Abstract
Since apoptosis defects limit efficacy of anti-cancer agents, autophagy has been proposed as a novel strategy for radiotherapy enhancement. We previously showed that caspase-3/7 inhibition induces autophagy and promotes radiosensitivity in vitro and in vivo. Therefore, we further investigated the mechanism by which radiation triggers autophagy in caspase-3/7 deficient cells, and found the involvement of Endoplasmic Reticulum (ER) stress. The ER activates a survival pathway, the unfolded protein response, which involves ER-localized transmembrane proteins PERK, IRE1, and ATF6. In this study, we found that PERK is essential for radiation-induced autophagy and radiosensitivity in caspase-3/7 double-knockout cells. Irradiation of these cells increased expression of phosphorylated-elf2α. Similar results were seen following administration of tunicamycin (TM), a well known ER stressor. Importantly, we found that the administration of TM with radiation in MCF-7 breast cancer cells, which are lacking functional caspase-3 and relatively resistant to many anti-cancer agents, enhances radiation sensitivity. Our findings reveal ER stress as a novel potential mechanism of radiation-induced autophagy in caspase-3/7 deficient cells and as a potential strategy to maximize efficiency of radiation therapy in breast cancer.
Keywords: autophagy, cancer, ER stress, PERK, radiation
Introduction
Most cancer therapies, including radiotherapy induce cancer cell death, such as apoptosis. However, the capacity of cancer cells to evade apoptosis limits efficacy of current treatment strategies. In our previous studies, we showed that caspase-3/7 inhibition increased autophagy and cancer radiosensitivity in vitro and in vivo (Kim et al., 2008c). Indeed, it has been shown that autophagy is promoted by radiation (Daido et al., 2005), particularly following mTOR inhibition (Kim et al., 2008b), and in cancer cells independently of apoptosis (Paglin et al., 2001). Autophagy is a survival mechanism when its primary function is to degrade long-lived proteins and recycle cellular components such as mitochondria or endoplasmic reticulum (ER). Under excessive stress, however, autophagy leads to cell death by depletion of cellular contents (Mizushima et al., 2002).
The specific mechanisms by which radiation triggers autophagy and results in enhanced radiosensitivity remain unclear in caspase-deficient cells. Therefore, we further investigated radiation-induced autophagy signaling and found that ER stress participates in the regulation of autophagy and radiosensitivity. Indeed, cellular stress from cytotoxic agents may result in survival mechanisms in different cellular compartments, including ER. Disruption of any function of ER causes ER stress and activates a cytoprotective signaling cascade called unfolded protein response (UPR) (Kim et al., 2009). In mammalian cells, the UPR is mediated by three ER-localized transmembrane proteins acting as sensors: RNA-activated protein kinase-like ER kinase (PERK), inositol-requiring enzyme-1 (IRE1), and activating transcription factor-6 (ATF6) (Kim et al., 2008a; Lai et al., 2007). PERK belongs to a family of eukaryotic translation initiation factor 2α (eIF2α) kinases that regulate translational control during UPR. Phosphorylation of eIF2α by activated PERK leads to transient attenuation of global protein synthesis, but induces translation of activating transcription factor 4 (ATF4). Activated-IRE1 and ATF6 trigger signaling cascades promoting transcription factors such as XBP-1 and CHOP, and ER chaperones such as GRP78/BiP. During ER stress, the loss of cyclin-D1 results in G1-arrest and subsequently provides a window to restore cell homeostasis. However, prolonged or excessive ER stress triggers programmed-cell death pathway, usually apoptosis (Dahmer, 2005; Hitomi et al., 2004; Ma et al., 2002). Among ER stressors, tunicamycin (TM), a naturally occurring antibiotic, induces ER stress by blocking biosynthesis of N-linked oligosaccharides and inhibiting protein glycosylation (Elbein, 1987). Under prolonged accumulation of misfolded non-glycosylated proteins in ER, cells are unable to restore cell homeostasis and undergo apoptotis (Kaufman, 1999). More recently, it has been shown that ER stress also induces autophagy through activation of autophagosome formation with LC3-conversion in yeast (Yorimitsu et al., 2006), and a growing body of evidence adds to the upregulation of ER stress proteins being directly involved in autophagy (Kim et al., 2008a; Kouroku et al., 2007).
Here, we first show that radiation-induced autophagy is mediated by ER stress, and more specifically by PERK. Using our caspase-3/7 knock-out cells that are defective in apoptosis, we then show that the PERK-eIF2α branch of ER stress regulates radiosensitivity. Irradiation of these cells elevated levels of phosphorylated-eIF2α, one of the substrates for activated caspase-3. Similar results were seen following TM administration. Importantly, we found that TM in MCF-7 breast cancer cells, which lack functional caspase-3 and are relatively insensitive to many anti-cancer agents (Devarajan et al., 2002), enhances radiosensitivity. Our findings reveal ER stress as a novel mechanism of radiation-induced autophagy in caspase-deficient cells and as a potential strategy to enhance efficiency of radiotherapy in breast cancer.
Materials and Methods
Cell culture and reagents
Primary mouse embryonic fibroblasts (MEF) derived from WT and caspase-3/7-double-knockout (DKO) mice and immortalized by transfection with SV40-T-antigen containing plasmid, were generously provided by Dr. Richard Flavell (Yale University, New Haven, CT). MEFs were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen Corporation) supplemented with 1mM non-essential amino acids, 10% fetal bovine serum, 1% penicillin-streptomycin, and 0.5µmol/L 2-mercaptoethanol (kindly provided by Dr. Ronald Wek, Indiana University, Indianapolis). Human breast cancer MCF-7/neo and MCF-7/casp3 cells were kindly provided by Dr. Marja Jäättelä (Danish Cancer Society, Copenhagen, Denmark)(Janicke et al., 1998; Ostenfeld et al., 2005) and cultured in RPMI 1640 containing 10% fetal bovine serum, 1% penicillin-streptomycin, and 400µg/ml G418 (Research Products International Corp., IL). Tunicamycin was purchased from Sigma (St. Louis, MO).
siRNA transfection
siRNA eIF2α, siRNA PERK, siRNA ATF6α, siRNA IRE1α (mouse), and siRNA control were purchased from Santa Cruz (SABT). Cells were transfected with 25nM siRNAs using Lipofectamine 2000. Transfected cells were used for subsequent experiments 24h later.
Immunoblotting
Cells (0.5×106) were treated with radiation and drugs, and collected at various time points, and then washed with ice-cold PBS twice before addition of lysis buffer (M-PER Mammalian Protein Extraction reagent, Pierce) which included protease inhibitor cocktail and phosphatase inhibitor cocktail I (Sigma, 5µl/ml). Protein concentration was quantified by Bio-Rad. Equal amounts of protein were loaded into each well and separated by 12.5% SDS-PAGE gel, and transfered onto PVDF-membranes (BIO-RAD). Membranes were blocked with 5% nonfat dry milk in PBS-T for 1h at room temperature. Blots were incubated with caspase-3 (Cell Signaling), P-PERK (Cell Signaling), PERK (SABT), P-eIFα (ser51,Upstate), eIFα (Cell Signaling), IRE1α (SABT), ATF6α (SABT), calrecticulin (Cell Signaling), GRP78 (SABT), and actin antibodies for 1h at 4°C. Goat anti-rabbit IgG secondary antibody (1:5000,SABT) was incubated for 45min at room temperature. Western blots were developed using chemoluminescence detection system (PerkinElmer) according to manufacturer’s protocol, and autoradiography.
Immunoprecipitation
Cells (5×105) received 5Gy (137Cs irradiator). After 30min, they were harvested and lysed in isotonic immunoprecipitation (IP) buffer (142.5mM KCl, 5mM MgCl2, 10mM HEPES, and 0.25% Nonidet P-40) which included protease inhibitor cocktail and phosphatase inhibitor cocktail I (Sigma, 5µl/ml). Cell lysates (400µg) were incubated with PERK-antibody overnight and precipitated with protein A/C Sepharose for 2h. Equal amounts of protein were fractionated by 8% SDS-PAGE, and transferred to PVDF-membranes (BIO-RAD). Western blots were developed using chemoluminescence detection system (PerkinElmer) according to manufacturer’s protocol, and autoradiography.
Clonogenic Assay
Cells were irradiated with 0-6Gy (dose rate of 1.8Gy/min) using 137Cs irradiator (J.L. Shepherd and Associates, Glendale, CA). After irradiation, cells were incubated at 37°C for 8–10 days. Cells were fixed for 15min with 3:1 methanol:acetic acid and stained for 15min with 0.5% crystal violet (Sigma) in methanol. After staining, colonies were counted by naked eye (cutoff of 50 viable cells). Surviving fraction was calculated as (mean colony counts)/(cells inoculated)×(plating efficiency (PE)), with PE defined as (mean colony counts)/(cells inoculated for irradiated controls). The dose enhancement ratio (DER) was calculated as the dose (Gy) for radiation alone divided by the dose (Gy) for radiation plus additional agent (normalized for agent toxicity) necessary for a surviving fraction of 0.25.
Autophagy assay
Cells were transfected with 2.5µg GFP-LC3 (a gift from Dr. Mizushima), elF2αwt and elF2αmt-(Ser51) plasmids using lipofectamine reagent (Invitrogen Life Technologies). After 24h, cells were treated with 5Gy. After 48h, GFP-LC3 fluorescence was observed under a confocal fluorescence microscope. Cells with punctate-GFP signaling were counted as autophagic cells because of the characteristic lysosomal localization of LC3-protein during autophagy (Mizushima et al., 2001). Punctate-GFP cells were quantified by randomly selecting three separate 100X fields and counting the number of punctate-GFP cells/field. The percent of punctate-GFP cells per total GFP-transfected cells was calculated (experiments conducted in triplicate).
Statistical analysis
All statistical analyses were performed with a Student's t-test (unpaired, 2-tailed, n=3). Differences were considered statistically significant when p<0.05. Values are expressed as mean±S.D.
Results
Radiation-induced autophagy is a PERK-dependent ER stress process in caspase-3/7 DKO cells
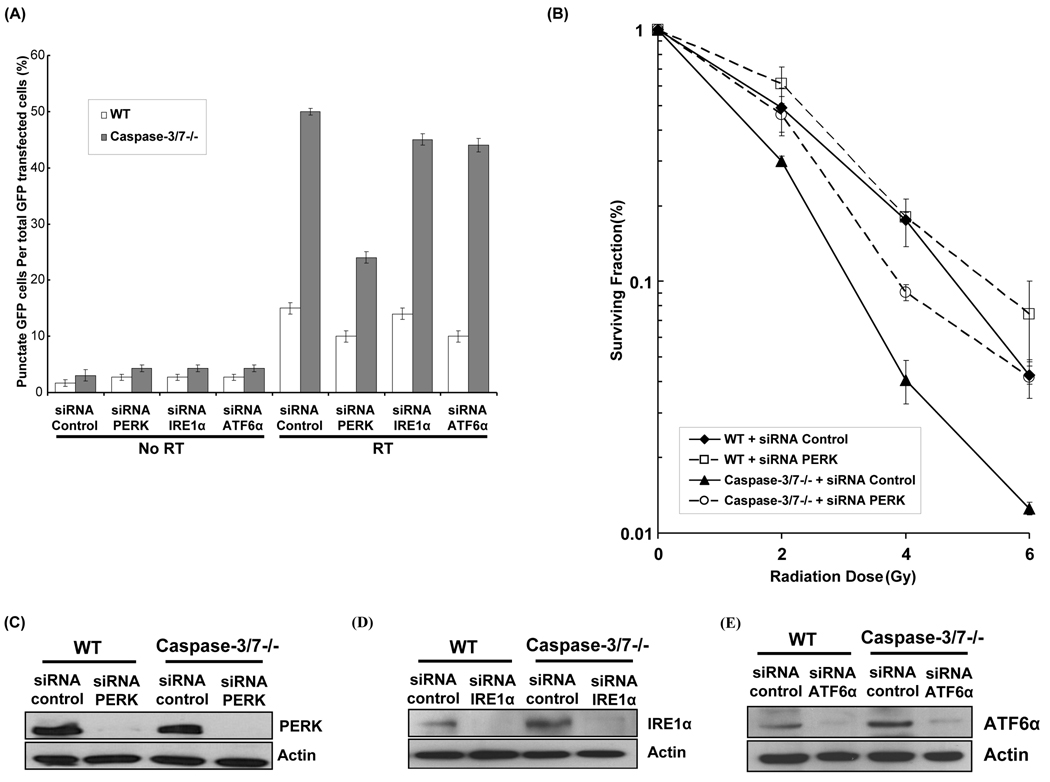
We have previously showed an increased radiosensitivity in caspase-3/7 deficient cells, which is mediated by autophagy (Kim et al., 2008c). To evaluate whether ER stress regulates radiation-induced autophagy in these cells and to determine which ER stress resident proteins are involved in the process, WT and caspase-3/7 DKO cells were first transfected with siRNA control or siRNA against PERK, IRE1α and ATF6α in presence or absence of radiation (5Gy). Autophagy was determined by counting cells exhibiting GFP-LC3 punctate pattern (Mizushima et al., 2002). As shown Figure 1A, PERK knock-down in caspase-3/7 DKO cells significantly decreased punctate GFP-LC3 expression compared to cells treated with siRNA control after irradiation (24±1 vs. 50±0.5, p=0.0007). In contrast, knock-down of IRE1α and ATF6α in caspase-3/7 DKO cells resulted in smaller reduction in GFP-LC3 punctate expression compared to siRNA control (p=0.005 and p=0.009, respectively). The efficacy of siRNAs PERK, IRE-1α, and ATF6α in reducing their intended target proteins is shown in Figures 1C, D and E. To more specifically determine the role of PERK in the regulation of radiosensitivity, we performed clonogenic assays with WT and caspase-3/7 DKO cells, which were transfected with siRNA control or siRNA PERK. As shown in Figure 1B, a significant increase of survival was observed in caspase-3/7 DKO cells treated with siRNA PERK as compared to siNRA control (DER=0.79, p=0.005). Treatment with siRNA PERK, however, had no significant radiosensitizing effects in WT cells as compared to control (DER=1, p=0.06). These data suggest that ER stress protein PERK is a key mediator in radiation-induced autophagy in caspase-3/7 DKO cells and an important regulator of radiosensitivity in absence of caspase-3/7.
Figure 1. Radiation-induced autophagy is PERK-dependent in caspase-3/7 DKO MEF cells.
(A) Wild-type (WT) and caspase-3/7 DKO MEF cells (caspase-3/7−/−) were transfected with 25nM siRNA against PERK, IRE1α and ATF6α. After 5hrs, cDNA GFP-LC3 (3µg) transfection was performed. The transfected cells were irradiated with 0 Gy or 5 Gy. After 48 hrs the percentage of cells with characteristic punctate GFP-LC3 fluorescence pattern was calculated relative to all GFP-positive cells. This was done in triplicate and error bar is shown as mean ± S.D. (B) Wild-type (WT) and caspase-3/7 DKO MEF cells were transfected with 25nM siRNA against PERK for 24hrs and were irradiated (0–6 Gy). After 8 days, surviving colonies were stained and scored. Values shown are the means ± S.D. of three separate repeated experiments. (C) PERK expression levels were determined by Western blotting using lysates from WT and caspase-3/7 DKO MEF cells treated with siRNA against control and PERK. Actin was probed to demonstrate equal loading. (D) IRE1α expression levels were determined by Western blotting using lysates from WT and caspase-3/7 DKO MEF cells treated with siRNA against control and IRE1α. Actin was probed to demonstrate equal loading. (E) ATF6α expression levels were determined by Western blotting using lysates from WT and caspase-3/7 DKO MEF cells treated with siRNA against control and ATF6α. Actin was probed to demonstrate equal loading.
PERK mediates radiation-induced autophagy via eIF2α in caspase-3/7 DKO cells
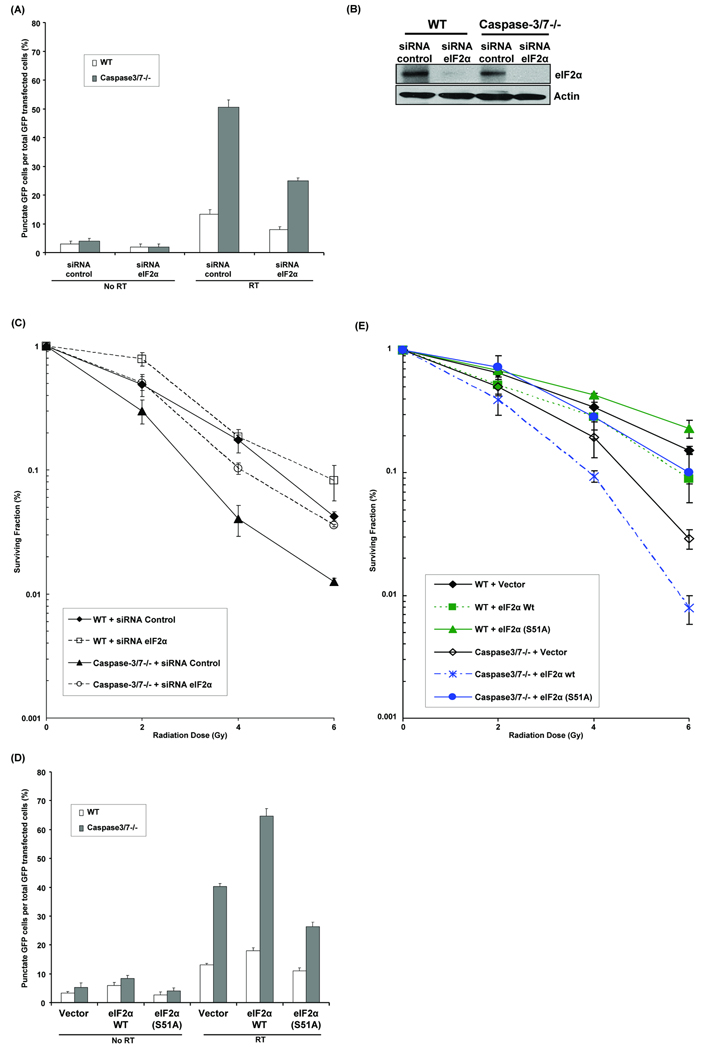
Earlier work has revealed that LC3 conversion leading to autophagy is associated with eIF2α phosphorylation by PERK in C2C5 and MEF cells treated with polyglutamine aggregates, an ER stressor (Kouroku et al., 2007). To determine whether radiation-induced autophagy in caspase-3/7 DKO cells is dependent on eIF2α, WT and caspase-3/7 DKO cells were transfected with siRNA control or siRNA eIF2α, then were transfected with GFP-LC3 plasmid, and finally irradiated or not. As shown in Figure 2A, siRNA eIF2α reduced the percentage of cells with GFP-LC3 punctates by half in irradiated caspase-3/7 DKO cells as compared to siRNA control (25±1 vs. 56±2.5, p=0.003). The resulting decrease in eIF2α expression after siRNA eIF2α transfection is shown in Figure 2B. In addition, radiosensitivity was explored using clonogenic assays (Figure 2C). siRNA eIF2α abolished the sensitizing effects of caspase-3/7 deletion as compared to siRNA control in caspase-3/7 DKO cells (DER=0.74, p=0.001), and to a similar level in WT cells treated with siRNA control. In WT cells transfected with eIF2α siRNA, however, there was a survival advantage compared to WT with siRNA control (DER=0.94, p=0.01).
Figure 2. eIF2α mediates radiation-induced autophagy in caspase-3/7 DKO MEF cells.
(A) Wild-type (WT) and caspase-3/7 DKO MEF cells were transfected with 25nM siRNA against eIF2α. After 5hrs, cDNA GFP-LC3 (3µg) was transfected in cells and then irradiated with 0Gy or 5Gy. After 48hrs the percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. This was done in triplicate and error bar is shown as mean ± S.D. (B) eIF2α expression levels were determined by Western blotting using lysates from WT and caspase-3/7 DKO MEF cells treated with siRNA against control and eIF2α. Actin was probed to demonstrate equal loading. (C) Wild-type (WT) and caspase-3/7 DKO MEF cells were transfected with 25nM siRNA against eIF2α for 24hrs. They were then irradiated with 0–6 Gy. After 8 days, colonies were stained and scored. Values shown are the means ± S.D. of three separate repeated experiments. (D) Wild-type (WT) and caspase-3/7 DKO MEF cells were transfected with eIF2α dominant-negative mutant (S51A) or eIF2α plasmids. After 5hrs, cDNA GFP-LC3 (3µg) was transfected in cells and then irradiated with 0Gy or 5Gy. After 48hrs the percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. This was done in triplicate and error bar is shown as mean ± S.D. (E) Wild-type (WT) and caspase-3/7 DKO MEF cells were transfected with eIF2α dominant-negative mutant (S51A) or eIF2α plasmids for 24hrs. They were then irradiated with 0–6 Gy. After 8 days, colonies were stained and scored. Values shown are the means ± S.D. of three separate repeated experiments.
To confirm the siRNA experiments results, we used a dominant-negative mutant allele of eIF2α that encodes a protein with single-amino acid substitution at position-51 (S51A) that inhibit or delay phosphorylation of endogenous eIF2α (Scheuner et al., 2001). As shown in Figure 2D, transfection with eIF2α (S51A) mutant plasmid reduced significantly the percentage of cells with GFP-LC3 punctates in irradiated caspase-3/7 DKO cells compared to vector control (26.3±1.5 vs. 40.3±1, p=0.0004). In contrast, overexpression of eIF2α wt in caspase-3/7 DKO cells significantly increased the percentage of cells with GFP-LC3 punctates compared to vector control (64.7±2.5 vs 40.3±1, p=0.0001). We also used the eIF2α (S51A) mutant in clonogenic assays (Figure 2E), which showed that caspase-3/7 DKO cells expressing eIF2α mutant were more resistant to radiation compared to the ones transfected with empty vector plasmid (DER=0.78, p=0.009). Conversely, overexpression of eIF2α wt in caspase-3/7 DKO cells increased significantly clonogenic cell death compared to vector control (DER=1.53, p=0.008). Together, these results suggest that eIF2α mediates radiation-induced autophagy and radiosensitivity in absence of caspase-3/7, similarly to PERK.
Phosphorylation of eIF2α by PERK and ER stress markers are induced in response to radiation in caspase-3/7 DKO cells
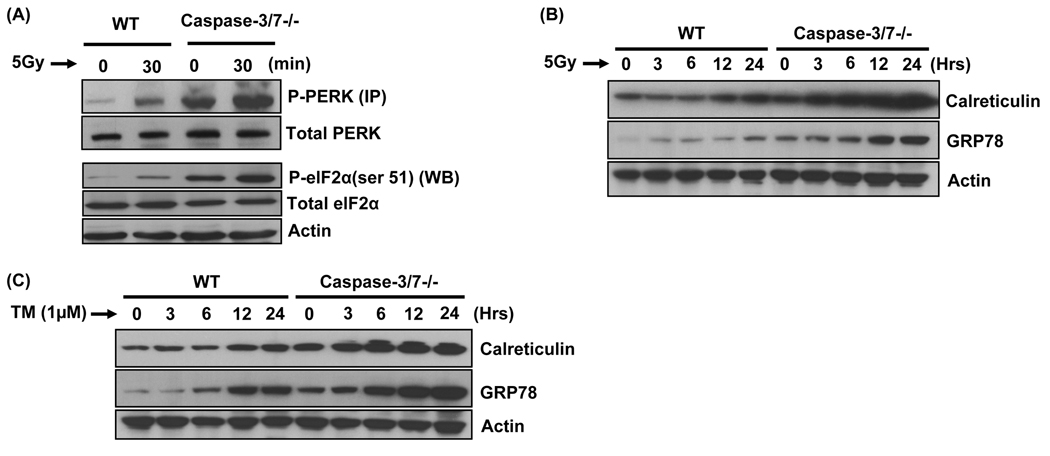
The above results lead to the hypothesis that PERK mediates radiation-induced autophagy by eIF2α phosphorylation. We therefore evaluated the contribution of PERK to eIF2α phosphorylation in WT and caspase-3/7 DKO cells after irradiation. Levels of phosphorylated-(activated) PERK (P-PERK) and phosphorylated-(deactivated) eIF2α (P-eIF2α) were determined by immunoprecipitation and immunoblotting, respectively (Figure 3A). As expected, radiation triggered PERK activation and induced eIF2α phosphorylation. These data suggest that radiation results in robust PERK/eIF2α signaling in caspase-3/7 deficient cells and at lower levels in WT cells. In addition, Figure 3B shows that spontaneous levels of P-eIF2α are elevated in caspase-3/7 DKO cells as compared to WT cells, suggesting that the lack of caspase-3/7 promotes eIF2α phosphorylation.
Figure 3. Radiation induces ER stress markers and phosphorylation of eIF2α.
(A) Wild-type (WT) and caspase-3/7 DKO MEF cells were irradiated (5Gy). Then, PERK was immunoprecipitated (IP) with anti-PERK antibody from WT and caspase-3/7 DKO lysates and the PERK immunocomplexes were separated by SDS-PAGE and immunoblotted with P-PERK and total PERK. Wild-type (WT) and caspase-3/7 DKO MEF cells were irradiated (5Gy) and the cells were then harvested after 30 min for immunoblotting analyses for P-eIF2α (ser 51) and total eIF2α. Actin was probed to demonstrate equal loading. (B) Wild-type (WT) and caspase-3/7 DKO MEF cells were irradiated (5Gy) and the cells were then harvested after 30 min for immunoblotting analyses for Calreticulin and GRP78. Actin was probed to demonstrate equal loading. (C) Wild-type (WT) and caspase-3/7 DKO MEF cells were treated with tunicamycin (1µg/ml) and the cells were then harvested after 30 min for immunoblotting analyses for Calreticulin and GRP78. Actin was probed to demonstrate equal loading.
To further validate whether radiation induces ER stress in our model, we assessed the levels of GRP78 (Lee, 2005) and calreticulin (Gelebart et al., 2005), two well-know ER stress markers. As shown in Figure 3B, radiation was able to robustly increase calreticulin levels in caspase-3/7 DKO cells and to a lower extent in WT cells. Similarly, GRP78 levels were significantly promoted by radiation in caspase-3/7 DKO cells at 12h and 24h, while the marker showed a modest increase already after 3h and more significantly at 24h in WT cells. We compared these findings using tunicamycin (TM), a classical inducer of ER stress (Figure 3C). As expected, TM increased GRP78 and calreticulin expression in a time-dependent manner in both cells. Interestingly, effects of TM on both markers were more pronounced in caspase-3/7 DKO cells as compared to WT cells, suggesting an association between absence of caspase-3/7 and promotion of ER stress. Taken together, these results demonstrate that radiation induces ER stress in caspase-3/7 DKO cells.
Tunicamycin induces P-eIF2α, resulting in autophagy and radiosensitization of caspase-3/7 DKO cells
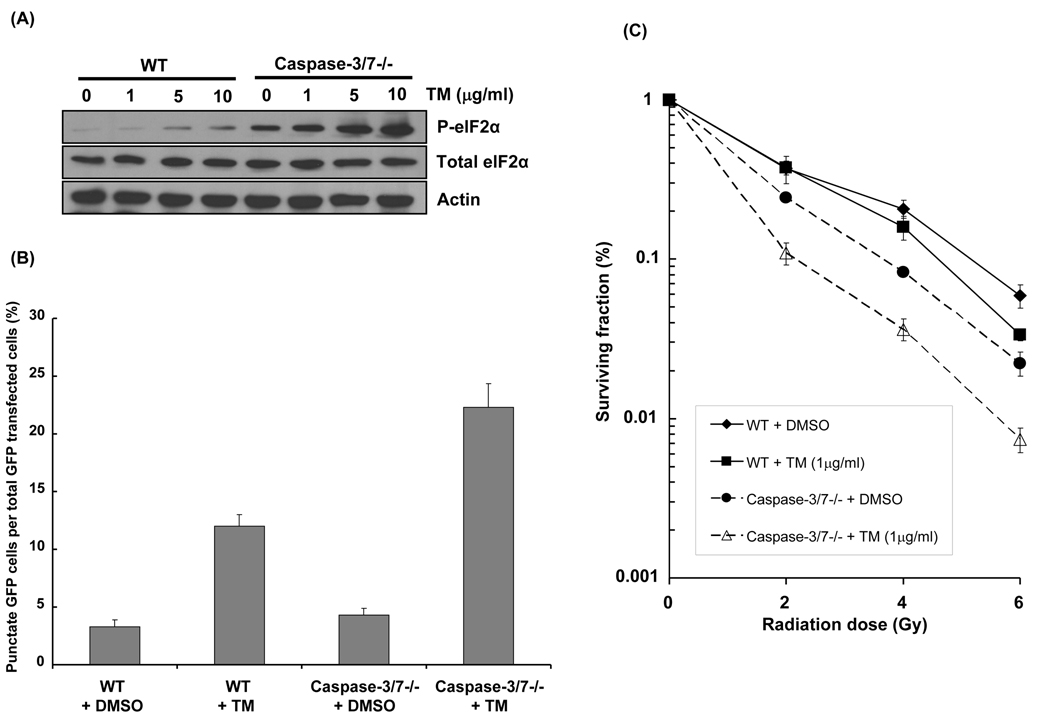
We confirmed our results by investigating the effects of TM, an ER stress-inducer. Figure 4A shows P-eIF2α expression 30min after administration of 0–10µg/ml TM in WT and caspase-3/7 DKO cells. As expected, TM induced P-eIF2α in WT cells. Moreover, TM resulted in higher, more robust increase in P-eIF2α levels in caspase-3/7 DKO cells compared to WT cells. Using a GFP-LC3 assay, we then determined the effect of TM on autophagosomes formation (Figure 4B). In caspase-3/7 DKO cells, 22.3% of cells treated with TM exhibited a characteristic GFP-LC3 punctate pattern, compared to 4.3% in DMSO group (p=0.007). Although to a lesser extent, the percentage of cells exhibiting the punctate pattern was increased by TM in WT cells as well (3.3±0.58 vs. 12±1, p=0.006). These findings suggest that TM-induced ER stress results in increased autophagy via eIF2α phosphorylation, particularly high in caspase-3/7 deficient cells. To test TM effects on radiosensitivity, WT and caspase-3/7 DKO cells were used in clonogenic survival assays. As shown in Figure 4C, induction of ER stress by TM significantly radiosensitized WT cells (DER=1.14, p=0.02) compared to DMSO. However, TM resulted in the greatest enhancement in radiosensitivity in caspase-3/7 DKO cells (DER=1.57, p=0.04) compared to DMSO. Taken together, these data confirm that radiation and TM induce robust eIF2α activation in caspase-3/7 deficient cells and suggest that TM enhances both autophagy and radiation sensitivity in both WT and caspase-3/7 deficient cells.
Figure 4. Tunicamycin (TM), an ER stressor induces P-eIF2α, resulting in autophagy and radiosensitization of caspase-3/7 DKO MEF cells.
(A) Wild-type (WT) and caspase-3/7 DKO MEF cells were treated with TM (0–10 µg/ml). Cells were then harvested after 30 min for western analyses detecting P-eIF2α and total eIF2α. Actin was probed to demonstrate equal loading. (B) Wild-type (WT) and caspase-3/7 DKO MEF cells were transfected with cDNA GFP-LC3 (3µg) for 24h and were treated with TM (1µg/ml) for 30 min. After 48h, the percentage of cells with characteristic punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. This was done in triplicate and error bar is shown as means ± S.D. (C) Wild-type (WT) and caspase-3/7 DKO MEF cells were treated with TM (1 µg/ml) and then irradiated with 0–6 Gy. After 8 days, colonies were stained and scored. Values shown are the means ± S.D. of three separate repeated experiments.
Tunicamycin increases radiation-induced autophagy and enhances radiosensitivity of MCF-7 breast cancer cells
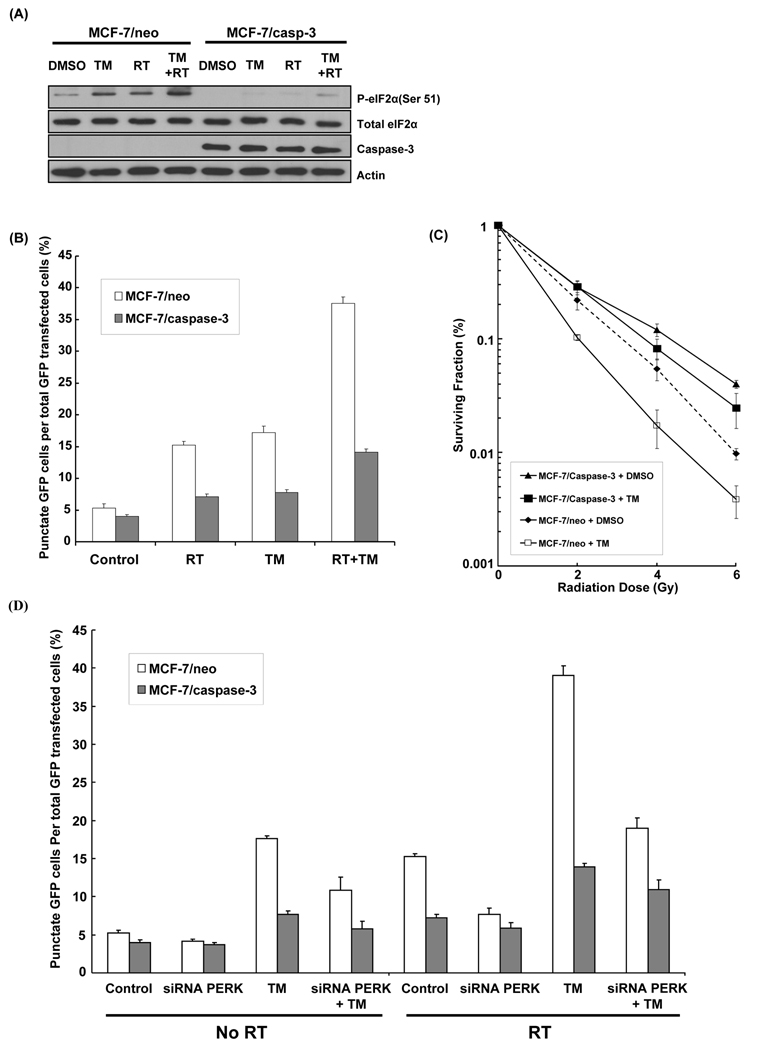
Since the MCF-7/neo cancer cell line lacks caspase-3 (Janicke et al., 1998; Ostenfeld et al., 2005), these cells are relatively resistant to radiation-induced apoptosis (Essmann et al., 2004). Therefore, we applied our findings by investigating the effects of radiation and TM on MCF-7/neo and MCF-7/casp3 (expressing ectopic caspase-3) cells. First, P-eIF2α levels were compared in MCF-7/neo and MCF-7/casp3 following radiation (5Gy) or TM (1µg/ml), or combination of both (Figure 5A). Both agents increased levels of P-eIF2α in MCF-7/neo as compared to MCF-7/casp-3, consistent with earlier data showing an increase in P-eIF2α in caspase-3/7 DKO vs. WT cells (Figure 3A). Combination of radiation and TM further increased P-eIF2α levels in MCF-7/neo cells as compared to radiation alone. In MCF-7/casp-3 cells, P-eIF2α increased after combination treatment but remained lower than in MCF-7/neo cells. Then, we determined the effects of TM on autophagy in both MCF-7 cell lines (Figure 5B). Combined radiation/TM resulted in the highest percentage of positive GFP-LC3 punctate cells in the MCF-7/neo group (37.5%) compared to radiation (15.2%, p=0.001) or TM alone (17.2%, p=0.0001), while only 5.3% autophagic cells were detected in control group. In contrast, autophagic cells were detected in a maximum of 14.3% (p=0.0006) of MCF-7/casp-3 cells upon radiation/TM treatment. Finally, clonogenic assays showed that MCF-7/neo cells were more radiosensitive than MCF-7/casp-3 cells (DER=1.31, p=0.01) (Figure 5C). Treatment with TM increased sensitivity of MCF-7/neo cells (DER=1.47, p=0.02) compared to DMSO, while sensitivity of MCF-7/casp-3 cells remained similar. These data suggest that TM, an ER stressor, increases radiation-induced autophagy and enhances radiosensitivity of MCF-7 cancer cells, which lack caspase-3.
Figure 5. Effects of TM on radiation-induced autophagy and radiosensitivity in a MCF-7 breast cancer cell model.
(A) MCF-7/neo and MCF-7/caspase-3 cells were treated with TM (1 µg/ml), radiation (5 Gy), or combination of both, and were then harvested after 30 min for immunoblotting analyses for P-eIF2α (ser 51), total eIF2α and caspase-3. Actin was probed to demonstrate equal loading. (B) MCF-7/neo and MCF-7/caspase-3 cells were transfected with cDNA GFP-LC3 (3 µg) for 24 hrs and were treated with TM (1 µg/ml for 30 min), radiation (5 Gy) and combination TM (1 µg/ml for 30 min) plus radiation (5 Gy). After 48 hrs, the percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. This was done in triplicate and error bar is shown as means ± S.D. (C) MCF-7/neo and MCF-7/caspase-3 cells were treated with TM (1 µg/ml) and then irradiated with 0–6 Gy. After 8 days, colonies were stained and scored. Values shown are the means ± S.D. of three separate repeated experiments. (D) MCF-7/neo and MCF-7/caspase-3 cells were transfected with cDNA GFP-LC3 (3 µg) for 24 hrs and were treated with TM (1 µg/ml for 30 min), radiation (5 Gy), siRNA PERK, and combination of these agents. After 48 hrs, the percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. This was done in triplicate and error bar is shown as means ± S.D.
We next assessed whether the effects of TM on radiation-induced autophagy and radiosensitivity in MCF-7 cancer cells are processes dependent on PERK. Autophagy assays showed that TM alone was able to increase autophagosome formation from 5% to 18% in MFC-7/neo cells, while PERK knockdown reduces TM-induced autophagy to 10% (Figure 5D). When combined to radiation, TM results in 40% autophagic cells (vs. 15% for radiation alone control group) and siRNA PERK significantly reduced radiation and TM-induced autophagy to 18% in MFC-7/neo cells. In contrast, PERK knockdown did not significantly reduce TM-induced autophagy in MCF-7/casp-3 cells treated with radiation or not. These results suggest the importance of PERK-mediated autophagy in MCF-7/neo cells treated with radiation and TM. In clonogenic assays (Figure 6), the ablation of PERK with siRNA in MCF-7/neo cancer cells abolished the effects of radiation and TM on cell survival (DER=0.52, p=0.01), suggesting PERK dependence for radiosensitization after treatment with TM and radiation.
Figure 6. Effects of PERK knockdown on TM-induced radiosensitization of MCF-7 breast cancer cells.
MCF-7/neo and MCF-7/caspase-3 cells were treated with TM (1 µg/ml), siRNA PERK, or combination of both, and then irradiated with 0–6 Gy. After 8 days, colonies were stained and scored. Values shown are the means ± S.D. of three separate repeated experiments.
Discussion
The purpose of this study was to explore the mechanisms of radiation-induced autophagy in caspase-3/7 deficient cells. Our findings revealed that ER stress, mainly through activation of the PERK/eIF2α axis, regulates radiation sensitivity in caspase-3/7 deficient cells and to a much lower extent in WT cells as well. Importantly, we also demonstrate that MFC-7 breast cancer cells, which are relatively resistant to radiation-induced apoptosis because they lack caspase-3, are more sensitive to radiation when an ER stressor, in this case TM, is administered concurrently.
The ability of tumor cells to evade apoptosis is a hallmark of cancer, which relative resistance to apoptosis constitutes an important clinical problem. As an alternative cell death pathway to apoptosis, autophagy is currently an important research target for therapy, mainly because many anti-cancer agents induce autophagy and its implication in tumorigenesis. We previously demonstrated that caspase-3/7 inhibition increased autophagy and subsequently enhanced cancer radiosensitivity in vitro and in vivo (Kim et al., 2008c). Here, we show that ER stress signaling is a mechanism participating in radiation-induced autophagy. Several recent reports have shown that ER stress is associated to autophagy, and mainly as an attempt of cell survival (Bernales et al., 2006; Ding et al., 2007; Kouroku et al., 2007; Ogata et al., 2006; Yorimitsu et al., 2006). Although the opposite outcome is not yet well explored, we and others have shown that excessive or prolonged accumulation of stress results in autophagic cell death, especially if apoptosis is unavailable (Cao et al., 2006; Kim et al., 2008b; Kim et al., 2008c; Kim et al., 2006; Ullman et al., 2008). Here, we also confirmed that ER stress-induced autophagy would result in radiosensitization in absence of caspase-dependent apoptosis. Indeed, PERK knock-down resulted in more radioresistance in WT and caspase-3/7 DKO cells compared to their respective controls (Figure 1B). Similarly, eIF2α knock-down (Figure 2C) or overexpression of eIF2α (S51A) mutant (Figure 2E) increased radioresistance in WT and caspase-3/7 DKO cells compared to controls. These data are consistent with a recent study showing that ER stress can result in necrosis-like cell death promoted by autophagy in Bax/Bak DKO cells or Bcl-xL-overexpressing cells that are thus apoptosis defective (Ullman et al., 2008). We also previously reported that Bax/Bak DKO cells were more radiosensitive through autophagy and that autophagy inhibition using ATG5/Beclin-1 siRNAs induces radioresistance (Kim et al., 2006). Additionally, our prior studies showed that autophagy modulates radiosensitivity of MDA-MB-231 breast and H460 lung cancer cells (Kim et al., 2008c; Kim et al., 2006). Consistently, we here report that, in absence of caspase-3/7, TM increased autophagy by five-fold and enhanced radiosensitivity with a DER of 1.57 (Figure 4). In scenarios when autophagy leads to cell survival, caspase-3, in addition to its role of apoptosis executioner, may prevent protection by autophagy in order to accelerate cell death. Indeed, one function of autophagy is to eliminate protein aggregates and misfolded proteins in order to limit ER stress response and subsequent apoptosis (Ding et al., 2007). Thus, one may speculate that absence of caspase-3/7 allows the promotion of autophagy and lead to more survival. However, when stress is excessive or unable to resolve, autophagy becomes a self-destructive process, as illustrated in our study. Nevertheless, autophagy and its cross-talk with apoptosis is complex (Eisenberg-Lerner et al., 2009) and deserves further investigation for better characterization.
Regarding the signaling of ER stress-induced autophagy, it is still unclear which UPR transducer has a role in mammalian cells. Indeed, Yorimitsu et al. showed that ER stress (induced by dithiothreitol or TM) triggers autophagy in yeast cells, through IRE1-signaling (Yorimitsu et al., 2006). Consistently, Ogata et al. reported that IRE1 is required for ER stress-induced autophagy in SK-N-SH cells treated with TM or thapsigargin (Ogata et al., 2006). This is contrasting with our findings, which indicate that mainly PERK knock-down significantly decreased autophagosome formation (Figure 1A), particularly in caspase-3/7 DKO cells. Accordingly, decrease in autophagosome formation correlated with increased radioresistance, suggesting that PERK/eIF2α is required for radiation-induced autophagy (Figure 1B and Figure 2C). This discrepancy may suggest a different mechanism for autophagy activation in caspase-3/7 deficient cells as opposed to yeast or SK-N-SH cells. Nevertheless, our findings are consistent with the study by Kouroku et al., which demonstrated that PERK-eIF2α, and not IRE1, induces autophagy by ER stress in MEF and C2C5 cells treated with polyglutamine-aggregate (Kouroku et al., 2007). Of note, the association between eIF2α- phosphorylation and starvation-induced autophagy was previously reported in yeast and mammalian cells (Talloczy et al., 2002). As shown in Figure 4A, TM increased P-eIF2α, in WT and caspase-3/7 DKO cells, with a particularly robust induction in the latter. Similarly, P-eIF2α levels were induced by TM in MCF-7/neo cancer cells compared to MCF-7/casp-3 cells (Figure 5A). Therefore, among the two UPR pathways, the specific signaling of ER stress-mediated autophagy appears to vary depending on cell type and stress stimulus.
Interestingly, our study suggests the presence of a spontaneous level of P-PERK and P-eIF2α in caspase-3/7 deficient cells regardless of radiation, as opposed to WT cells (Figure 3A). As we know, PERK mediates its effects by phosphorylating (inactivating) eIF2α (Harding et al., 1999). In the context of ER stress, inactive P-eIF2α has been shown to reduce ATF4, which mRNA translation is dependent on eIF2α phosphorylation at Ser51 (Scheuner et al., 2001). In phosphorylated serine absence, ATF4 mRNA is transcribed, but not translated. More recently, Kouroku et al. demonstrated that Ser51-phosphorylation is necessary for LC3 conversion, and thus autophagosome formation (Kouroku et al., 2007). Consistently, we found decreased autophagosome formation and radiosensitvity using an eIF2α (S51A) mutant in irradiated caspase-3/7 DKO cells (Figures 2D and 2E). Conversely, eIF2α overexpression further increased radiation-induced autophagy and radiosensitivity in caspase-3/7 DKO cells. These results support the idea that the enhanced sensitivity of caspase-3/7 deficient cells is mediated by PERK/eIF2α phosphorylation.
It has been previously shown that active caspase-3 cleaves eIF2α at its C-terminal in Saos-2, HeLa, K562, and Jurkat T cells (Marissen et al., 2000; Satoh et al., 1999). Therefore, another hypothesis that we tested is whether caspase-3 cleaves eIF2α in our model. To detect the endogenous cleavage of eIF2α, we used two commercially available antibodies: one antibody (Ab-A) recognizing the C-terminus, the other one (Ab-B) recognizing the N-terminus. So, the event of cleavage will eliminate the C-terminus and make the cleaved product be recognized by Ab-B but not Ab-A. We induced active recombinant caspase-3 in WT cells, and found a reduction of uncleaved-eIF2α and total-eIF2α levels (Supplementary Figure 2A). This is consistent with previous findings that caspase-3 cleaves eIF2α at C-terminus, altering its function (Marissen et al., 2000; Satoh et al., 1999). We then found that eIF2α cleavage by caspase-3 is promoted by TM in WT cells and, although to a lesser extent, radiation as well (Supplementary Figure 2B). Similarly to experiment shown in Supplementary Figure 2B, we also tested eIF2α cleavage by active caspase-3 after apoptosis induction by radiation and TM in caspase-3/7 DKO cells (Supplementary Figure 2C). As expected, caspase-3 was not detected in caspase-3/7 DKO cells, and high levels of uncleaved-eIF2α were detected as opposed to WT cells. These results suggest that radiation, similarly to TM, reduces uncleaved-eIF2α levels in WT cells, and not in caspase-3/7 deficient cells. Thus, these data suggest that active caspase-3 is associated with cleavage of eIF2α after exposure to stressors such as radiation and that cleaved-eIF2α could potentially be an additional factor limiting radiation-induced autophagy in WT cells.
Function of ER is disrupted in many diseases, including cancer, thus activating UPR, mainly to resolve cellular stress. In addition, tumor microenvironment is characterized by a “baseline” level of ER stress response that promotes tumor development and metastasis (Lee, 2007; Romero-Ramirez et al., 2004). It has previously been shown by immunohistochemistry studies that caspase-7 is downregulated in 85% of colon cancer samples compared with normal mucosa (Palmerini et al., 2001). Similarly, in a study examining caspase-3 expression in malignant breast tissue samples, it was showed that 75% of breast tumor samples lacked caspase-3 transcript and expression, while remaining samples showed substantial decreases in caspase-3 expression (Devarajan et al., 2002). These results suggest that defects in caspase-3 or caspase-7 are prevalent in human breast and colon cancer. Therefore, we wanted to translate our findings from caspase-deficient MEFs cells to cancer models that harbor defects in caspase-3. MCF-7 human breast carcinoma cells have been used to study ER stress mechanisms (Delom et al., 2007a; Delom et al., 2007b), though not related to autophagy. In our study, we looked at autophagy levels in these cells as well as MCF-7/casp-3 cells following treatment with TM and radiation (Figure 5). Data showed almost 40% of cells lacking caspase-3 underwent autophagy following combination treatment, as opposed to only 15% of cells expressing caspase-3. As anticipated, absence of caspase-3 also correlated with increased radiosensitization, consistent with our observations in caspase-3/7 DKO cells (Figure 4C). Taken together, these data support the use of an ER stressor, such as TM, to sensitize MCF-7 cancer cells to radiation. Of note, classic agents inducing ER stress such as TM are broadly toxic and subsequently not suitable for use in cancer patients. However, drugs including proteasome inhibitors, selenium, and fatty acid synthase inhibitors were shown to induce ER stress in addition to their specific targeted effects, and, therefore, are more indicated for clinical trials.
The current study provides novel findings that radiation-induced ER stress mediates autophagy. Additionally, our data suggest that caspase-3 has a critical role in modulating the PERK/eIF2α pathway following radiation. To our knowledge, this is the first report of radiation-induced autophagy through UPR mechanisms. Many cancers exhibit multiple deregulations in cell death pathways, allowing for the subsequent promotion of tumor cell survival. As we showed here, there is a potential for novel anticancer strategies to overcome resistant cancer cells with defective apoptosis machinery in order to improve overall therapeutic outcomes.
Supplementary Material
Representative photographs in wild-type (WT) and caspase-3/7 DKO MEF cells show that the characteristic punctate appearance of GFP-LC3, indicative of autophagosome formation, increases with radiation in both cell types.
(A) Cell extracts from WT MEF cells were incubated with active recombinant caspase-3 for 0, 1 and 6h. eIF2α with intact C-terminus or N-terminus were then probed with the respective antibodies. (B) Wild-type (WT) MEF cells were treated by tunicamycin (TM) (1µg/ml for 12h or 24h) or radiation (10 Gy for 24h and 48h) and were then harvested for immunoblotting analyses for caspase-3, cleaved caspase-3, uncleaved eIF2α, and total eIF2α. Actin was probed to demonstrate equal loading. (C) Caspase-3/7 DKO MEF cells were treated by tunicamycin (TM) (1µg/ml for 12 hrs or 24 hrs) or radiation (10 Gy for 24 hrs and 48 hrs) and were then harvested for immunoblotting analyses for caspase-3, cleaved caspase-3, uncleaved eIF2α, and total eIF2α. Actin was probed to demonstrate equal loading.
Acknowledgments
This work was supported in part by NCI 1R01 CA125842-01A1 and DOD BC030542.
Footnotes
Conflict of Interest statement
Authors declare no competing interests.
References
- Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- Dahmer MK. Caspases-2, -3, and -7 are involved in thapsigargin-induced apoptosis of SH-SY5Y neuroblastoma cells. J Neurosci Res. 2005;80:576–583. doi: 10.1002/jnr.20471. [DOI] [PubMed] [Google Scholar]
- Daido S, Yamamoto A, Fujiwara K, Sawaya R, Kondo S, Kondo Y. Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy. Cancer Res. 2005;65:4368–4375. doi: 10.1158/0008-5472.CAN-04-4202. [DOI] [PubMed] [Google Scholar]
- Delom F, Emadali A, Cocolakis E, Lebrun JJ, Nantel A, Chevet E. Calnexin-dependent regulation of tunicamycin-induced apoptosis in breast carcinoma MCF-7 cells. Cell Death Differ. 2007a;14:586–596. doi: 10.1038/sj.cdd.4402012. [DOI] [PubMed] [Google Scholar]
- Delom F, Fessart D, Chevet E. Regulation of calnexin sub-cellular localization modulates endoplasmic reticulum stress-induced apoptosis in MCF-7 cells. Apoptosis. 2007b;12:293–305. doi: 10.1007/s10495-006-0625-4. [DOI] [PubMed] [Google Scholar]
- Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Essmann F, Engels IH, Totzke G, Schulze-Osthoff K, Janicke RU. Apoptosis Resistance of MCF-7 Breast Carcinoma Cells to Ionizing Radiation Is Independent of p53 and Cell Cycle Control but Caused by the Lack of Caspase-3 and a Caffeine-Inhibitable Event. Cancer Res. 2004;64:7065–7072. doi: 10.1158/0008-5472.CAN-04-1082. [DOI] [PubMed] [Google Scholar]
- Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, et al. Chemical Biology Investigation of Cell Death Pathways Activated by Endoplasmic Reticulum Stress Reveals Cytoprotective Modulators of ASK1. J Biol Chem. 2009;284:1593–1603. doi: 10.1074/jbc.M807308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008a;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha YI, Lu B. Autophagy upregulation by inhibitors of caspase-3 and mTOR enhances radiotherapy in a mouse model of lung cancer. Autophagy. 2008b;4:659–668. doi: 10.4161/auto.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Moretti L, Lu B. M867, a novel selective inhibitor of caspase-3 enhances cell death and extends tumor growth delay in irradiated lung cancer models. PLoS ONE. 2008c;3:e2275. doi: 10.1371/journal.pone.0002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Mutter RW, Cao C, Albert JM, Freeman M, Hallahan DE, et al. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006;281:36883–36890. doi: 10.1074/jbc.M607094200. [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Lee AS. GRP78 Induction in Cancer: Therapeutic and Prognostic Implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Marissen WE, Guo Y, Thomas AA, Matts RL, Lloyd RE. Identification of caspase 3-mediated cleavage and functional alteration of eukaryotic initiation factor 2alpha in apoptosis. J Biol Chem. 2000;275:9314–9323. doi: 10.1074/jbc.275.13.9314. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective Tumor Cell Death by {sigma}-2 Receptor Ligand Siramesine Involves Lysosomal Leakage and Oxidative Stress. Cancer Res. 2005;65:8975–8983. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- Palmerini F, Devilard E, Jarry A, Birg F, Xerri L. Caspase 7 downregulation as an immunohistochemical marker of colonic carcinoma. Human Pathology. 2001;32:461–467. doi: 10.1053/hupa.2001.24328. [DOI] [PubMed] [Google Scholar]
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee A-H, Yoshida H, et al. XBP1 Is Essential for Survival under Hypoxic Conditions and Is Required for Tumor Growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- Satoh S, Hijikata M, Handa H, Shimotohno K. Caspase-mediated cleavage of eukaryotic translation initiation factor subunit 2alpha. Biochem J. 1999;342(Pt 1):65–70. [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Talloczy Z, Jiang W, Virgin HWt, Leib DA, Scheuner D, Kaufman RJ, et al. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman E, Fan Y, Stawowczyk M, Chen HM, Yue Z, Zong WX. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ. 2008;15:422–425. doi: 10.1038/sj.cdd.4402234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative photographs in wild-type (WT) and caspase-3/7 DKO MEF cells show that the characteristic punctate appearance of GFP-LC3, indicative of autophagosome formation, increases with radiation in both cell types.
(A) Cell extracts from WT MEF cells were incubated with active recombinant caspase-3 for 0, 1 and 6h. eIF2α with intact C-terminus or N-terminus were then probed with the respective antibodies. (B) Wild-type (WT) MEF cells were treated by tunicamycin (TM) (1µg/ml for 12h or 24h) or radiation (10 Gy for 24h and 48h) and were then harvested for immunoblotting analyses for caspase-3, cleaved caspase-3, uncleaved eIF2α, and total eIF2α. Actin was probed to demonstrate equal loading. (C) Caspase-3/7 DKO MEF cells were treated by tunicamycin (TM) (1µg/ml for 12 hrs or 24 hrs) or radiation (10 Gy for 24 hrs and 48 hrs) and were then harvested for immunoblotting analyses for caspase-3, cleaved caspase-3, uncleaved eIF2α, and total eIF2α. Actin was probed to demonstrate equal loading.