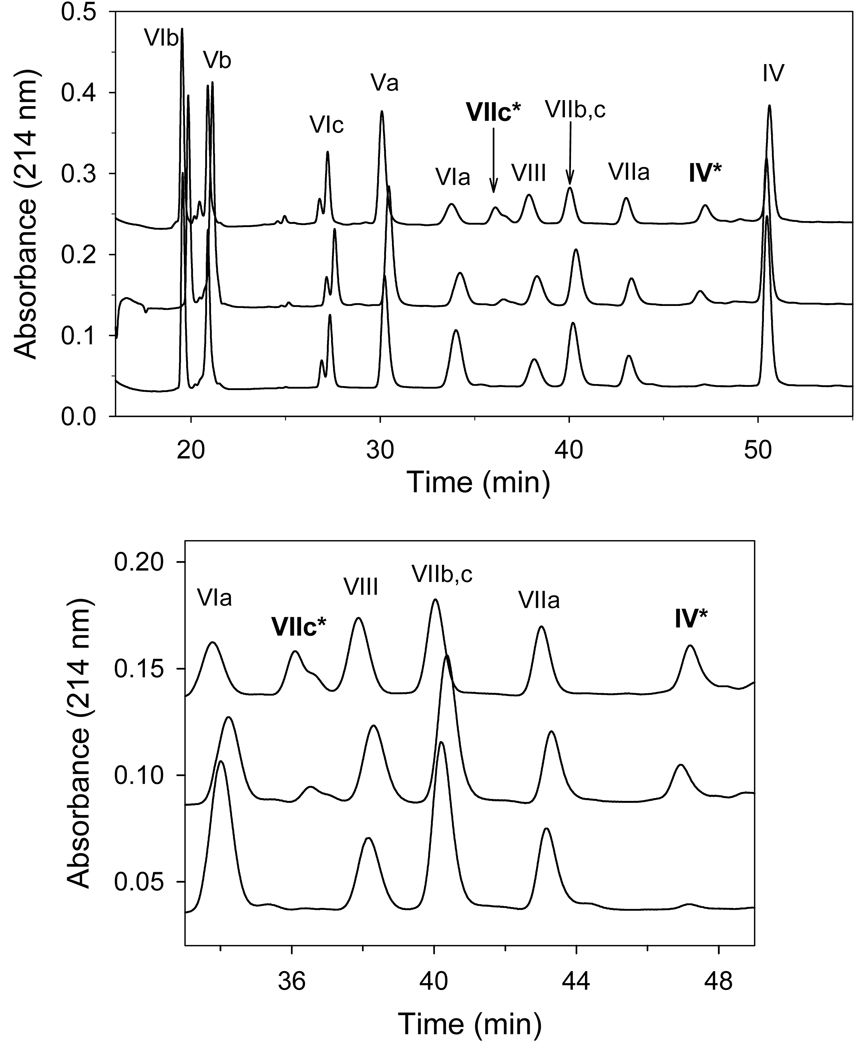
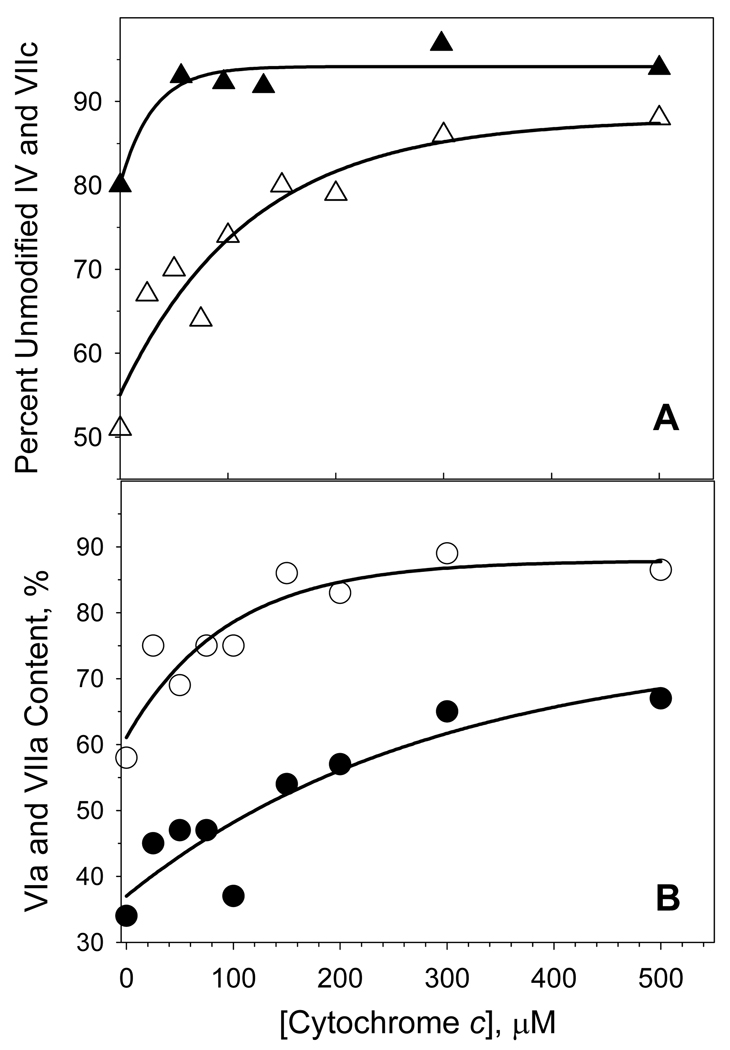
The reaction of H2O2 with detergent-solubilized CcO is known to cause a time- and concentration-dependent inactivation of the enzyme [16]. The electron-transport activity of H2O2-treated CcO decreases 50–80% after reaction with 500 µM H2O2 for 30 min. at RT (inset to Figure 1). However, significant protection from H2O2-induced inactivation occurs when at least a 20-fold molar excess of cyt c3+ is added to the reaction mixture, e.g., only 5 % inactivation occurs if 200 µM cyt c3+ is present (Figure 1). Excess cyt c3+ also prevents two other H2O2-induced effects: 1) oxidation of Trp48,IV and Trp19,VIIc and 2) partial dissociation of subunits VIa and VIIa (Figures 2 & 3). When excess cyt c3+ is not included, ~20% of IV ~50% of VIIc elute with altered elution times due to oxidation of a single tryptophan (Trp48,IV and Trp19,VIIc) within each subunit [16]. The RP-HPLC elution peaks corresponding to the two oxidized subunits are absent if excess cyt c3+ is present during H2O2 exposure, i.e., > 90% of VIIc and nearly 100% of subunit IV remain unmodified (Figure 2 & 3). H2O2-induced oxidation of Trp48,IV and Trp19,VIIc without cyt c3+ and the absence of tryptophan oxidation in presence of cyt c3+ was also monitored by mass spectrometry as described previously [16,17]. In addition, excess cyt c3+ prevents dissociation of subunits VIIa and VIa, which normally accompanies H2O2-induced oxidative damage to CcO [16]. Only ~15% of subunit VIIa dissociates from CcO when it is exposed to H2O2 in the presence of at least a 20-fold molar excess of cyt c3+, rather than >40% dissociation of this subunit in its absence. Dissociation of subunit VIa is considerably reduced as well (Figure 3, panel B).
Figure 1. Ferricytochrome c protection of CcO from inactivation by H2O2.
Main panel: Electron transfer activity of CcO after its exposure to H2O2 in the presence of different concentrations of cyt c3+. Cytochrome c oxidase (10 µM) was reacted with 500 µM H2O2 for 30 min in the presence of 0–500 µM ferricytochrome c. The reaction was stopped by removal of H2O2 using anion-exchange chromatography, and the remaining CcO activity determined spectrophotometrically. Data were fitted to a single-exponential rise to a maximum (solid line). Inset panel: Inactivation of CcO by H2O2 in the absence of cyt c3+. Cytochrome c oxidase (10 µM) was reacted with each concentration of H2O2 for 30 min., excess H2O2 removed by anion-exchange chromatography, and the remaining CcO activity determined spectrophotometrically. Data were fitted to a single-exponential decay (solid line). Assays were performed in triplicate using three different CcO preparations. The standard deviation in experiments was estimated to be ± 5.0%.
Figure 2. Cytochrome c oxidase nuclear-encoded subunit composition after exposure to H2O2 in the presence or absence of ferricytochrome c.
Cytochrome c oxidase (10 µM) was reacted with 500 µM H2O2 in the presence of either 0, 300, or 500 µM cyt c3+ (top, middle and lower chromatograms). After 30 min., H2O2 and cyt c3+ were removed by HiTrap Q anion exchange chromatography and the nuclear-encoded subunit composition quantified by RP-HPLC analysis of 0.5 nmol of CcO [27]. Upper panel: RP-HPLC elution profile for all 10 of the nuclear-encoded subunits. Lower panel: Expanded profile between 33 to 49 min to better illustrate the elution of the H2O2 modified subunits (IV* and VIIc*). The data represents typical RP-HPLC profiles of three experiments using three different CcO preparations.
Figure 3. Ferricytochrome c protection of CcO subunits from H2O2-induced modifications.
Panel A: Protection of CcO subunits IV (filled triangles) and VIIc (open triangles) from H2O2-induced oxidations by increasing cyt c3+ concentration. Panel B: Protection of CcO subunits VIa (filled circles), and VIIa (open circles) from H2O2-induced dissociation by increasing cyt c3+ concentration. The percent modification of each subunit was calculated from the areas of the relevant RP-HPLC elution peaks. Differences between chromatograms were normalized on the assumption that the area under the RP-HPLC peak corresponding to subunit Va remained constant. Solid lines are single-exponential fits to the data. The results are presented as an average of three measurements using three different preparations of CcO. The deviation in experiments was estimated to be ± 4.4%.
Ferricytochrome c Protects Cardiolipin from Hydrogen Peroxide-Induced Peroxidation
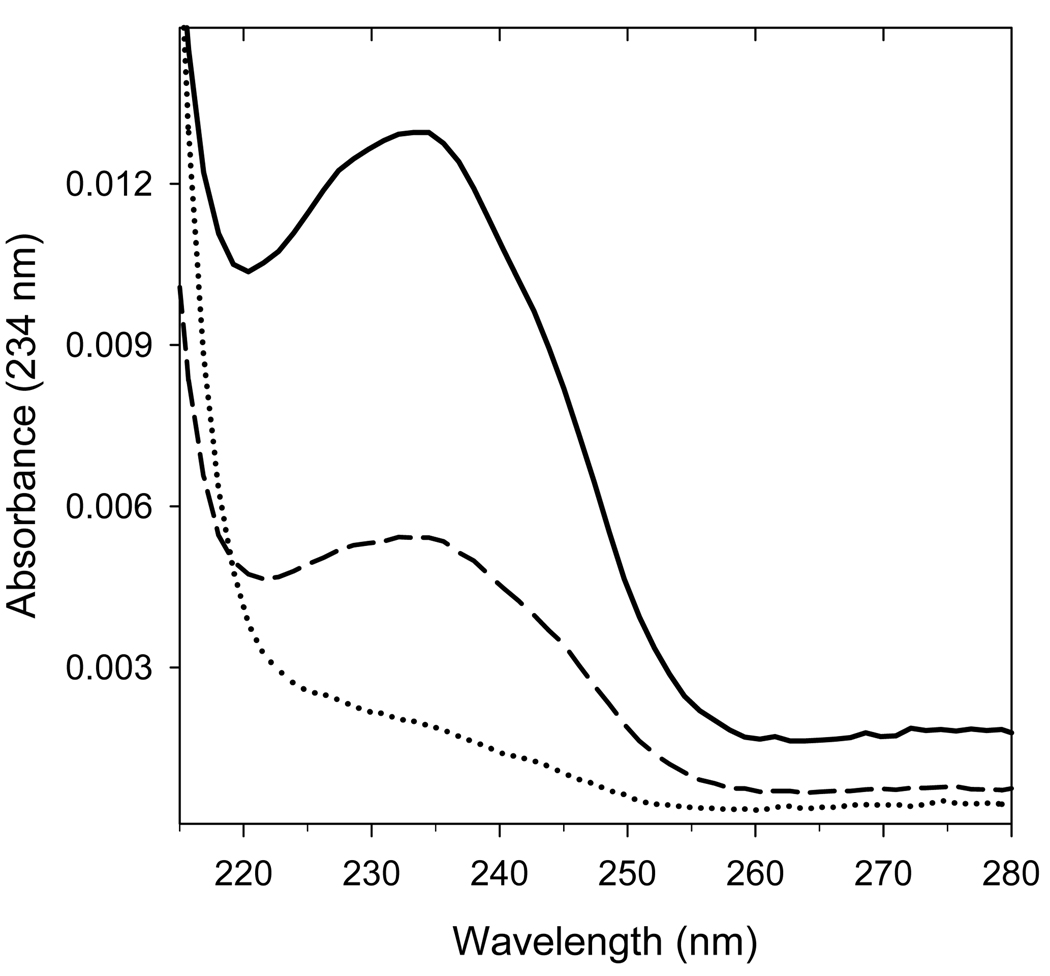
Inclusion of cyt c3+ also protects cardiolipin that is bound to CcO from H2O2-induced peroxidation. If either pure or protein-bound cardiolipin is exposed to peroxides, a significant portion of the non-conjugated double bonds is converted into conjugated dienes with characteristic absorbance at 234 nm (Figure 4, thick line). However, if CcO is exposed to high concentrations of H2O2 in the presence of excess cyt c3+, the double bonds are well protected from oxidation as evident from the significantly reduced absorbance at 234 nm (Figure 4, dashed line).
Figure 4. Excess cyt c3+ protects CcO-bound cardiolipin from H2O2-induced peroxidation.
Peroxidized cardiolipin has a characteristic absorbance maximum at 234nm due to the presence of conjugated dienes; therefore, the extent of peroxidation can be determined from the UV spectrum of cardiolipin. The UV spectrum of HPLC-purified CL before (dotted line) and after exposure of CcO to 500 µM H2O2 in either the presence (dashed line), or absence (thick solid line) of excess cyt c3+. The UV spectrum was acquired online using a diode-array UV HPLC detector. In each case, cardiolipin was extracted from 2 nmole of CcO, dissolved in ethanol, and purified by HPLC. Nearly identical results were obtained for three different CcO preparations.
Hydrogen Peroxide-induced Spectral Changes for Cytochrome c Oxidase and Ferricytochrome c
Reaction of H2O2 with CcO results in the formation of two oxy-intermediates, the “peroxy-” or P-form” and the “ferryl-” or F-form, both of which are detected by visible spectroscopy [36]. Inclusion of cyt c3+ does not alter either the relative amounts or rates at which the two intermediates are generated. Reaction of 1.2 µM CcO with 500 µM H2O2, either in the presence or absence of 8 µM cyt c3+, produces a mixture that contains 0.36–0.48 µM P-form, and 0.68–0.76 µM F-form, i.e., 35 – 40% and 60%–65%, respectively (Figure 5). The pseudo-first order rate constants for the formation of the oxy-intermediates are also nearly identical in the presence or the absence of 100 µM cyt c3+ i.e., 0.041 s−1 and 0.039 s−1, respectively (data not shown).
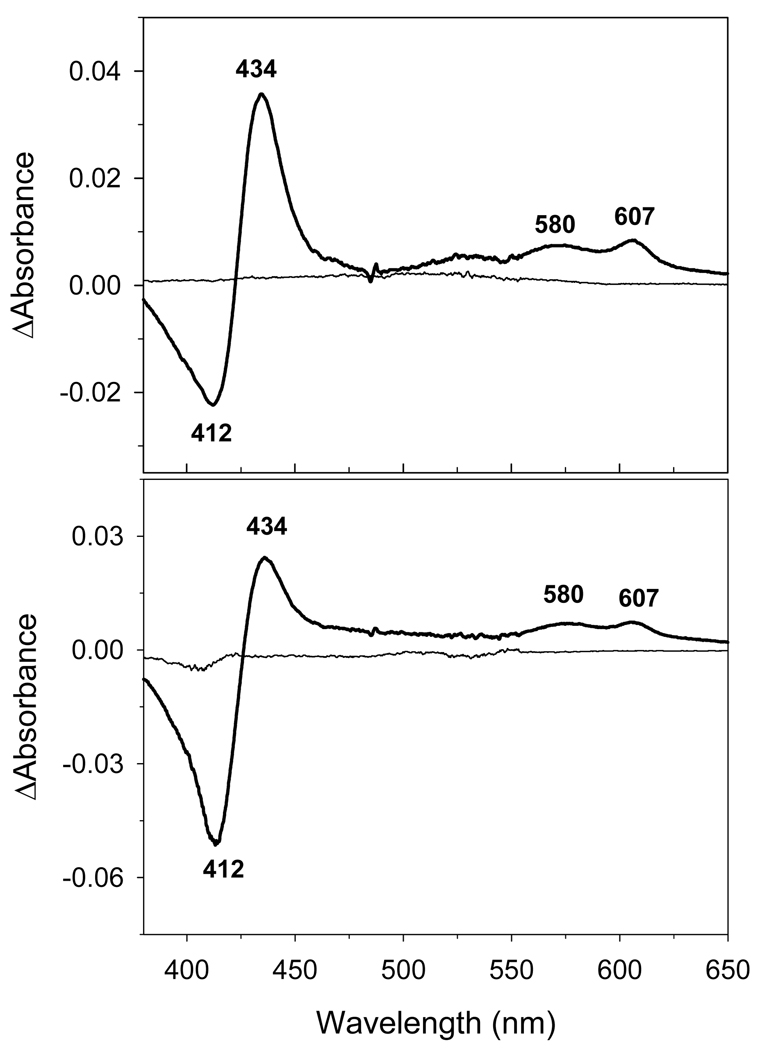
Figure 5. Generation of the H2O2-induced CcO oxy-intermediates is unaffected by the presence of excess cyt c3+.
CcO P- and F-oxy-intermediates have a characteristic CcOP+F – CcOoxid difference spectrum with a minimum at 412 nm and maxima at 434, 580 and 607 nm. The difference spectrum for CcO was generated in silico by subtracting the digitized visible spectrum of 1.2 µM oxidized CcO in 20 mM Tris-Cl buffer, pH 7.4, with 2 mM dodecyl maltoside, from the digitized spectrum acquired after its reaction with 500 µM H2O2 for 30 min in absence and presence of cyt c3+. Upper Panel: H2O2-induced difference spectrum of CcO acquired in the absence of ferricytochrome c. Lower Panel: H2O2-induced difference spectral changes of cytochrome c oxidase in presence of 8 µM ferricytochrome c. In this case, the spectrum of ferricytochrome c was recorded, set to zero and was used as a reference (thin line). CcO was then added to make the solution 1.2 µM in CcO and the difference spectrum generated as described above. Concentrations of P- and F- forms were calculated using ΔΔε607–630 = 11 mM−1cm−1 for the P-form, and ΔΔε580–630 = 5.3 mM−1cm−1 for the F-form [32]. In both A and B forms of CcO hydrogen peroxide produces essentially the same amount of intermediates.
Hydrogen peroxide also reacts with cyt c3+, but the spectral effects are minimal under the conditions used. For example, maximum absorbance in the Soret region decreases by <5% when 500 µM cyt c3+ is exposed to 500 µM of H2O2 for 30 min (Figure 6, main panel). Such exposure slightly perturbs the charge-transfer bond between the heme iron and Met80 sulfur [44] since very small absorbance changes also occur at 695 nm (Figure 6, inset). However, H2O2 does not induce any major structural changes in cyt c3+ as evidenced by the very small changes in the molar ellipticity at 417 nm (3–5%) and no significant alteration in either the far-UV or aromatic regions of the spectrum (data not shown).
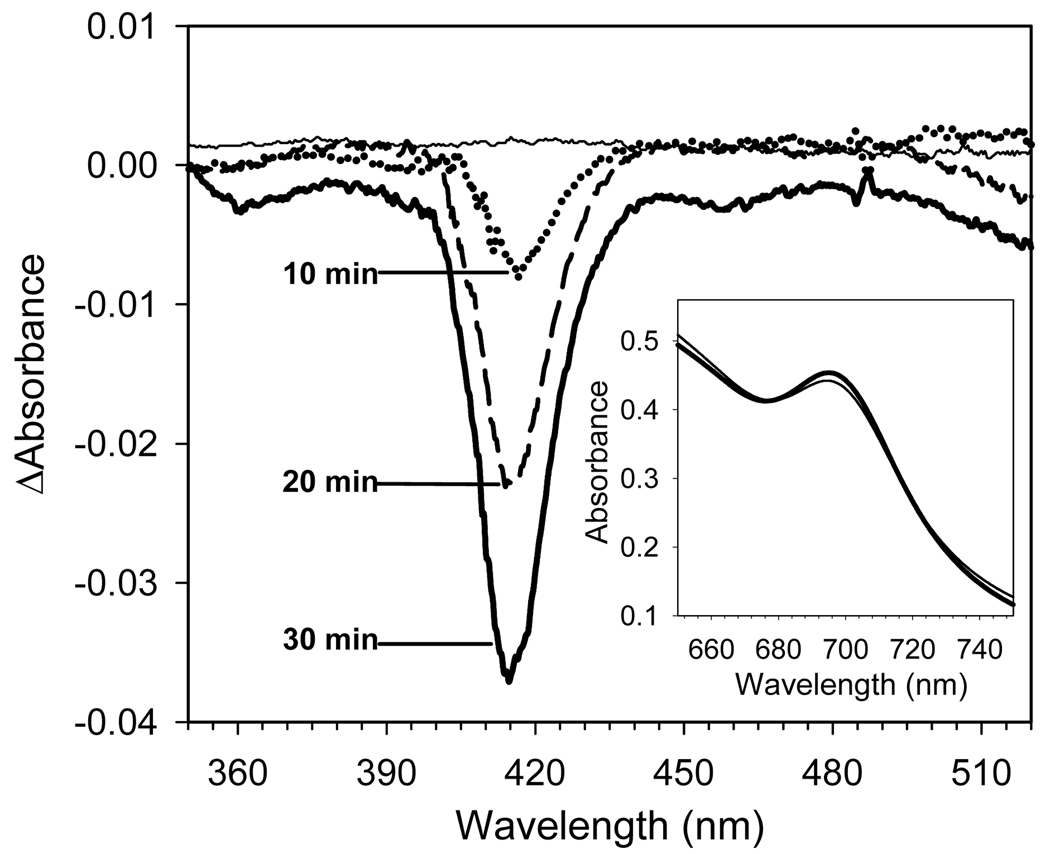
Figure 6. H2O2-induced bleaching of the visible spectrum of cyt c3+.
Main Panel: Time-dependence of H2O2 –induced changes in the Soret difference spectrum of ferricytochrome c. Ferricytochrome c (500 µM) in 20 mM Tris-Cl pH 7.4 buffer containing 2 mM dodecyl maltoside was reacted with 500 µM H2O2 at room temperature. After 0, 10, 20 and 30 min, 10 µL aliquots were diluted 100-fold with reaction buffer and the absolute spectra were recorded. The absorption maxima in absolute spectra were 0.530, 0.527, 0.512, and 0.502, respectively. The difference spectrum was generated by subtracting the digitized visible spectrum of cyt c3+ before addition of H2O2 from the digitized spectrum acquired after its reaction with H2O2. Maximum absorbance in the Soret region decreased by only 0.6%, 3.5%, and 5.3% after 10, 20 and 30 min reaction of cyt c3+ with H2O2, dotted, dashed, and solid line, respectively.
Inset Panel: H2O2-induced changes in the far visible spectrum of a mixture of cyt c3+ and CcO. Difference spectra were recorded for 500 µM cyt c3+ and 10 µM CcO versus 10 µM CcO before (thick line) and after 30 min reaction with 500 µM H2O2 (thin line). Measurements were done in triplicate with the nearly identical results.
H2O2-Induced Free Radical Generation within Cytochrome c Oxidase: Effect of Ferricytochrome c
Exposure of CcO to H2O2 generates a free radical EPR signal that is characterized by the g = 2.006 with a peak-to-trough width of 12 G (Figure 7, panel A). At 109 K this signal is saturated at a microwave power above 4 mW and no hyperfine structure is detected using amplitude modulation smaller than 10 G. A maximum yield of radical occurs 10 sec after addition of H2O2 and corresponds to ~14% of the CcO population. Inclusion of cyt c3+ suppresses the radical signal in a concentration- and time-dependent manner (Figure 7, panel B). This free radical is generated as H2O2 reacts with the catalytic binuclear center of CcO since the EPR signal is missing if the samples containing only ferricytochrome c and hydrogen peroxide, or if the catalytic site of CcO is first blocked with cyanide.
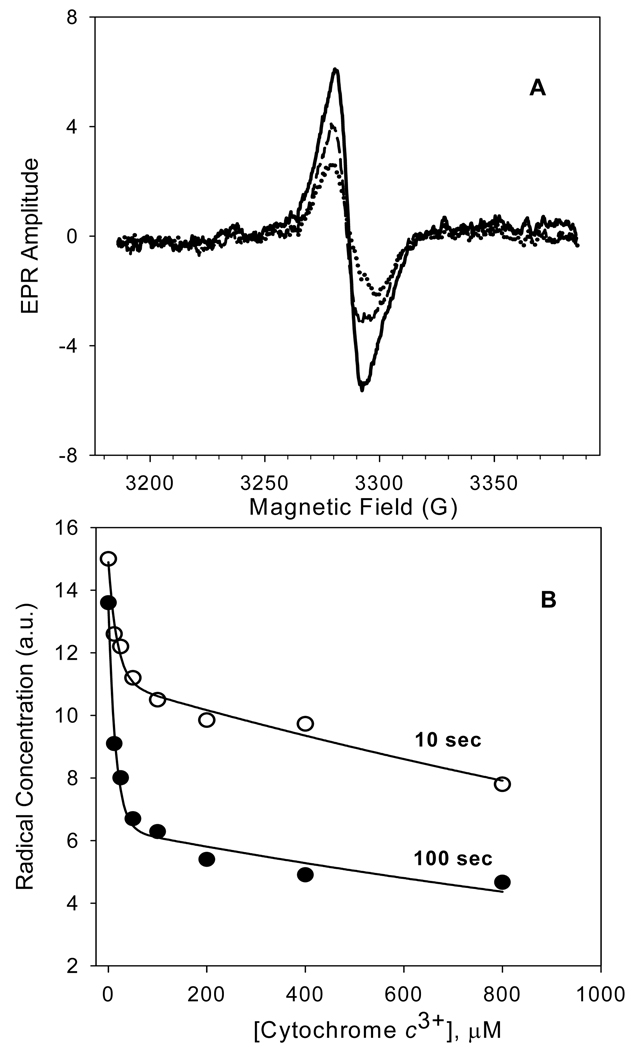
Figure 7. Ferricytochrome c suppression of the H2O2-induced free radical EPR signal of CcO.
Upper Panel: Difference EPR spectra for CcO in either the absence (solid line), or presence of 100 µM (dashed line), or 800 µM f cyt c3+ (dotted line). All samples were frozen within 10 seconds after addition of 500 µM H2O2. Each difference spectrum was calculated by subtracting the oxidized CcO spectrum from that of the H2O2 treated enzyme. Lower Panel: Dependence of the CcO radical yield upon the cyt c3+ concentration for samples reacted with H2O2 for either 10, or 100 seconds. Solid lines through each set of data are non-linear regression fits of the data to a two-term exponential decay. The reaction was initiated by addition of 500 µM H2O2 to 48.3 µM CcO solubilized in 20 mM Tris-Cl buffer, pH 7.4, containing 2 mM dodecyl maltoside and 4 mM K2SO4. Refer to Experimental Procedures for EPR experimental details. The results are presented as an average of three experiments employing two different preparations of CcO.
Decomposition of Hydrogen Peroxide by Ferricytochrome c and Cytochrome c Oxidase
Cyt c3+ exhibits peroxidase/catalase-type activity, which would be expected to decrease the H2O2 concentration [24,58]. To determine if this activity is sufficient to affect oxidative damage to CcO, the peroxidase/catalase-like activities of cyt c3+, CcO, and a mixture of both proteins were determined by monitoring the catalytic destruction of H2O2. Both proteins catalyze a single exponential decrease in the H2O2 concentration with time (Figure 8). The activity of CcO is more than ten-times greater than cyt c3+, with corresponding second order rate constants of 63.2 ± 2.5 M−1s−1 compared to a rate of 5.1 ± 1.0 M−1s−1 for cyt c3+. Using these values, a pseudo-first order rate of (3.2 ± 0.6) ×10−3 s−1 is predicted for a mixture of 10 µM CcO and 500 µM cyt c3+, which is within experimental error of the value experimentally determined, i.e., (3.8 ± 0.7) × 10−3 s−1. The peroxidase/catalase-like activities of cyt c3+ and CcO are, however, completely inhibited if proteins are first reacted with cyanide (Figure 8). Presumably, cyanide reacts with a five-ligand form of cyt c3+ to produce a cyano – Fe3+ complex, which prevents subsequent reaction of the iron with H2O2.
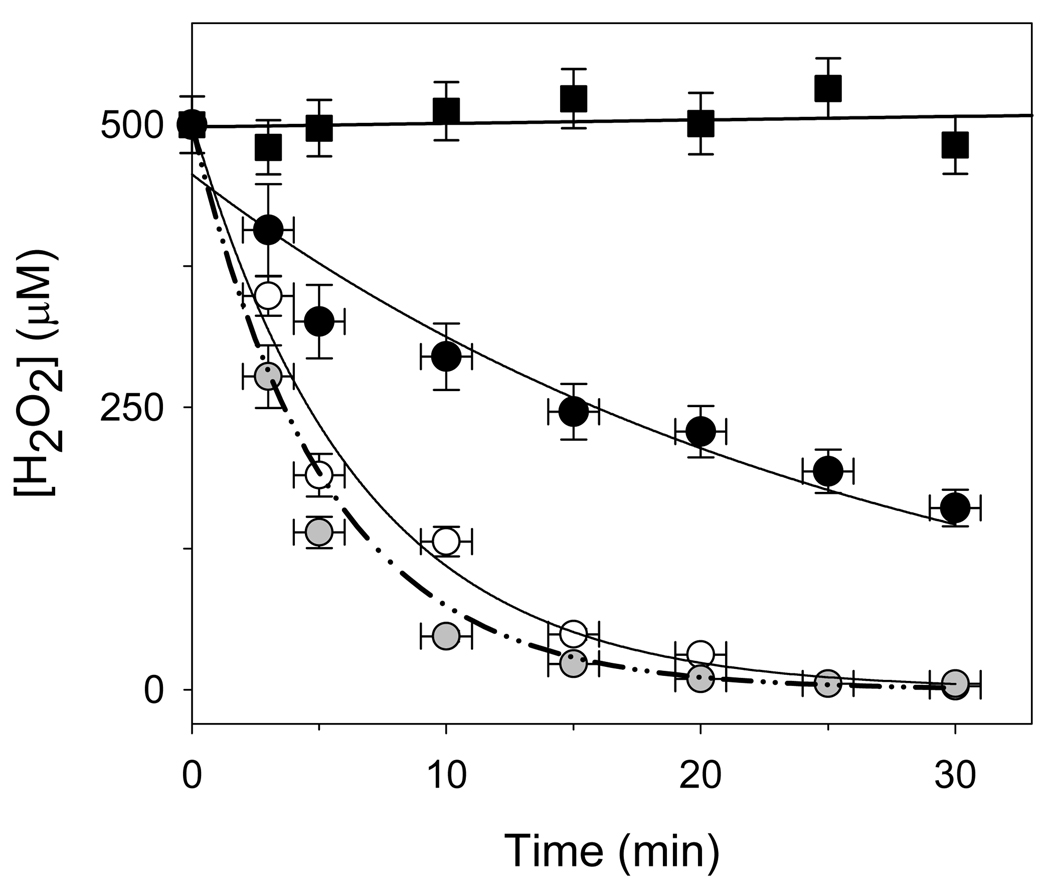
Figure 8. Decomposition of H2O2 by cyt c3+, CcO, and a mixture of cyt c and CcO.
The peroxidase activity of 500 µM cyt c3+ (unfilled circles), 500 µM cyt c3+ that previously had been reacted with 10 mM KCN for 1 hour at RT (black-filled squares), 10 µM CcO (black-filled circles), and a mixture of 500 µM cyt c3+ and 10 µM CcO (grey-filled circles) were quantified by following the destruction of 500 µM H2O2 as a function of time using the FOX2 assay. The solid lines are non-linear regression fits of the cyt c3+ and CcO data to single exponentials, with second order rate constants of 5.1 M−1s−1 and 63.2 M−1s−1, respectively (the rate constant for KCN reacted cyt c3+ was essentially zero). The dot-dash line is the theoretical first order rate for a mixture of 500 µM cyt c3+ and 10 µM CcO using the two second order rate constants. All reactions were done in 20 mM Tris-Cl, pH 7.2 buffer, containing 2 mM dodecyl maltoside. The provided data are mean values of three independent experiments using three different CcO preparations and the error bars correspond to standard deviations of the measurements.
DISCUSSION
H2O2-induced modifications to CcO are largely prevented by high concentrations of cyt c3+. In contrast to results obtained in the absence of cyt c3+, exposure of CcO to H2O2 in the presence of excess cyt c3+ leaves the electron-transport activity of CcO unaffected. Additionally, Trp19,VIIc and Trp48,IV both remain unmodified; dissociation of subunit VIIa is significantly reduced; peroxidation of cardiolipin is prevented; and the observed free radical concentration within CcO is reduced. The only H2O2-induced alteration not prevented by excess cyt c3+ is the dissociation of subunit VIa, which still occurs up to ~30%. The protective effects of cyt c3+ against time- and concentration-dependent H2O2-induced modification of CcO are the consequence of two quite different effects: (1) cyt c3+ catalyzed decomposition of H2O2, and (2) cyt c3+-induced quenching of a peroxide-induced free radical within CcO.
Cyt c3+ catalyzed decomposition of H2O2
The mechanism for cyt c3+ catalyzed decomposition of H2O2 appears similar to either that of a classical peroxidase or catalase. Such activity would seem to be unlikely since cytochrome c contains six coordinate iron structure. However, the Met80 ligand bond is relatively weak and can be displaced by rather mild perturbants, e.g., changes in ionic strength or pH, allowing external ligands, such as cyanide, azide, imidazole and peroxide, to bind to the iron of cyt c3+ [24,45,46,47,48,49,50,51]. A small portion of cyt c3+ must be present in an “open” conformation in which the 6th axial coordination bond with Met80 is broken [52] to permit such binding. Since equilibrium between the “closed” and “open” cyt c3+ favors the “closed” 6-coordinate heme species H2O2 is expected to react slowly with cyt c3+. Consistent with an “open” conformation hypothesis is the complete inhibition of peroxidase/catalase activity when cyt c3+ is first reacted with cyanide (Figure 8). Cyanide forms a strong coordination bond with any heme having an open 6th ligand position; therefore, cyanide inhibition clearly demonstrates that catalytic destruction of H2O2 by cyt c3+ requires a direct interaction of H2O2 with the iron of cyt c3+.
A peroxidase type mechanism raises the issue of an absence of reducing equivalents to complete the catalytic cycle. The small percentage of cyt c3+ in the “open” conformation would be expected to react with peroxide to form the typical peroxidase intermediate, compound I, containing ferryl oxygen, Fe(IV)═O, and a radical porphyrin cation, R•+. It is not obvious how the 2-electron reduction of compound I to regenerate cyt c3+ and water would occur, but a second H2O2 molecule may be the source of reducing equivalents, resulting in the disproportion of two peroxide molecules, i.e., a catalase-type reaction. Such a mechanism has been used to explain the H2O2-induced reduction of ferryl-oxygen compounds in both met-myoglobin [53,54] and cytochrome P450 [55]. Alternatively, the side chains of aromatic acids of protein itself may donate two electrons, i. e a peroxidase-type reaction.
Regardless of the mechanism for the peroxidase/catalase-like activity of cyt c3+, there is no doubt that cyt c3+ catalyzes the slow decomposition of H2O2 with the second order rate of 5.1 ± 1.0 M−1s−1 at pH 7.4. This rate is in good agreement with previously reported values of 4–11 M−1s−1 [56,57,58]. Although the peroxidase activity of cyt c3+ is ~6–7 orders of magnitude less than that of the 5-coordinate Fe structure in peroxidases, it is sufficient to significantly decrease the H2O2 concentration at high cyt c3+ concentration.
Cyt c3+-Induced Quenching of a CcO free radical
The peroxidase/catalase activity of cyt c3+ satisfactorily explains a diminished effect of H2O2 on CcO due to partial destruction of H2O2, but it cannot explain the rapid decrease in the H2O2-induced free radical content of CcO. Inclusion of a 2–16 fold molar excess of cyt c3+ decreases the amount of the EPR-detectable radical by 30–45% 10 s after addition of peroxide. The slow peroxidase/catalase-like activity of cyt c3+ cannot appreciably alter the H2O2 concentration during such a short time interval (refer to Fig. 7).
The origin of the EPR-detectable free radical signal is unknown, but stoichiometric amounts of radicals are generated in proximity to the heme a3-CuB site of CcO coincident with the formation of the peroxy- and ferryl-oxygen mixture. With both intermediates, the iron of heme a3 is converted to an oxo-ferryl state with simultaneous generation of a radical cation, often assigned to Y244 (e.g. Fea3IV═O CuBII Y244•+). The Y244 radical is not responsible for the EPR signal since electronic coupling of the radical to the iron and/or copper adjacent iron-copper binuclear center makes Y244•+ EPR-silent. However, radical migration from Y244 to a site distant from the binuclear center would make it EPR visible and would explain the EPR signal. We previously postulated such radical migration away from Y244 along an “aromatic wire” to explain H2O2-induced oxidative damage to CcO-tryptophans located far from the binuclear center [16,17]. The yield of EPR detectable radical(s) in these distant site(s) is sub-stoichiometric with a maximum yield of only ~14%, but any radicals remaining near the binuclear center would go undetected. Alternatively, the steady state level of radicals may be less than stoichiometric because H2O2 acts as a 2-electron reductant to destroy the Y244•+ radical cation before it can migrate away from the binuclear center (refer to prior discussion of peroxidase/catalase mechanism.
Three feasible mechanisms could explain the quenching of the EPR-detectable free radicals by cyt c3+, but none of them is entirely satisfactory. First, cyt c3+ may form a complex with CcO that sufficiently alters the conformation of near the binuclear center [26,27,28] to suppress a radical migration away from the catalytic center (Y244) to the secondary distant site(s). Consistent with this explanation is the decreased yield of the observed radical together with the inhibition of the modifications of the Trp19,VIIc, Trp48,IV and cardiolipin in the presence of ferricytochrome c. This mechanism, however, is not completely acceptable since the cyt c3+-induced CcO conformation would be expected to exhibit altered H2O2 reaction kinetics, which does not occur (generation of the oxy-intermediates and CcO peroxidase activity are nearly identical with, or without cyt c3+; refer to Figures 5 & 7).
Second, cyt c3+ may participate in protein to protein free radical transfer in which case a protein of cyt c3+ would serve as an electron donor to a radical that had migrated to the surface of CcO, e.g., Trp19,VIIc or Trp48,IV. We were unable to detect any such alterations to cyt c3+ although detection of such sub-stoichiometric amounts of radicals in a 20–50 fold excess of cyt c would be difficult.
Third, cyt c3+ could function as a shuttle of electrons to decrease free radical content. However, in absence of electron donors in our experimental conditions this mechanism is unlikely. Moreover, if such electron transfer is occurred it would have also affect the rate of formation of P and F intermediates during the reaction of oxidized enzyme with H2O2. Yet both the rates and amount of oxy-intermediates are the same in the presence and the absence of cytochrome c (Fig. 6).
We conclude that cyt c3+ assists in two ways to shield CcO against oxidative damage. First, cyt c3+ protects CcO by an external mechanism: catalytic removal of H2O2 by cyt c3+. Although the rate of peroxidase activity of cyt c3+ is lower than for a specialized catalase or peroxidase, its location and high concentration in the mitochondrion (up to 0.7 mM [59]) potentially make cyt c3+ a significant contributor in the catalytic destruction of peroxide. Moreover, according to Kagan et al., interaction of cyt c3+ with mitochondrial cardiolipin significantly increases its peroxidase activity, which can become as high as ~200 M−1s−1 [21,60].
Second, cyt c3+ may also protect CcO from oxidative damage by suppression or elimination of detrimental free radical(s) that are generated as part of normal CcO turnover. In contrast to the purified enzyme, CcO within the mitochondrion is surrounded by cyt c on the matrix side of the inner membrane and, therefore, is well protected against H2O2-induced oxidative damage. In fact, CcO in vivo is relatively resistant to H2O2–induced oxidative damage. For example, with heterozygous Mn-superoxide dismutase knockout mice, mitochondrial H2O2 increases by 40–50% with significantly reduced complex I and complex V activities, but almost no detectable change in CcO electron-transport activity [61]. Furthermore, increasing the H2O2 concentration within isolated rat heart mitochondria, (i.e., conditions that are similar to those with knockout mice), does not lead to diminished CcO activity [62]. Purified CcO, however, is susceptible to hydrogen peroxide because it is separated from these defensive mechanisms. Altogether the present study suggests that cyt c3+ may have an important role in protecting the cytochrome oxidase and consequently mitochondrion as well against oxidative impairment.
ACKNOWLEDGMENT
This work was supported by grants from UTHSCSA (University Research Council grant for AM), National Institute of Health (NIH GM024795 for NCR and NIH GM0843348 for MF), and the Robert A. Welch Foundation (AQ1481 for NCR). Mass spectrometry data were acquired in the Institutional Mass Spectrometry Laboratory of the University of Texas Health Science Center at San Antonio. The authors thank Dr. Susan Weintraub for her expert mass spectrometry analysis, Dr. Rastislav Varhač for invaluable discussions regarding these data, Ms. Tiffany McDonald-Marsh for excellent technical assistance and Dr. LeAnn K. Robinson for editorial help in preparing the manuscript.
ABBREVIATIONS
- ROS
reactive oxygen species
- CcO
bovine heart cytochrome c oxidase
- P-and F-form
peroxy- and ferryl cytochrome c oxidase oxy-intermediates
- cyt c3+
ferricytochrome c
- CL
cardiolipin (diphosphatidylglycerol)
- DM
dodecyl maltoside
- RT
room temperature
- FOX2
ferrous ion oxidation-xylenol orange assay
- HPLC
high-performance liquid chromatography
- ESI/MS
electrospray ionization mass spectrometry
- MALDI-TOF/MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- Trp48,IV
tryptophan 48 within CcO subunit IV
- Trp19,VIIc
tryptophan 19 within CcO subunit VIIc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Advances in Enzymol. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch. Biochem. Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Murphy ME, Kherer JP. Oxidation state of tissue thiol groups and content of protein carbonyl groups in chickens with inherited muscular dystrophy. Biochem. J. 1989;260:359–364. doi: 10.1042/bj2600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, cooper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J. Biol. Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 5.Cohen G. Oxidative stress, mitochondrial respiration, and Parkinson’s disease. Ann. N.Y. Acad. Sci. 2000;899:112–120. doi: 10.1111/j.1749-6632.2000.tb06180.x. [DOI] [PubMed] [Google Scholar]
- 6.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by Complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 8.Bratton MR, Pressler MA, Hosler JP. Suicide inactivation of cytochrome c oxidase: catalytic turnover in the absence of subunit III alters the active site. Biochemistry. 1999;38:16236–16245. doi: 10.1021/bi9914107. [DOI] [PubMed] [Google Scholar]
- 9.Musatov A, Carroll CA, Liu Y-C, Henderson GI, Weintraub ST, Robinson NC. Identification of 4-Hydroxynonenal-Modified Subunits of Bovine Heart Cytochrome c Oxidase. Biochemistry. 2002;41:8212–8220. doi: 10.1021/bi025896u. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJA. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- 11.Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 2007;43:1423–1438. doi: 10.1016/j.freeradbiomed.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Schenker S, Frosto TA, Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE) Biochim. Biophys. Acta. 1998;1380:336–344. doi: 10.1016/s0304-4165(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 13.Ksenzenko MYu, Vygodina TV, Berka V, Ruuge EK, Konstantinov AA. Cytochrome oxidase-catalyzed superoxide generation from hydrogen peroxide. FEBS Lett. 1992;297:63–66. doi: 10.1016/0014-5793(92)80328-e. [DOI] [PubMed] [Google Scholar]
- 14.Rich PR, Rigby SEJ, Heathcote P. Radicals associated with the catalytic intermediates of bovine cytochrome c oxidase. Biochim. Biophys. Acta. 2002;1554:137–146. doi: 10.1016/s0005-2728(02)00228-1. [DOI] [PubMed] [Google Scholar]
- 15.Wilson MT, Jensen P, Aasa R, Malmström PG, Vänngård T. An investigation by EPR and optical spectroscopy of cytochrome oxidase during turnover. Biochem. J. 1982;203:483–492. doi: 10.1042/bj2030483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musatov A, Hebert E, Carroll CA, Weintraub ST, Robinson NC. Specific modification of two tryptophans within the nuclear-encoded subunits of bovine cytochrome c oxidase by hydrogen peroxide. Biochemistry. 2004;43:1003–1009. doi: 10.1021/bi0358925. [DOI] [PubMed] [Google Scholar]
- 17.Lemma-Gray P, Weintraub ST, Carroll CA, Musatov A, Robinson NC. Tryptophan 334 Oxidation in Bovine Cytochrome c Oxidase Subunit I Involves Free Radical Migration. FEBS Lett. 2007;581:437–442. doi: 10.1016/j.febslet.2006.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Kim CN, Yang Y, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 19.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Lin H, Ye S, Liu Q-Y, Meng Z, Zhang C-M, Xia Y, Margoliash E, Rao Z, Liu X-J. Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis. Proc. Natl. Acad. Sci. 2006;103:8965–8970. doi: 10.1073/pnas.0603327103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Wang Z-B, Xu J-X. Effect of cytochrome c on the generation and elimination of O2- and H2O2 in mitochondria. J. Biol. Chem. 2003;278:2356–2360. doi: 10.1074/jbc.M209681200. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Xu JX. The operation of the alternative electron-leak pathways mediated by cytochrome c in mitochondria. Biochem. Biophys. Res. Commun. 2004;317:980–987. doi: 10.1016/j.bbrc.2004.03.144. [DOI] [PubMed] [Google Scholar]
- 24.Deterding LJ, Barr DP, Mason RP, Tomer KB. Characterization of cytochrome c free radical reactions with peptides by mass spectrometry. J. Biol. Chem. 1998;273:12863–12869. doi: 10.1074/jbc.273.21.12863. [DOI] [PubMed] [Google Scholar]
- 25.Pereverzev MO, Vygodina TV, Konstantinov AA, Skulachev VP. Cytochrome c, an ideal antioxidant. Biochem. Soc. Trans. 2003;31:1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- 26.Musatov A, Konstantinov AA. Conformational change of cytochrome a3 induced by oxidized cytochrome c. FEBS Lett. 1988;238:295–299. doi: 10.1016/0014-5793(88)80500-3. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrandt P, Heimburg T, Marsh D, Powell GL. Conformational Changes in Cytochrome c and Cytochrome Oxidase upon Complex Formation: A Resonance Raman Study. Biochemistry. 1990;29:1661–1668. doi: 10.1021/bi00458a044. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt P, Vanhecke F, Buse G, Soulimane T, Mauk A. G Resonance Raman Study of the Interactions between Cytochrome c Variants and Cytochrome c Oxidase. Biochemistry. 1993;32:10912–10922. doi: 10.1021/bi00091a047. [DOI] [PubMed] [Google Scholar]
- 29.Robinson NC, Neumann J, Wiginton D. Influence of detergent polar and apolar structure upon the temperature dependence of beef heart cytochrome c oxidase activity. Biochemistry. 1985;24:6298–6304. doi: 10.1021/bi00343a039. [DOI] [PubMed] [Google Scholar]
- 30.Soulimane T, Buse G. Integral cytochrome-c oxidase. Preparation and progress towards a three-dimensional crystallization. Eur. J. Biochem. 1995;227:588–595. doi: 10.1111/j.1432-1033.1995.tb20429.x. [DOI] [PubMed] [Google Scholar]
- 31.Fabian M, Palmer G. Hydrogen peroxide is not released following reaction of cyanide with several catalytically important derivatives of cytochrome c oxidase. FEBS Lett. 1998;422:1–4. doi: 10.1016/s0014-5793(97)01561-5. [DOI] [PubMed] [Google Scholar]
- 32.Baker GM, Noguchi M, Palmer G. The Reaction of Cytochrome Oxidase with Cyanide. J. Biol. Chem. 1987;262:59544. [PubMed] [Google Scholar]
- 33.Sedlák E, Robinson NC. Phospholipase A(2) digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Biochemistry. 1999;38:14966–14972. doi: 10.1021/bi9914053. [DOI] [PubMed] [Google Scholar]
- 34.Margoliash E, Frohwirt N. Spectrum of horse-heart cytochrome c. Biochem J. 1959;71:570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergmayer HU, Gawehn K, Grassl M. Enzymes as Biochemical Reagents in Methods of Enzymatic Analysis. In: Bergmayer HU, editor. Engl. Transl. Vol. 1. Weinheim: Verlag Chemie; 1974. p. 425. [Google Scholar]
- 36.Fabian M, Palmer G. The interaction of cytochrome oxidase with hydrogen peroxide: the relationship of compound P and compound F. Biochemistry. 1995;34:13802–13810. doi: 10.1021/bi00042a011. [DOI] [PubMed] [Google Scholar]
- 37.Wikstrom M, Morgan JE. The dioxygen cycle. Spectral, kinetic, and thermodynamic characteristics of ferryl and peroxy intermediates observed by reversal of the cytochrome oxidase reaction. J. Biol. Chem. 1992;267:10266–10273. [PubMed] [Google Scholar]
- 38.Liu Y-C, Sowdal LH, Robinson NC. Separation and quantification of cytochrome c oxidase subunits by Mono-Q fast protein liquid chromatography and C18 reverse-phase high performance liquid chromatography. Arch. Biochem. Biophys. 1995;324:135–142. doi: 10.1006/abbi.1995.9917. [DOI] [PubMed] [Google Scholar]
- 39.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 40.McDonald-Marsh T, Carroll CA, Robinson NC, Musatov A. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis of cardiolipin extracted from detergent-solubilized mitochondrial electron transfer complexes. Anal. Biochem. 2006;359:262–263. doi: 10.1016/j.ab.2006.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 42.Palmer G. Electron paramagnetic resonance. Methods Enzymol. 1967;10:594–609. [Google Scholar]
- 43.Nourooz-Zadeh J. Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol. 1999;300:58–62. doi: 10.1016/s0076-6879(99)00113-5. [DOI] [PubMed] [Google Scholar]
- 44.Schejter A, George P. The 695-nm band of ferricytochrome and its relationship to protein conformation. Biochemistry. 1964;3:1045–1049. doi: 10.1021/bi00896a006. [DOI] [PubMed] [Google Scholar]
- 45.George P, Tsou CL. Reaction between hydrocyanic acid, cyanide ion and ferricytochrome c. Biochem. J. 1952;50:440–448. doi: 10.1042/bj0500440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varhač R, Tomášková N, Fabián M, Sedlák E. Kinetics of cyanide binding as a probe of local stability/flexibility of cytochrome c. Biophys. Chem. 2009;144:21–26. doi: 10.1016/j.bpc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Varhač R, Antalík M. Correlation of acid-induced conformational transition of ferricytochrome c with cyanide binding kinetics. J. Bio. Inorg. Chem. 2008;13:713–721. doi: 10.1007/s00775-008-0357-8. [DOI] [PubMed] [Google Scholar]
- 48.Bren KL, Gray HB, Banci L, Bertini I, Turano P. Paramagnetic 1H NMR spectroscopy of the cyanide derivative of Met80Ala-iso-1-cytochrome c. J. Am. Chem. Soc. 1995;117:8067–8073. [Google Scholar]
- 49.Schejter A, Aviram I. The reaction of cytochrome c with imidazole. Biochemistry. 1969;8:149–153. doi: 10.1021/bi00829a021. [DOI] [PubMed] [Google Scholar]
- 50.Sutin N, Yandell JK. Mechanisms of the reaction of cytochrome c. J. Biol. Chem. 1972;247:6932–6936. [PubMed] [Google Scholar]
- 51.Barr DP, Mason RP. Mechanism of radical production from the reaction of cytochrome c with organic hydroperoxides. J. Biol. Chem. 1995;270:12709–12716. doi: 10.1074/jbc.270.21.12709. [DOI] [PubMed] [Google Scholar]
- 52.Cheng G, Wysocki VH, Cusanovich MA. Local stability of Rhodobacter capsulatus cytochrome c2 probed by solution phase hydrogen/deuterium exchange and mass spectrometry. J. Am. Soc. Mass. Spectrom. 2006;17:1518–1525. doi: 10.1016/j.jasms.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 53.Brittain T, Baker AR, Butler CS, Little RH, Lowe DJ, Greenwood C, Watmough NJ. Reaction of variant sperm-whale myoglobins with hydrogen peroxide: the effects of mutating a histidine residue in the haem distal pocket. Biochem. J. 1997;326:109–115. doi: 10.1042/bj3260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies MJ. Identification of a globin free radical in equine myoglobin treated with peroxides. Biochim. Biophys. Acta. 1991;1077:86–90. doi: 10.1016/0167-4838(91)90529-9. [DOI] [PubMed] [Google Scholar]
- 55.Schlichting I, Berendzen J, Chu K, Stock AM, Maves SA, Benson DE, Sweet RM, Ringe D, Petsko GA, Sligar SG. The catalytic pathway of cytochrome P450cam at atomic resolution. Science. 287:1615–1622. doi: 10.1126/science.287.5458.1615. [DOI] [PubMed] [Google Scholar]
- 56.Hamachi I, Fujita A, Kunitake T. Enhanced N-demethylase activity of cytochrome c bound to a phosphate-bearing synthetic bilayer membrane. J. Am. Chem. Soc. 1994;116:8811–8812. [Google Scholar]
- 57.Rosei MA, Blarzino C, Coccia R, Foppoli C, Mosca L, Cini C. Production of melanin pigments by cytochrome c/H2O2 system. Int. J. Biochem. Cell. Biol. 1998;30:457–463. doi: 10.1016/s1357-2725(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 58.Diederix RE, Ubbink M, Canters GW. The peroxidase activity of cytochrome c-550 from Paracoccus versutus. Eur. J. Biochem. 2001;268:4207–4216. doi: 10.1046/j.1432-1327.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- 59.Hackenbrock CR, Chazotte B, Gupte SS. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr. 1986;18:331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- 60.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transition of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang T-T, Epstein CJ, Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech. Ageing. Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 2001;276:23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]