Abstract
Aim:
To investigate the influence of trimetazidine, which is known to be an antioxidant and modulator of metabolism, on cardiac function and the development of diabetic cardiomyopathy in db/db mouse.
Methods:
Trimetazidine was administered to db/db mice for eight weeks. Cardiac function was measured by inserting a Millar catheter into the left ventricle, and oxidative stress and AMP-activated protein kinase (AMPK) activity in the myocardium were evaluated.
Results:
Untreated db/db mice exhibited a significant decrease in cardiac function compared to normal C57 mice. Oxidative stress and lipid deposition were markedly increased in the myocardium, concomitant with inactivation of AMPK and increased expression of peroxisome proliferator-activated receptor coactivator-1α (PGC-1α). Trimetazidine significantly improved systolic and diastolic function in hearts of db/db mice and led to reduced production of reactive oxygen species and deposition of fatty acid in cardiomyocytes. Trimetazidine also caused AMPK activation and reduced PGC-1α expression in the hearts of db/db mice.
Conclusion:
The data suggest that trimetazidine significantly improves cardiac function in db/db mice by attenuating lipotoxicity and improving the oxidation status of the heart. Activation of AMPK and decreased expression of PGC-1α were involved in this process. Furthermore, our study suggests that trimetazidine suppresses the development of diabetic cardiomyopathy, which warrants further clinical investigation.
Keywords: cardiac protection, cardiomyopathy, diabetes, diabetic cardiovascular complications, mice, mitochondria, heart, oxidative stress
Introduction
The prevalence of diabetes mellitus is rapidly increasing worldwide, as well as within China. Patients with diabetes mellitus are at an increased risk of developing cardiovascular disease; cardiovascular complications are therefore the leading cause of diabetes-related morbidity and mortality1. Diabetes mellitus also can affect cardiac structure and function in the absence of coronary artery disease, a condition known as diabetic cardiomyopathy. This term was introduced 30 years ago by Rubler et al2, who reported a series of four diabetic patients with congestive heart failure, normal epicardial coronary arteries, and no other known conditions that might have led to congestive heart failure.
The increased generation of reactive oxygen species (ROS) and impaired antioxidant defenses both cause oxidative stress, which is a contributing factor to the development and progression of diabetic cardiomyopathy3, 4. Several groups have shown that ROS are overproduced in both type 1 and type 2 diabetes5, 6, 7, 8. Increased ROS generation may trigger maladaptive signaling pathways leading to the activation of JNK, p38 kinase, and Akt, as well as to the further activation of ERK1/2, which could contribute to the pathogenesis of diabetic cardiomyopathy9. Increased ROS generation might also contribute to mitochondrial uncoupling, which could impair myocardial energetics in diabetes. Strategies that either reduce ROS or augment myocardial antioxidant defense mechanisms might have therapeutic value in improving myocardial function in diabetes mellitus patients3, 10, 11, 12, 13.
Altered myocardial substrate and energy metabolism also contribute significantly to the development of diabetic cardiomyopathy14, 15. The heart uses a variety of substrates for energy production, including fatty acids (FA) and glucose. The diabetic heart is characterized by reduced glucose and lactate metabolism and enhanced FA metabolism16, 17. Diabetic db/db mice have elevated rates of FA oxidation, which further increase as the supply of FA increases18, 19. Ectopic fat deposition and the presence of high ROS levels in diabetes resulted in lipid peroxidation20. Data from several animal models indicate that myocardial FA deposition and subsequent lipid peroxidation by ROS precede contractibility dysfunction6.
Metabolic modulation to sustain glucose use appears to prevent cardiac dysfunction in models of severe type 2 diabetes mellitus21, 22. Similar results have been observed in human patients with type 2 diabetes, suggesting that pharmacological interventions that can reduce cardiac FA utilization may improve cardiac performance23, 24, 25.
Trimetazidine [1-(2,3,4-trimethoxybenzyl)piperazine dihydrochloride, or TMZ] is a metabolism-modulating agent that inhibits FA oxidation secondary to an inhibition of long-chain 3-ketoacyl coenzyme A thiolase, reducing FA oxidation and increasing glucose oxidation26. In diabetic patients with ischemic heart disease, trimetazidine added to standard medical therapy improves left ventricular volumes and the left ventricular ejection fraction compared to a placebo27. Some studies have shown that trimetazidine also has antioxidant activity and decreases the formation of free radicals to improve mechanical function in the post-ischemic isolated rat heart28.
Here, we found that reduced cardiac contractile and diastolic function are accompanied by increased ROS formation and ectopic fat deposition, perhaps as a consequence of lipotoxicity in the diabetic heart. Furthermore, our results suggest that TMZ might attenuate diabetic cardiomyopathy and improve mechanical recovery of the diabetic heart by modifying the oxidation status.
Materials and methods
Animals
Experimental protocols complied with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Academy of Sciences of China. Control and diabetic mice C57BL/KsJ mice were used in this study. Homozygous C57BL6/KsJ-leprdb (db/db) and respective wild-type controls C57(+/+) were males between ten and twelve weeks of age and were supplied by the SLAC Experimental Animal Center in Shanghai, China. The animals were housed in groups of four to five in a room maintained at a temperature of 23±1 °C and 55%±5% humidity with a 12 h light/dark cycle and access to food and water ad libitum. The db/db mice were divided randomly into three groups. One group of mice (n=10) was given a low dose (10 mg·kg−1·d−1) of TMZ (Servier, France) by gavage while a second group (n=8) was given a high dose (30 mg·kg−1·d−1). Wild-type and db/db control mice were treated with ultrapure water.
Determination of in vivo hemodynamic parameters
Mice were anesthetized by intraperitoneal administration of pentobarbital at 50 mg/kg body weight, and hemodynamic variables were monitored using a catheter tip manometer (AD Instruments) advanced from the right carotid artery via the aortic arch into the left ventricle (LV) at the end of the treatment period. Global systolic function was measured as LV peak pressure (LVPSP), LV end-diastolic pressure (LVEDP), and the maximum-minimum rate of pressure increase (dp/dtmax). Global LV end systole was defined as the point of minimum dp/dt and LV end diastole as the beginning of the sharp upward incline of the LV dp/dt tracing. Continuous parameters were recorded on a chart recorder and in digitalized form on computer disk for averaging.
Analysis of tissue homogenates
Superoxide dismutase (SOD) activity and malondialdehyde (MDA) concentration were detected in, respectively, 1% and 10% homogenates of mouse heart tissue. SOD activity was determined using a kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The concentration of MDA, an indicator of lipid peroxidation, was measured using thiobarbituric acid-reactive substances using a kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Analysis of serum metabolites
Serum levels of glucose, triglycerides (TG), and insulin were determined for control diabetic (db/db) and trimetazidine-treated db/db mice under normal diet. Blood was collected and centrifuged in an Eppendorf centrifuge at 14 000 r/min for 10 min. The resulting serum sample was stored at -80 °C for subsequent analysis. Blood glucose was determined using the glucose oxidase method with one-touch test strips (Lifescan; Johnson & Johnson Co, Milpitas, CA). Triglyceride concentrations were determined in 10 μL serum samples using a kit from the Biosino Bio-Technology and Science Incorporation (Beijing, China). Plasma insulin levels were determined using an ELISA kit (Linco Research, St Charles, MO, USA) according to the manufacturer's recommendations.
Histological analysis and in situ localization of ROS
To detect neutral lipids, 10 μm frozen sections of mouse heart tissue were stained with oil red O, counterstained with hematoxylin, and analyzed by fluorescence microscopy. For in situ localization of ROS, frozen 10 μm sections of heart tissue from wild-type, db/db, and TMZ-treated mice were incubated with 5- (and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (DCF-DA, 10 μmol/L, Molecular Probes) fluorophores, which are sensitive to hydrogen peroxide (H2O2). DCFH-DA (Sigma-Aldrich, St Louis, MO, USA) was deacetylated intracellularly by nonspecific esterase and was furthered oxidized by H2O2 to form the fluorescent compound DCF. Fluorescence was detected by confocal microscopy (Bio-Rad 1024, Hemel Hempstead, UK). Cells were incubated with 10 μmol/L DCFH-DA at 37 °C for 20 min, and DCF fluorescence was detected immediately by fluorescence microscopy. Images were transferred to ImageJ software and converted to grayscale with a range from 0 to 255 arbitrary units (0 represents complete staining; 255 represents no staining). Oil red O staining was quantified and expressed as a percentage of total area, and the DCF fluorescence was expressed as a percentage of relative grayscale value. Four mice from each group were analyzed and two tissue sections from each mouse were imaged.
Western analysis
Protein samples (15–20 μL, 2 mg/mL) were separated by SDS-PAGE (Bio-Rad) using an 8% or 10% (wt/vol) acrylamide gel. Proteins were transferred to nitrocellulose membranes using a Semi-Dry Transfer Cell (Bio-Rad). Nonspecific sites were blocked with 5% nonfat milk, and membranes were then incubated with primary antibodies (Santa Cruz, CA) at dilutions of 1:1000 in blocking solution, washed, and treated with horseradish peroxidase-linked secondary antibodies at dilutions of 1:5000 and 1:10 000. Bound antibody was detected by autoradiography using an enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL, USA) according to the manufacturer's recommendations. The bands were scanned and quantified by densitometric analysis using Quantity One software.
Statistical analysis
All data are represented as the group mean±standard error of the mean (SEM). Data were analyzed using the statistical program Instat. One-way ANOVA was used to compare values among groups. A Tukey-Grammar post hoc test was used to confirm intergroup differences; P<0.05 was considered significant. Differences between means were considered statistically significant when the P values were <0.05.
Results
Systemic metabolic parameters
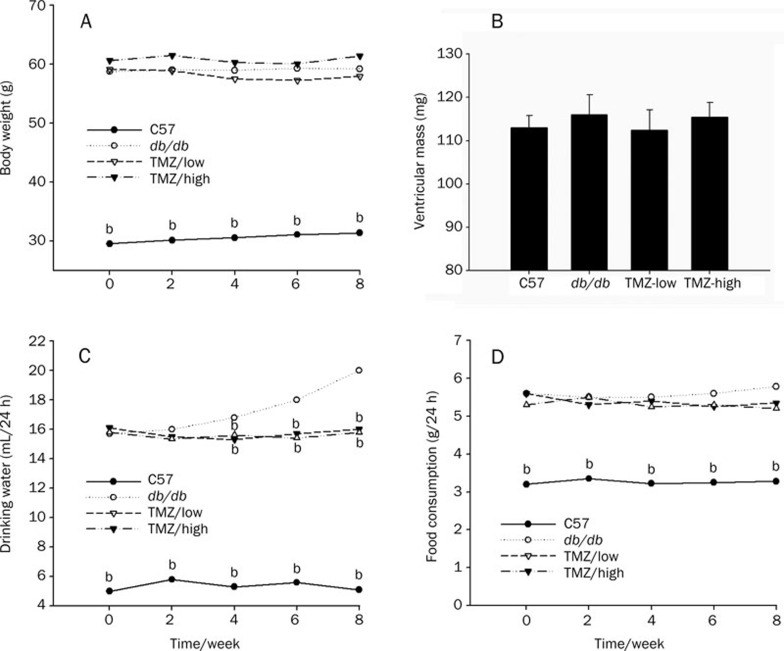
Mice were monitored every two weeks for eight weeks for changes in body mass, food consumption, and water consumption. Diabetic (db/db) mice weighed significantly more than C57 mice at the end of the experiment. The increase in body weight was primarily due to increased fat deposition. Despite the differences in body mass, ventricular (right and left) mass did not differ between the diabetic and control groups (Figure 1). These results are consistent with those of a previous study29. Neither the low nor the high dose of TMZ had an effect on body weight.
Figure 1.
Systemic metabolic parameters of control, diabetic and trimetazidine-treated mice. Body weight (A), water consumption (C), and food consumption (D) were measured in control (C57, n=8), diabetic (db/db, n=12), low-dose trimetazidine-treated (TMZ-low, n=10), and high-dose trimetazidine-treated (TMZ-high, n=8) mice. Ventricular mass (mg) (B) was measured at the time of death. Values represent means from at least five animals in each group. bP<0.05 compared to the db/db group.
The food and water consumption of db/db mice was much higher than that of C57 mice, consistent with the polydipsia and hyperphagia typically associated with diabetes (Figure 1C, 1D). The food consumption of TMZ-treated mice did not differ from that of control mice. However, TMZ treatment significantly reduced the water consumption of db/db mice.
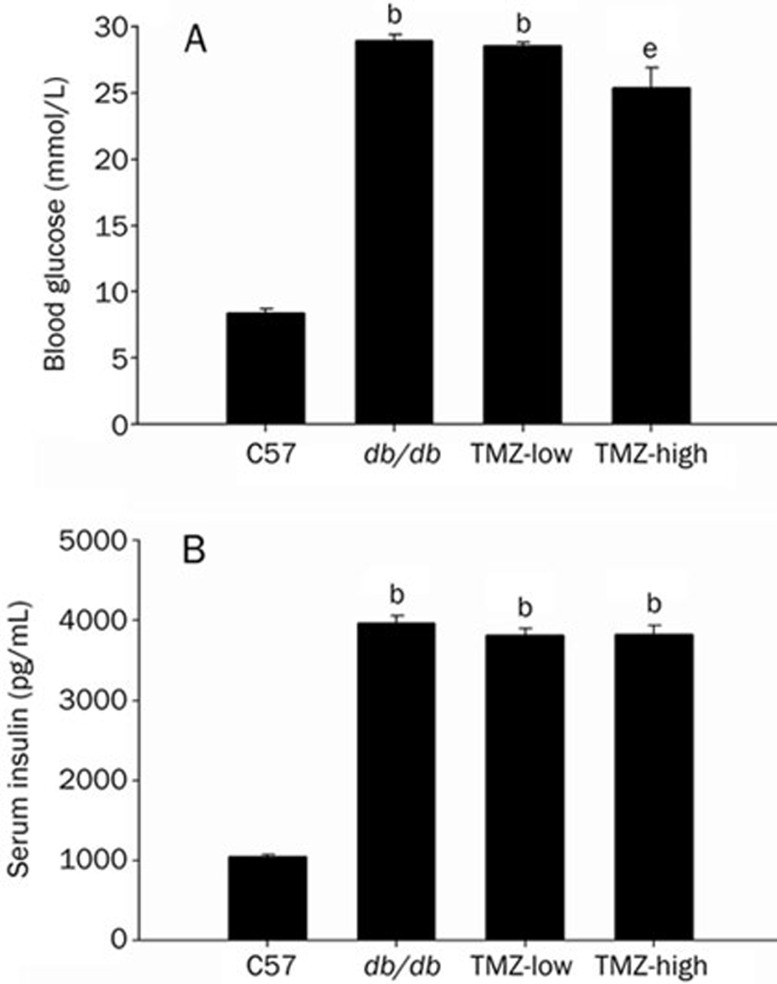
As expected22, diabetic mice had significantly elevated blood glucose levels (28.9±0.50 mmol/L vs 8.36±0.37 mmol/L), an indicator of their diabetic status. These mice also had significantly elevated levels of serum insulin (3960±92.0 pg/mL vs 1050±30.2 pg/mL, P<0.05) compared to C57 mice, indicating insulin resistance (Figure 2). The high-dose TMZ treatment did not affect the level of insulin, but blood glucose was slightly significantly decreased by this treatment (25.4±1.53 mmol/L vs 28.9±0.50 mmol/L, P<0.05, Figure 2).
Figure 2.
Blood glucose and serum insulin levels. (A) Blood glucose was determined using ONE-TOUCH test strips in C57, db/db, TMZ-low, and TMZ-high mice. (B) Serum insulin was measured by ELISA. Values represent means from at least five animals in each group. Vertical bars indicate the standard error of the mean (SEM). bP<0.05 compared to the C57 control group; eP<0.05 compared to the db/db group.
In vivo cardiac performance
To determine in vivo therapeutic effects of TMZ treatment on cardiac dysfunction, we directly measured hemodynamic parameters in anesthetized mice using the Millar cardiac catheter system. Table 1 shows that db/db mice exhibit a significant increase in heart rate, left ventricle maximum systolic pressure, left ventricle end systolic pressure (LVESP), and left ventricle end diastolic pressure (LVEDP), as well as reduction in stroke volume and cardiac output, compared to C57 controls. TMZ treatment reversed these parameters (P<0.05). In contrast, the rate of increase in LV pressure during systole (+dp/dt) and the rate of LV relaxation (-dp/dt) were significantly higher in db/db mice than in C57 mice. There were no differences in +dp/dt or -dp/dt between db/db and TMZ-treated mice. In our study, the hemodynamic parameters were increased in the TMZ treated group, indicating that trimetazidine affects hemodynamics and improves cardiac function.
Table 1. In vivo hemodynamic characterization of C57, db/db, TMZ-low, and TMZ-high mice. Data shown represent the mean±SEM for control (C57, n=8), diabetic (db/db, n=10), low-dose TMZ-treated (TMZ-low, n=6) and high-dose TMZ-treated (TMZ-high, n=6) mice. bP<0.05 compared to the C57 mice group. eP<0.05 compared to the db/db mice group.
| C57 | db/db | TMZ-low | TMZ-high | |
|---|---|---|---|---|
| Heart rate (beat/min) | 300±8.05 | 356±21.7b | 283±16.2e | 295±8.12e |
| Maximum volume (μL) | 18.1±0.39 | 14.3±0.22b | 14.9±0.42b | 16.7±0.65be |
| LVESP (mmHg) | 94.6±2.33 | 129±2.42b | 115±7.44be | 112±3.42be |
| LVEDP (mmHg) | 5.76±0.32 | 124±3.31b | 110±7.10be | 108±4.20be |
| LVEDP (mmHg) | 5.76±0.32 | 10.4±0.92b | 7.63±0.67e | 7.89±0.96e |
| Stroke volume (μL) | 3.86±0.14 | 3.00±0.15b | 3.35±0.21 | 3.98±0.11e |
| Cardiac output (μL/min) | 735±41.9 | 559±30.0b | 775±63.0e | 739±24.0e |
| dp/dtmax (mmHg/s) | 3190±278 | 7440±434b | 6730±488b | 6270±525b |
| dp/dtmin (mmHg/s) | −2860±101 | −4040±324b | −4160±181b | −3990±364b |
Hemodynamic parameters were measured with a cardiac catheter tip manometer advanced from the right carotid artery via the aortic arch into the left ventricle. LVESP, LV end systolic pressure; LVEDP, LV end diastolic pressure; dp/dtmax and dp/dtmin, positive and negative first derivative of LV contraction and relaxation, respectively.
Attenuation of serum triglyceride and fat deposition in myocardium by TMZ
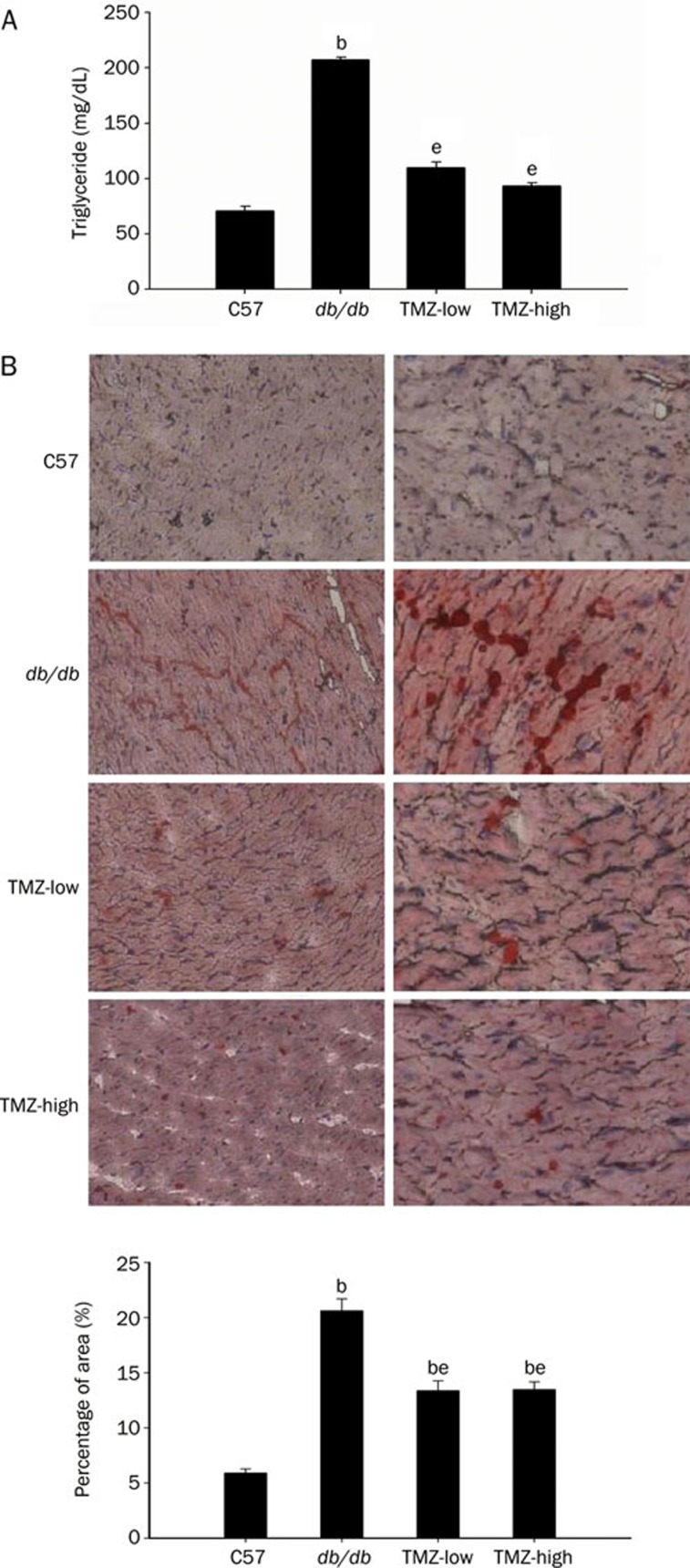
We assayed serum triglyceride levels and myocardial fat deposition to determine the in vivo effects of TMZ treatment on lipid metabolism. The level of serum triglycerides observed in db/db mice was markedly elevated compared to that of C57 mice (207.1±2.55 mg/dL vs 70.9±4.00 mg/dL), consistent with diabetic hyperlipemic status. Nevertheless, treatment with TMZ significantly lowered serum triglyceride levels in db/db mice at both low and high dose (109.8±5.06 and 93.1±3.12 mg/dL, respectively; Figure 3A). One histologic signature of the diabetic heart is lipid accumulation in myocytes due to increased FA import. Oil red O staining revealed substantial neutral lipid droplet accumulation in the cardiac myocytes of db/db mice, whereas C57 myocytes were primarily oil red O-negative. Frozen sections of cardiac tissue from TMZ-treated mice showed that lipid deposition was reduced in these animals compared to db/db mice (Figure 3B). These data suggest that TMZ treatment attenuates the effects of pathologic lipid metabolism in diabetes.
Figure 3.
Effect of trimetazidine(TMZ) treatment on serum and intramyocardial lipid accumulation. (A) Serum triglyceride levels were reduced in TMZ-treated mice compared to diabetic db/db mice. bP<0.05 compared to C57 group; eP<0.05 compared to db/db group. (B) Photomicrographs showing the histologic appearance of ventricular tissue from C57, diabetic (db/db), low-dose trimetazidine-treated (TMZ-low), and high-dose trimetazidine-treated (TMZ-high) mouse heart at low (upper panels) and high (lower panels) magnification. Frozen tissue sections were stained with oil red O. Red droplets indicate neutral lipids. Oil red O staining quantification is expressed as a percentage of total area. bP<0.05 compared to C57 group. eP<0.05 compared to db/db group.
Reduction of ROS generation and lipid peroxidation by TMZ
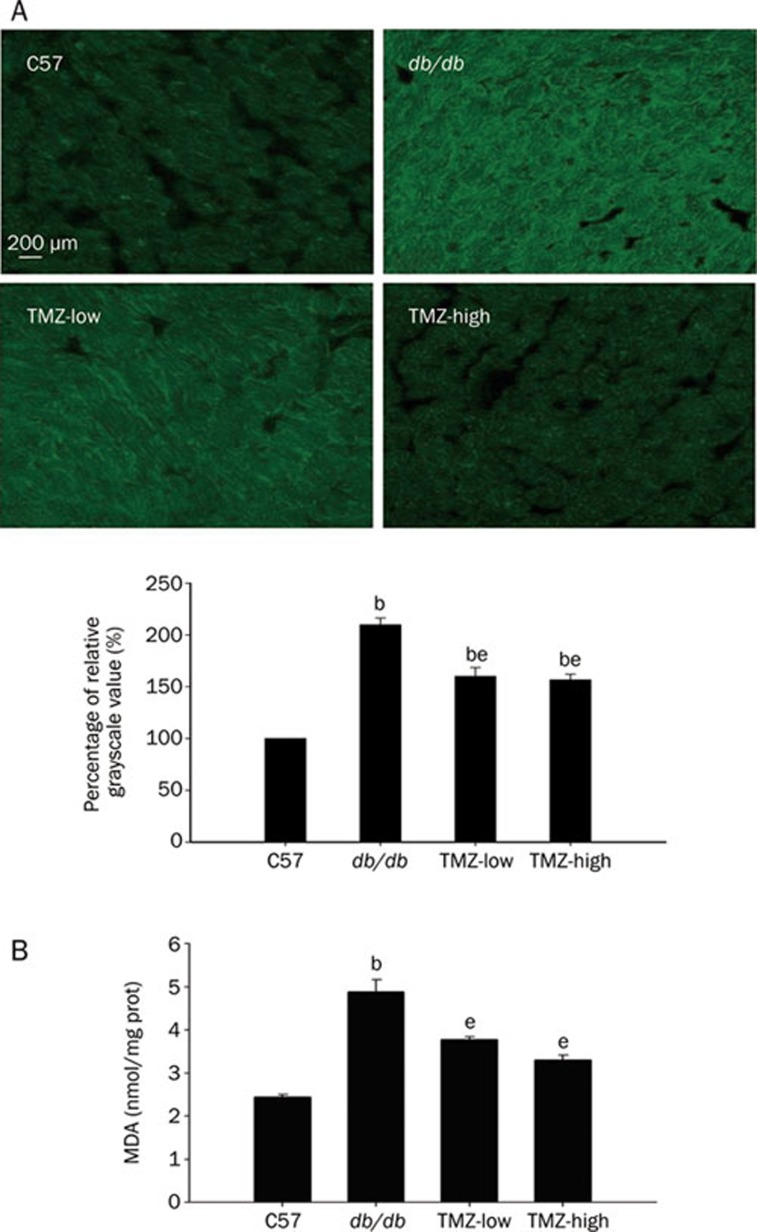
Lipid accumulation and increased ROS generation act together to promote lipotoxicity in diabetic cardiomyopathy. Therefore, we analyzed ROS in cardiac tissue sections with the fluorescence probe DCF-DA and determined the level of lipid peroxidation by measuring malondialdehyde (MDA) to investigate the effects of TMZ treatment on ROS generation as a possible mechanism of its cardioprotective effects. We found that fluorescent ROS staining was increased in the myocardium of diabetic mice, indicating that ROS generation was elevated. TMZ treatment at both low and high doses dramatically reduced ROS accumulation in the myocardium, suggesting that ROS generation was attenuated. MDA was simultaneously and markedly increased in db/db mouse heart homogenates as a consequence of lipid peroxidation, reflecting the severe lipotoxicity in the diabetic heart. After 8 weeks of treatment with low or high doses of TMZ, mice had significantly decreased tissue levels of MDA (Figure 4). These data suggest that chronic administration of TMZ could reduce cardiotoxicity by reducing ROS production and concomitant lipid peroxidation.
Figure 4.
Attenuation of oxidative damage by trimetazidine(TMZ) treatment. (A) DCF staining of frozen heart tissue sections from each group suggests that treatment with trimetazidine reduced the ROS generation in db/db mice hearts. DCF staining quantification is expressed as a percentage of relative grayscale value. bP<0.05 compared to C57 group; eP<0.05 compared to db/db group. (B) Malondialdehyde (MDA) levels in heart homogenates from TMZ-treated groups were markedly reduced compared to the db/db group. bP<0.05 compared to the C57 group; eP<0.05 compared to the db/db group.
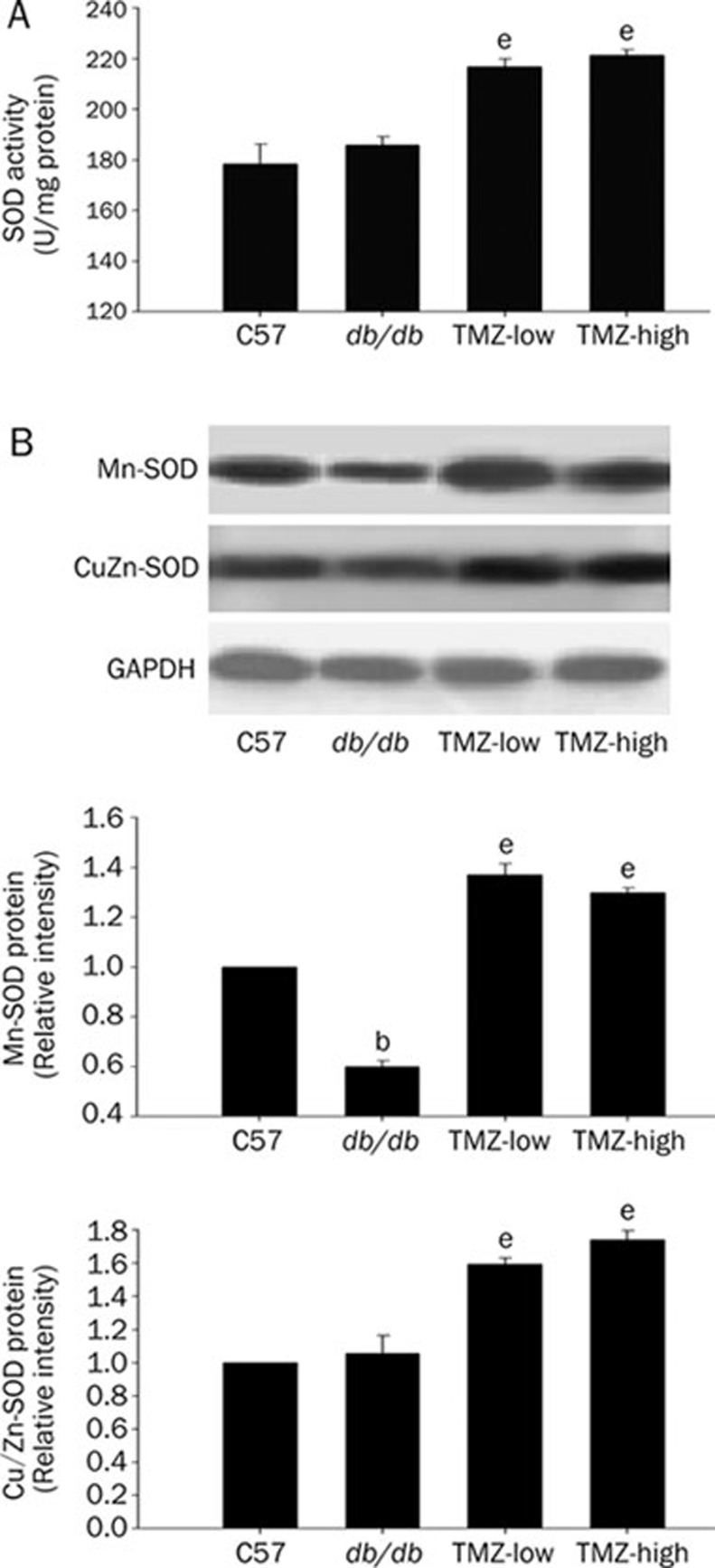
Improvement of antioxidant capability
Increased ROS generation and impaired antioxidant defenses could both contribute to oxidative stress in the diabetic heart. Superoxide dismutase (SOD) is an antioxidase that exists in several forms, including the mitochondrial protein MnSOD and the cytoplasmic protein Cu/ZnSOD. To determine cardiac antioxidant capability in the four groups of mice, we measured the total myocardial SOD activity in each group. Figure 5 shows that although db/db mice had only slightly higher antioxidant activity than did C57 mice, both low- and high-dose TMZ treatments significantly enhanced SOD activity (Figure 5A). We also used Western blot analysis to investigate changes in the expression of Cu/ZnSOD and MnSOD as alternative mechanisms by which myocardial O2 · generation could be reduced. The levels of Cu/ZnSOD protein expression in myocardia from db/db and C57 mice were very similar, but chronic treatment with TMZ had a significant effect on Cu/ZnSOD expression. MnSOD protein expression was decreased in the hearts of db/db mice compared to C57 mice, suggesting mitochondrial impairment. However, TMZ treatment inhibited the decrease in MnSOD protein expression, indicating that TMZ might protect the mitochondria from damage (Figure 5). Taken together, these findings indicate that TMZ improved the antioxidant capability of the diabetic heart by increasing the expression and activity of SOD.
Figure 5.
Effect of trimetazidine(TMZ) treatment on the antioxidant capability of superoxide dismutase (SOD). (A) SOD activity was increased in low- or high-dose TMZ-treated mice compared to db/db mice. (B) MnSOD and Cu/ZnSOD in myocardial homogenates from each group were analyzed by Western blotting; the data suggest that both low- and high-dose treatment with TMZ increased the expression of MnSOD and Cu/ZnSOD. Values represent the mean±SEM relative densitometric intensity normalized to actin and expressed as % control ±SEM from three experiments. bP<0.05 compared to the C57 group; eP<0.05 compared to the db/db group.
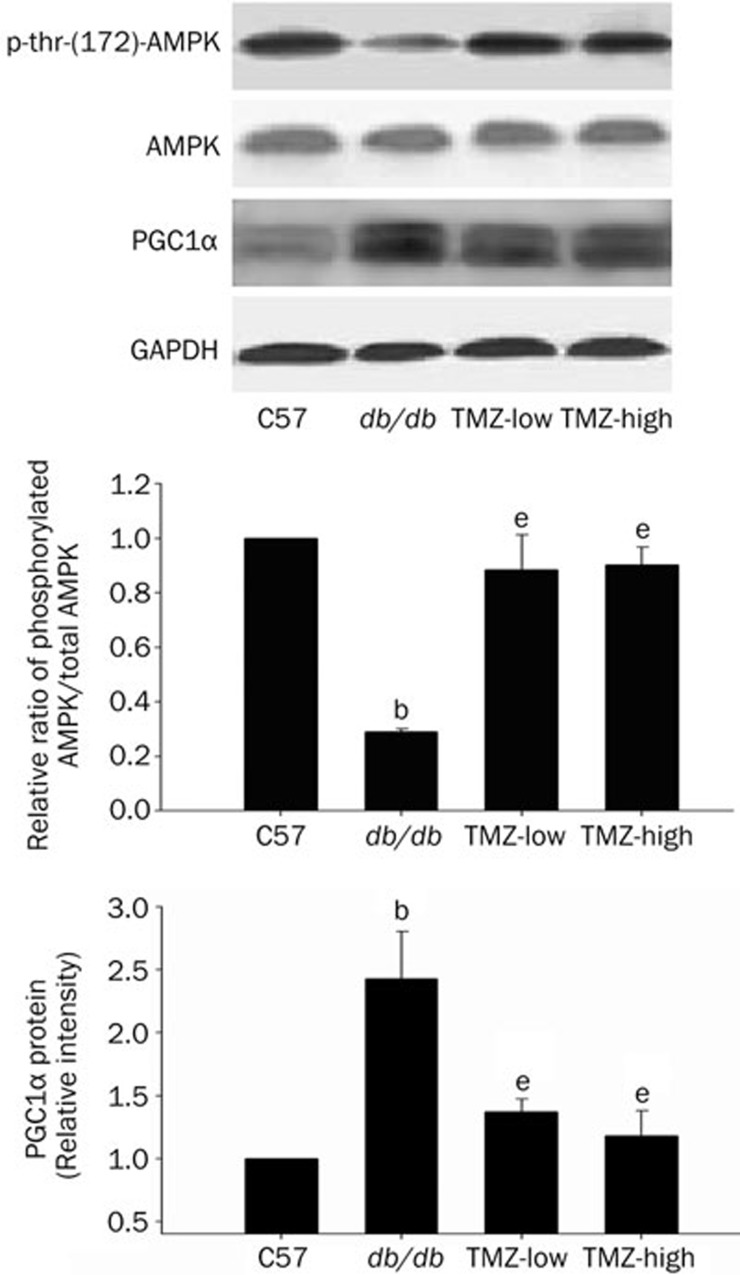
Role of AMPK and PGC1α in the cardioprotection induced by TMZ
AMPK is reportedly involved in mediating protective effects in diabetic hearts30. The expression of peroxisome proliferator-activated receptor coactivator-1α (PGC-1α), the primary regulator of glycometabolism and lipid metabolism genes, is increased in the db/db mouse heart31. Therefore, we investigated whether protection of the diabetic myocardium by TMZ in the present study could be explained by an effect of the drug on AMPK and PGC1α expression. Western blot analysis showed that the level of phosphorylated AMPK (Tyr172) in db/db mice hearts was significantly lower than that in C57 mice but was significantly enhanced by TMZ treatment, suggesting that this treatment increases AMPK activity in the diabetic heart. The protein expression level of PGC1α was significantly higher in diabetic mouse heart than in C57 heart. In contrast to the findings for AMPK, TMZ treatment attenuated the expression of PGC1α. These observations suggest that the mechanisms of TMZ-mediated cardiocyte protection may involve the activation of AMPK and inhibition of PGC1α expression.
Discussion
Diabetic cardiomyopathy is a complex multifactorial pathology that includes altered substrate metabolism and increased oxidative stress. Unlike other cardioprotective drugs, TMZ has protective effects that are not related to a direct hemodynamic effect32, 33. Rather, it has been proposed that TMZ acts by directly improving myocardial energy metabolism, resulting in cytoprotection26. Meanwhile, several studies have revealed that TMZ has an indirect antioxidant effect34, 35.
In this study, we have presented compelling evidence that TMZ protects the heart against lipotoxicity and oxidative stress in diabetic individuals, which would lead to improved cardiac function. This cardioprotective effect, manifested by reduced lipid accumulation in tissues and triglyceride levels in serum together with reduced ROS production, is associated with an enhancement of SOD protein expression and activity. Increased AMPK activity and inhibition of PGC1α protein expression are also potentially involved in the signaling pathway underlying cardioprotection.
Diabetes mellitus is a complex metabolic syndrome associated with systemic abnormalities such as obesity, polydipsia, hyperphagia, hyperglycemia, and hyperinsulinemia. These symptoms were all manifested in db/db mice, and ventricular mass did not differ among groups, consistent with the findings of a previous study22. The present study revealed that TMZ treatment was ineffective in treating these disease features, similar to earlier findings for STZ-induced mice36. Interestingly, however, blood glucose was decreased slightly in the group treated with high-dose TMZ. We presume that the db/db mouse is a model of severe diabetes and that the dose of TMZ used here has no effect on systemic abnormalities. Meanwhile, TMZ treatment improved cardiac function in db/db mice, particularly at a high dose. In contrast to previous studies, in which an isolated, working heart was perfused with TMZ37, we measured hemodynamic parameters in db/db mice in vivo using LV catheterization following chronic TMZ administration. It should be noted that in this study, LV contractility as determined by dp/dt, left ventricle maximum systolic pressure, and left ventricle end systolic pressure were increased in db/db mice. In previous study in humans38, patients with heart failure, especially those who are also diabetic, trimetazidine administration was shown to significantly increase hemodynamic parameters, including left ventricular ejection fraction (LVEF). Meanwhile, a study in diabetic rat hearts using Langendorff perfusion showed that trimetazidine treatment significantly enhanced hemodynamic parameters37. In this study, mice in the db/db group exhibited a diminished left ventricle maximum volume and increased LV maximum pressure and LVESP as well as dp/dt (max and dp/dt min); the heart rate was also increased. One possible explanation for this observation is that increased plasma volume and sympathetic activation in db/db mice occurs due to cardiac dysfunction related to diabetes and obesity18, which could induce an accelerated heart rate and increased left ventricle maximum systolic pressure and left ventricle end systolic pressure, especially in early stages of heart failure. These observations were supported by Van den Bergh et al39. TMZ treatment restored these hemodynamic parameters to normal (control) levels, which further support our hypothesis. Meanwhile, stroke volume and cardiac output were decreased and LVEDP was increased in the db/db group; these values were also improved by TMZ treatment, especially in high dose group, suggesting that TMZ treatment could significantly improve cardiac function.
The diabetic heart is characterized by reduced glucose metabolism and enhanced FA metabolism16, 17. Therapeutic metabolic intervention in Zucker diabetic rats restores cardiac function and reverses lipotoxicity40, 41, 42. The changes in substrate use that characterize diabetic hearts directly contribute to impaired cardiac function43, 44. Studies in transgenic mouse models of lipotoxic cardiomyopathy have shown that reversal of myocardial steatosis normalizes cardiac function. In addition, metabolic modulation to sustain glucose use prevents cardiac dysfunction in a db/db mouse model of severe type 2 diabetes mellitus21, 22. TMZ was shown to inhibit long chain 3-ketoacyl CoA thiolase activity, resulting in reduced FA oxidation secondary to a stimulation of glucose oxidation26. Based on these data, we hypothesized that modulation of lipid metabolism by TMZ might have a substantial effect in improving cardiac function. In the present study, we showed that TMZ treatment did reduce lipid accumulation in the myocardium. Moreover, TMZ also reduced serum triglyceride levels, although its effect was subtle. Nonetheless, at this stage, we cannot exclude the possibility that the effect of reduced lipid deposition in the myocardium is secondary to a reduction of serum triglyceride levels. In addition to the mechanism of reducing FA oxidation demonstrated here, TMZ might also affect FA uptake. TMZ can decrease FA oxidation but also decrease TG accumulation (ie, decrease FA synthesis). It has also been suggested that TMZ inhibits FA synthesis, possibly through down-regulation of the rate limiting enzyme acetyl-CoA carboxylase-alpha (ACC) via AMPK activation45. When FA synthesis occurs more slowly than FA oxidation, the free FA is decreased and TG synthesis is also decreased. Thus, AMPK activation is also an important pathway through which TMZ can reduce lipotoxicity. However, the exact mechanisms involved are unclear at present and will require further study.
Increased oxidative stress in the diabetic heart is a contributing factor to the development and progression of diabetic cardiomyopathy3, 4. Increased ROS generation and impaired antioxidant defenses could both contribute to oxidative stress. Some studies have shown that ROS generation increases in both type 1 and type 2 diabetes5, 6, 7, 8. Studies in cardiac cells have suggested that chronic exposure to FA inhibits AMPK activation28, 46. In this study, the activity and protein expression of SOD were increased, and H2O2 levels were decreased upon TMZ administration. However, other antioxidant enzymes might be involved in this mechanism; for example, catalase converts H2O2 to H2O. Thus, we hypothesized that TMZ might attenuate ROS generation by enhancing antioxidant defenses.
The enzyme 5′-AMP-activated protein kinase (AMPK) has emerged as a key regulator of carbohydrate and fat metabolism, working as a “fuel sensor” in most tissues47. Two primary acute consequences of AMPK activation are an increase in glucose uptake by induction of translocation of the glucose 4 transporter from microvesicles in the cytoplasm to the plasma membrane, where fusion takes place, and an increase in FA oxidation by phosphorylation and inactivation of acetyl-CoA carboxylase (ACC), the rate-limiting enzyme in FA synthesis48, 49. Studies have suggested that chronic exposure to FA inhibits AMPK activation in cardiac cells50. Furthermore, levels of PGC-1 mRNA are significantly increased in the db/db mouse heart compared to controls30. In this study, serum triglyceride and lipid deposition in the myocardia of db/db mice were both increased while p-AMPK expression was decreased, confirming previous research. TMZ can decrease FA oxidation but also decrease TG accumulation, suggesting that TMZ inhibits TG or FA synthesis, which may be regulated by AMPK activation via phosphorylation and inactivation of ACC.
Peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1α) coactivates PPARα, which controls genes involved in cardiac FA oxidation and mitochondrial respiration51. The activation of PPARα can accelerate FA oxidation and the incorporation of lipids into triacylglycerol in many tissues. The activation of PPARα also increases the expression of pyruvate dehydrogenase kinase 4, which reduces glucose oxidation. Concomitant with this event, PPARα activation increases the expression levels of genes such as CD36, which regulates cellular FA uptake, and malonyl CoA decarboxylase, which degrades malonyl CoA, thereby derepressing carnitine palmitoyl transferase-1 and stimulating mitochondrial FA uptake31, 52, 53, 54. In the present study, db/db mouse heart exhibited decreased expression of p-AMPK and increased expression of PGC-1α. However, TMZ treatment enhanced AMPK activity and increased expression of PGC-1α, suggesting that AMPK and PGC-1α might be involved in the mechanism of TMZ-induced improvement of cardiac function. Levels of PGC-1 mRNA are significantly increased in the cardiac tissue of db/db mice compared to controls31. In this study, PGC-1α expression was increased in the db/db mouse heart, consistent with previous results from Finck et al. Upon TMZ treatment, PGC-1α was decreased in db/db mice but remained higher than in non-diabetic controls (C57 mice). Mitochondrial biogenesis is one function of PGC-1α55. We presumed that the mitochondria in db/db mice were damaged and that increased PGC-1α synthesis might be a compensatory response to increase mitochondrial activity. TMZ might protect the mitochondria from the injury that induced a lower level of compensatory response during the development of chronic diabetes. Thus, down-regulation of PGC-1α in TMZ-treated mice may be involved in the mechanism underlying their reduced lipid metabolism and increased glucose metabolism.
Taken together, the results presented here suggest that the formation of ROS and ectopic fat deposition both contribute to the development and progression of diabetic cardiomyopathy. Furthermore, TMZ treatment might improve the mechanical recovery of the diabetic heart by both decreasing the production of ROS and attenuating lipotoxicity. Our findings also suggest that TMZ treatment could be pursued as a novel therapeutic approach to diabetic cardiomyopathy.
Author contribution
Yuan-jing Li and Pei-hua WANG designed research; Yuan-jing LI and Chen CHEN performed research; Yuan-jing LI and Ming-hui ZOU analyzed data; Yuan-jing LI, Chen CHEN, and Dao-wen WANG wrote the paper.
Figure 6.
AMPK phosphorylation and PGC1α expression in C57, db/db, TMZ-low, and TMZ-high mouse heart tissue. Top panel: p-AMPK, AMPK, and PGC1α levels in hearts from each group were determined by Western blot analysis. Middle and bottom panels: quantitative analysis of p-AMPK, AMPK, and PGC1α in heart tissue. Values represent the mean±SEM of the relative densitometric intensity normalized to a control and expressed as % control±SEM from three experiments. bP<0.05 compared to the C57 group; eP<0.05 compared to the db/db group.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (No 2007CB512004), National Natural Science Foundation of China (No 30770882) and Wuhan City grants.
References
- Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–97. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Cai L. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med. 2006;41:851–61. doi: 10.1016/j.freeradbiomed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–9. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold LE, Ren J. Streptozotocin directly impairs cardiac contractile function in isolated ventricular myocytes via a p38 map kinase-dependent oxidative stress mechanism. Biochem Biophys Res Commun. 2004;318:1066–71. doi: 10.1016/j.bbrc.2004.04.138. [DOI] [PubMed] [Google Scholar]
- Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–48. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35:615–21. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–81. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- Matsushima S, Kinugawa S, Ide T, Matsusaka H, Inoue N, Ohta Y, et al. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am J Physiol Heart Circ Physiol. 2006;291:H2237–45. doi: 10.1152/ajpheart.00427.2006. [DOI] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, et al. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–59. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–33. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta. 2005;1734:112–26. doi: 10.1016/j.bbalip.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–9. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55:466–73. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53:1412–7. doi: 10.2337/diabetes.53.6.1412. [DOI] [PubMed] [Google Scholar]
- Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–82. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–13. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev. 2002;7:115–30. doi: 10.1023/a:1015320423577. [DOI] [PubMed] [Google Scholar]
- Fragasso G, Piatti Md PM, Monti L, Palloshi A, Setola E, Puccetti P, et al. Short- and long-term beneficial effects of trimetazidine in patients with diabetes and ischemic cardiomyopathy. Am Heart J. 2003;146:E18. doi: 10.1016/S0002-8703(03)00415-0. [DOI] [PubMed] [Google Scholar]
- Thrainsdottir IS, von Bibra H, Malmberg K, Ryden L. Effects of trimetazidine on left ventricular function in patients with type 2 diabetes and heart failure. J Cardiovasc Pharmacol. 2004;44:101–8. doi: 10.1097/00005344-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–8. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- Rosano GM, Vitale C, Sposato B, Mercuro G, Fini M. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol. 2003;2:16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambert S, Vergely C, Filomenko R, Moreau D, Bettaieb A, Opie LH, et al. Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol Cell Biochem. 2006;283:147–52. doi: 10.1007/s11010-006-2518-9. [DOI] [PubMed] [Google Scholar]
- Campbell FM, Kozak R, Wagner A, Altarejos JY, Dyck JR, Belke DD, et al. A role for peroxisome proliferator-activated receptor alpha (PPARalpha) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem. 2002;277:4098–103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- Kewalramani G, An D, Kim MS, Ghosh S, Qi D, Abrahani A, et al. AMPK control of myocardial fatty acid metabolism fluctuates with the intensity of insulin-deficient diabetes. J Mol Cell Cardiol. 2007;42:333–42. doi: 10.1016/j.yjmcc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detry JM, Sellier P, Pennaforte S, Cokkinos D, Dargie H, Mathes P. Trimetazidine: a new concept in the treatment of angina. Comparison with propranolol in patients with stable angina. Trimetazidine European Multicenter Study Group. Br J Clin Pharmacol. 1994;37:279–88. doi: 10.1111/j.1365-2125.1994.tb04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Volta S, Maraglino G, Della-Valentina P, Viena P, Desideri A. Comparison of trimetazidine with nifedipine in effort angina: a double-blind, crossover study. Cardiovasc Drugs Ther. 1990;4 Suppl 4:853–9. doi: 10.1007/BF00051292. [DOI] [PubMed] [Google Scholar]
- Iskesen I, Saribulbul O, Cerrahoglu M, Var A, Nazli Y, Sirin H. Trimetazidine reduces oxidative stress in cardiac surgery. Circ J. 2006;70:1169–73. doi: 10.1253/circj.70.1169. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M.Trimetazidine, oxidative stress, and cell injury during myocardial reperfusion Rev Esp Cardiol 200558895–7.Spanish. [PubMed] [Google Scholar]
- Ovide-Bordeaux S, Bescond-Jacquet A, Grynberg A. Cardiac mitochondrial alterations induced by insulin deficiency and hyperinsulinaemia in rats: targeting membrane homeostasis with trimetazidine. Clin Exp Pharmacol Physiol. 2005;32:1061–70. doi: 10.1111/j.1440-1681.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- Ikizler M, Erkasap N, Dernek S, Batmaz B, Kural T, Kaygisiz Z. Trimetazidine-induced enhancement of myocardial recovery during reperfusion: a comparative study in diabetic and non-diabetic rat hearts. Arch Med Res. 2006;37:700–8. doi: 10.1016/j.arcmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Gunes Y, Guntekin U, Tuncer M, Sahin M. Improved left and right ventricular functions with trimetazidine in patients with heart failure: a tissue Doppler study. Heart Vessels. 2009;24:277–82. doi: 10.1007/s00380-008-1118-x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh A, Flameng W, Herijgers P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail. 2006;8:777–83. doi: 10.1016/j.ejheart.2006.03.001. [DOI] [PubMed] [Google Scholar]
- McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–24. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–23. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Gao ZP, Forder JR.Impact of 1 wk of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation Am J Physiol 19992772 Pt 1): E342–51. [DOI] [PubMed] [Google Scholar]
- Golfman LS, Wilson CR, Sharma S, Burgmaier M, Young ME, Guthrie PH, et al. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab. 2005;289:E328–36. doi: 10.1152/ajpendo.00055.2005. [DOI] [PubMed] [Google Scholar]
- McFadden JW, Corl BA. Activation of AMP-activated protein kinase (AMPK) inhibits fatty acid synthesis in bovine mammary epithelial cells. Biochem Biophys Res Commun. 2009;390:388–93. doi: 10.1016/j.bbrc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Tritto I, Wang P, Kuppusamy P, Giraldez R, Zweier JL, Ambrosio G. The anti-anginal drug trimetazidine reduces neutrophil-mediated cardiac reperfusion injury. J Cardiovasc Pharmacol. 2005;46:89–98. doi: 10.1097/01.fjc.0000164091.81198.a3. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG.AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes Am J Physiol 19992771 Pt 1): E1–10. [DOI] [PubMed] [Google Scholar]
- Saha AK, Ruderman NB. Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol Cell Biochem. 2003;253:65–70. doi: 10.1023/a:1026053302036. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–70. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- Clark H, Carling D, Saggerson D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur J Biochem. 2004;271:2215–24. doi: 10.1111/j.1432-1033.2004.04151.x. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–78. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–17. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, et al. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–17. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]